Revista de la Facultad de Ciencias
Agrarias. Universidad Nacional de Cuyo. Tomo 56(1). ISSN (en línea) 1853-8665.
Año 2024.
Original article
Selection
of Rhizobium leguminosarum strains via symbiotic and production
variables in Pisum sativum L.
Selección
de cepas de Rhizobium leguminosarum por variables simbióticas y
productivas en Pisum sativum L.
Carolina Alba
Eöry2,
Gabriel María
Prieto3,
Daniela Adriana
Vallejo1,
Juan Martín
Enrico4,
Fernando
Salvagiotti4,
Alejandro
Perticari3
1INTA.
Instituto Nacional de Tecnología Agropecuaria. Laboratorio Bacterias Promotoras
del Crecimiento Vegetal (LBPCV). Instituto de Microbiología y Zoología Agrícola
(IMYZA). Nicolás Repetto y De Los Reseros s/n° - Hurlingham (B1686). Buenos
Aires. Argentina.
2Profesional
independiente. Arredondo 3488. Castelar (1712). Buenos Aires. Argentina.
3Agencia
de Extensión Rural Arroyo Seco. Estación Experimental Agropecuaria
Oliveros-INTA. San Martin 528. Arroyo Seco (2128). Santa Fe. Argentina.
4INTA.
Manejo de Cultivos, Suelos y Agua. Estación Experimental Agropecuaria Oliveros.
Ruta 11 km 353. 2206. Oliveros. Santa Fe. Argentina.
*piccinetti.carlos@inta.gob.ar
Abstract
Field pea (Pisum
sativum L.) is a winter symbiotic legume that associates with Rhizobium
leguminosarum sv viciae. This work aimed to evaluate strains of R.
leguminosarum for their infective ability and early-plant growth, BNF
contribution, biomass and grain yield. Seventy-eight specific strains and four
pea cultivars were evaluated in a growth chamber, five strains and three
cultivars were evaluated in a greenhouse, and three strains and two cultivars
were evaluated in a field experiment. Only 44-55% of all evaluated strains were
infective in the four cultivars. In the greenhouse, D70 and D156 strains showed
the best nodulation variables as well as higher N content and yield. The field
experiment showed D156 and D70 yielded a similar behavior for N content in
canopy biomass and individual nodule biomass, whereas D191 had a higher nodule number
per plant, aerial biomass and grain yields. D70 provided good nodulation, N
content in biomass, and yield in the growth chamber, greenhouse, and field
experiments, whereas D156 had a like or superior behavior in the greenhouse and
field experiments. Therefore, D156 could constitute a good candidate for
bacterial single-strain inoculants, as well as for formulating microbial
consortia.
Keywords: Field pea, Rhizobium
leguminosarum, strain selection, symbiotic efficiency
Resumen
La arveja (Pisum
sativum L.) es una leguminosa simbiótica del invierno que establece asociación
con Rhizobium leguminosarum sv viciae. Los objetivos de trabajo
fueron evaluar cepas de R. leguminosarum por infectividad y crecimiento
temprano, aporte de BNF, producción de biomasa y rendimiento. Setenta y ocho
cepas y cuatro cultivares se evaluaron en cámara de crecimiento, cinco cepas y
tres cultivares en invernadero, mientras que tres cepas y dos cultivares en
experimento de campo. Solo el 44-55% de las cepas fueron infectivas en los
cultivares. En invernadero, las cepas D70 y D156 mostraron los mejores valores de
nodulación y los mayores contenidos de N en biomasa y rendimiento. En campo,
D156 y D70 mostraron comportamientos similares que en invernadero, mientras que
D191 tuvo más nódulos por planta, mayor biomasa aérea y mayor rendimiento. La
cepa D70 mantuvo su comportamiento de nodulación, contenido de N en biomasa y
rendimiento en cámara de crecimiento, invernadero y campo; mientras que D156
tuvo igual o mejor comportamiento que D70 en invernadero y campo. Por lo tanto,
D156 podría ser una buena candidata para formular inoculantes bacterianos con
esta cepa o para formular consorcios microbianos.
Palabras clave: Arveja • Rhizobium
leguminosarum • selección de cepas • eficiencia simbiótica
Originales:
Recepción: 21/04/2023 - Aceptación: 28/11/2023
Introduction
Field pea (Pisum
sativum L.) is a winter symbiotic legume able to establish a specific
mutualistic association with Rhizobium leguminosarum symbiotic variant
(sv) viciae. This association contributes to satisfying part of the crop
nitrogen (N) demand via biological nitrogen fixation (BNF). From an agronomic
perspective, field peas may be included as an alternative winter crop in
agricultural rotations, with a shorter cycle duration and lower water
consumption than other winter crops like wheat or barley, and the N-fixing
ability (19). The N-fixing efficiency of
the legume-rhizobium relationship depends mainly on crop genotype (1, 2), rhizobium strain (23), and soil-water interactions associated with
management practices (10, 13, 14).
Contributions of BNF in field pea range between 45 and 286 kg N ha-1
(1, 13, 16, 22, 31, 36), representing
33-91% of total N uptake (1, 13, 16, 21, 22, 26,
31, 36).
Rhizobium
leguminosarum strains
have three distinct symbionts: (i) R. leguminosarum sv phaseoli,
which nodulates beans (Phaseolus vulgaris); (ii) R. leguminosarum sv
trifolii, which nodulates clovers (Trifolium sp); and (iii) R.
leguminosarum sv viciae, which nodulates field pea, vetch (Vicia sp),
lentil (Lens sp), faba beans (Vicia faba) and Lathyrus (Lathyrus
sp) (7, 8, 27). In field peas,
statistical interactions between strains and crop genotypes have been observed
for traits associated with BNF, plant growth and photosynthesis (11). Rhizobia strains have greater relevance on
BNF and N content, biomass and grains, than plant genotype (23). This strain-dependent behavior is
associated with the expression of genes related to R. leguminosarum sv viciae
nodulation, ensuring nodule establishment (7).
In Argentina,
although field pea is not a native plant, native soil N-fixing rhizobia can
nodulate this crop. Currently, the R. leguminosarum native strain D70 is
the elite strain inoculating field peas, vetches, and lentils. This strain can
nodulate all commercial cultivars and has shown greater BNF contribution in all
agro-ecological environments evaluated in Argentina (13). However, even showing good behavior
regarding grain yield and biomass production, it does not necessarily respond
equally in all agricultural environments. Newly incorporated commercial
cultivars still require evaluation regarding symbiotic behavior. In this
regard, strains of the collection of the Instituto de Microbiología y Zoología
Agrícola (IMyZA) of the Instituto Nacional de Tecnología Agropecuaria (INTA)
could potentially perform better than D70 and be used in new inoculant
formulations. Thus, this work aimed to i) evaluate Rhizobium sp. soil isolates and strains from the IMyZA-INTA collection for
infective ability and early growth promotion under controlled conditions
(growth chamber), ii) evaluate symbiotic efficiency of strains selected from
the growth chamber, under greenhouse conditions, and iii) evaluate inoculation
effects of the greenhouse selected strains on BNF contribution, nodulation, and
production variables under field conditions.
Materials
and methods
Growth
chamber experiments
Seventy-one to
seventy-eight specific strains and/or soil isolates from the IMyZA-INTA
collection (Additonal material 1) were combined with four
field pea cultivars (Facón, Bicentenario, Manantiales, and Pampa). Rhizobium
sp. inocula were grown in glass tubes (20 mm x 195
mm) with 10 ml of yeast extract mannitol (YEM) broth (35) on an orbital shaker (180 rpm, 48 h at
28°C). Each inoculum contained at least 3 x 108 colony-forming units
per milliliter (CFU ml-1). Field pea seeds were disinfected
superficially with 80% ethanol for 1 min, 4% sodium hypochlorite for 2 min, at
80% ethanol for 0.5 min, and rinsed 5 times with sterile distilled water. Then,
these seeds were placed in plastic trays with moistened absorbent paper for 72
h in an incubation stove at 28°C for germination. Two germinated seeds were
inoculated with 1 ml of inoculant and planted in a 220-ml pot with sterile
vermiculite substrate and daily irrigated with N-free nutrient solution (17). Each pot was kept at a constant temperature
of 25°C and 16 h of light. Each strain and cultivar combination was considered
a treatment, and a non-inoculated control was added for each field pea
cultivar. The experimental design was set as completely randomized with three
replications. Finally, the number of nodules per plant (N°Nod) and
biomass per plant (BiomTotal) were evaluated 25 days after planting
(DAP).
Greenhouse
experiment
Five strains
selected from the growth chamber experiment (D70, D156, D191, D192, and D193)
were evaluated for nodulation and early growth promotion abilities in the
greenhouse. The experiment was planted in 2 L pots on August 21st,
2013, using four seeds per pot and sterile vermiculite as substrate. Seeds were
disinfected as described above. Treatments consisted of a factorial combination
of the five selected strains plus a non-inoculated control, with three
commercial field pea cultivars (Viper, Facón, and Bicentenario) arranged in a
complete randomized block design totaling 17 replicates. The inoculant was
peat-based with a count of at least 1 x 108 CFU g-1 of
product. Sugared water (20% w/v) was used as adherent at a rate of 10 ml kg
seed-1. The inoculant was applied to seeds, mixed to homogenize, and
allowed to stand for 30 min before planting. Pots were irrigated three days a
week with an N-free nutrient solution (17).
Variables per plant were evaluated at 56 DAP and 78, 92, and 103 DAP per pot.
Root biomass (BiomRoot), aerial biomass (BiomAerial),
plant biomass (BiomPlant), number of nodules (N°Nod), dry
nodule biomass (BiomNod) and dry biomass per nodule (BiomNod-I)
were evaluated at two sampling moments (at 56 and 78 DAP), with additional
measurements such as root and aerial length (LengRoot and LengStem,
respectively) at 56 DAP. Then, the following variables were also evaluated:
vegetative, grain, and aerial biomass (BiomVeg, BiomGrain,
and BiomAerial, respectively), and plant yield components as pod
numbers, number of grains, number of grains per pod, and individual grain
biomass (N°Pod, N°Grain, N°Grain-pod, and BiomGrain-I,
respectively) at harvest (ca. 103 DAP). Additionally, N content (%Nupt)
and isotopic composition (δ15Nupt) on whole plant biomass were
determined at 92 DAP to estimate the β factor for each strain and field pea
cultivar combination according to Boddey et al. (2000).
Field
experiment
Strains D70,
D156, and D191, first evaluated in the greenhouse, were then compared in a
field experiment. All field treatments (i.e. the combinations between
these three strains and two pea cultivars, Facón and Viper) were carried out in
triplicate. The field experiment was performed in the locality of Rueda
(33°21’48.48” S and 60°27’39.12” W), in Santa Fe province (Argentina), on a
Typic Argiudoll soil. The experimental unit (plot) had six furrows (at 0.175 m
interrow) and 5 m in length. The experiment was sown on July 29th,
2014, with 119 plants m-2 (110-127 plants m-2) and
harvested on November 14th (108 DAP). Plants were fertilized with 100 kg ha-1
of monoammonium phosphate (i.e. 20 kg P ha-1). Seeds were
inoculated as described in the greenhouse experiment. Weeds and pests were
controlled. During the cycle, total rainfall was 284 mm and mean temperature
was 17.3°C.
At 60 DAP
(September 14th), 0.5 m of two rows (0.18 m-2) was
sampled and the number of nodules in each plant (N°Nod) was counted.
Then, plants were oven-dried at 65°C for 72 h to determine nodule biomass per
plant. At 95 DAP, 1 m2 of the total aboveground biomass of field pea
of each plot (N-fixing crop) plus one linear meter of wheat (non-fixing crop)
were sampled. These samples were dried in an air circulation stove at 65°C for
72 h. A subsample of each mentioned sample was ground with a Wiley mill to
determine N content in biomass (%Nupt) according to the
micro-Kjeldahl method. The N uptake (Nupt) was obtained by
multiplying %Nupt by BiomAerial. BNF was determined by
the 15N natural abundance method, using an elemental analyzer Carlo
Erba EA 1108 coupled to a ThermoScientific Delta V Advantage isotopic mass
spectrometer of continuous flow through a ConFLo IV interface. Then, %NFBN
was estimated according to Collino et al. (2015)
and NBNF was calculated by NBNF = %NBNF * Nupt.
At harvest (108
DAP), two samples of aboveground biomass (BiomAerial) were taken
from each plot of 1 m2. The, grained (BiomGrain) and
vegetative structures (BiomVeg) were separated, dried in an air
circulation stove at 65°C for 72 h and weighed. The harvest index was
calculated. Grain yield (YieldGrain) was adjusted to grain moisture
of 0.135 kg water kg grain-1.
Molecular
characterization
Three of the
strains evaluated (D70, D156 and D191) were characterized by partial sequencing
of the 16S rRNA gene by amplification reactions. Pure colonies were grown on
plates in YEM culture medium, then suspended in 50 μl of ultrapure water and
boiled in a water bath for 10 min to obtain DNA extracts. The universal primers
fD1 and rP2 proposed by Weisburg et al. (1991)
were used and amplification was carried out in a volume of 25 μl of the
reaction containing 2.5 ml 1X Buffer, 0.75 μl of 50 mM MgCl2, 0.5 μl
of 10 mM dNTP, 0.25 μl Taq DNA Polymerase, 0.5 μl of each primer (fD1 and rP2),
1 μl of tempered genomic DNA and 19 μl of ultrapure water. Amplification
conditions consisted of an initial denaturation of 2 min at 94°C, followed by
35 cycles of denaturation (94°C, 40 seconds), annealing (52°C, 40 seconds) and
extension (72°C, 1.5 min) and a final extension at 72°C for 10 min. A negative
control without template DNA was included. Electrophoresis was performed at 90
V. The amplified products were analyzed by 0.8% agarose electrophoresis stained
with SYBR Safe DNA gel stain (InvitrogenTM) for 30 min and then
purified with the comercial Gel Extraction kit QIAEX II (Qiagen). Partial
sequences of 16S rRNA were compared with those deposited in the NCBI BLAST database
(http://blast.ncbi.nlm.nih.gov/).
Data
analysis
Statistical
analysis of the growth chamber, greenhouse, and field experiments was carried
out using a two-way ANOVA. Means were compared with the DGC test (p≤0.05). All
analysis was performed using Infostat software version 2018p (Di Rienzo et al., 2018).
Results
Growth
chamber experiment
Only 44-55% of
all the strains tested (71-78) presented nodules in the four cultivars (i.e.
they were infective), varying according to the cultivar. Facón and Pampa
cultivars had the highest number of infectious strains (41), whereas Bicentenario and Manantiales had
the lowest (32 and 31, respectively).
Twenty-three
strains were able to infect the four cultivars evaluated. The isolates obtained
from nodules of the genus Lathyrus had a higher proportion of infective
and effective nodules in the four cultivars evaluated (60%), followed by those
from Lens, with 50%, and those from Vicia, with 29%, whereas the
isolates from Pisum, with 8%. Soil isolates had 38% nodulation ability
in the four cultivars.
Results from the
growth chamber showed highly significant (p˂0.001) variability in the N°Nod
per plant (i.e. strain x cultivar), whereas the effects of strains on
BiomTotal were significant at p=0.025 (Additional material
2). N°Nod ranged from 76 to 1. D70 presented the highest N°Nod
values, with an average of 43 nod pl-1, followed by D191, with 35
nod pl-1, and higher N°Nod than the rest. These responses
were 97% and 67% higher, on average, at D70 and D191, respectively (Additional material 1). BiomTotal ranged from 287
to 149 mg pl-1, with D156, D155, and D191 showing the highest values
(233-221 mg pl-1), i.e. 6% higher than the BiomTotal of D70
with the four cultivars (Additional material 1).
Based on these
results, greenhouse experiments included strain D156 selected for biomass
production ability, D191 for nodulation behavior, and D192 and D193 for fast
initial growth (not shown) as compared to the reference strain D70.
Greenhouse
experiment
Strains D70, D156,
D191, D192, and D193 were evaluated in the greenhouse experiment. At 56 DAP, a
highly significant interaction between strain and cultivar was detected in all
variables, except root biomass. Strain D192 had the best behavior in LengRoot
(358 mm pl-1) followed by D156 and D193 (322 mm pl-1, on
average) with cultivar Facón, with 28 and 15% differences in comparison to D70
and D191. D192 and D193 showed better LengAerial values, with 235 mm
pl-1, followed by D156 and D70 (206 mm pl-1), all with
cultivar Bicentenario, being 66% for 192 and D193 and 46% for D156 and D70.
Instead, strain D193 had higher LengPlant (515 mm pl-1),
followed by D192 (508 mm pl-1) with Bicentenario and Facón.
Likewise, strains D192 and D193 had higher BiomPlant (473 mg pl-1)
with Bicentenario, with significant differences with the rest of 55%. These
strains also had the highest BiomAerial values (271 mg pl-1),
followed by D156 and D70 (214 mg pl-1), all with Bicentenario. In
contrast, regarding nodule variables, D70 and D156 showed higher N°Nod
(32 nod pl-1 on average) with Viper, 100% higher than the rest, as
well as higher BiomNod (9 mg pl-1 on average), 141%
higher than the rest, and highest BiomNod-I (0.44 mg nod-1 in
average), 144% higher than the rest, all with Bicentenario.
At 78 DAP, a
significant interaction between strain and cultivar was observed in the
nodulation variables, except in individual nodule biomass. Strain D70 had the
highest N°Nod, together with strains D192 and D193, which were
better associated with Bicentenario (109, 129, and 110 nod pot-1,
respectively). Also, strain D70 showed better BiomNod with Viper (40
mg pot-1) and Bicentenario (38 mg pot-1), and the best
BiomNod-I (0.53 mg nod-1) concerning other strains (75%).
Differently, plant growth variables showed significant differences among
strains. Strain D191 showed the lowest values of BiomAerial (2.0 g
pot-1) and BiomRoot (0.7 g pot-1) with respect
to the rest (40 and 39%, respectively).
At harvest (103
DAP), the five strains were evaluated for BiomVeg, BiomGrain,
and BiomAerial. Only BiomVeg, showed cultivar and strain
interaction. All plant yield components (i.e. N°pod, N°Grain,
N°Grain-pod and BiomGrain) showed significant interaction
between both factors, although only measured in Facón and Viper with strains
D70, D156, and D191. Strain D70 produced 3.9 g pot-1 of BiomVeg with
Viper cultivar, significantly higher than the rest (45% on average), followed
by D156 (3.5 g pot-1) also with Viper cultivar. In contrast, strain
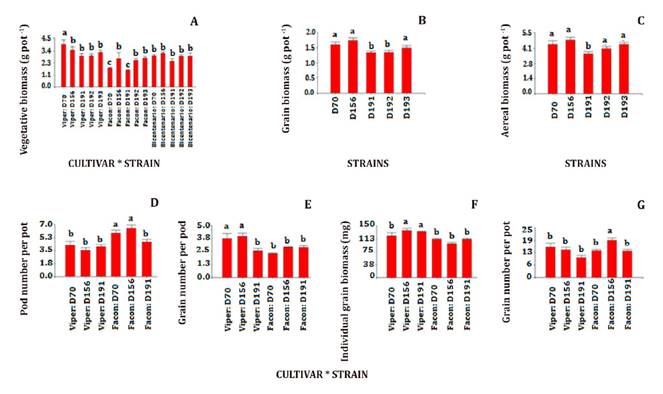
D191 showed the worst performance (1.6 g pot-1) with Facón (figure 1A).
A) vegetative
weight (g), B) grain weight (g), C) aerial biomass (g), D) pod
number, E) grain per pod, F) individual grain biomass (mg) and G)
grain number per pod. Different letters indicate significant differences
between treatments at p≤0.05. Results shown in panel D correspond to cultivars
Facón and Viper average values.
A) biomasa
de grano (g), B) biomasa vegetativa (g) y C) biomasa aérea total
(g), D) número de vainas por maceta, E) número de granos por
vaina, F) biomasa individual del grano (mg) y G) número de granos
por maceta. Letras diferentes indican diferencias significativas entre
tratamientos con valor p≤0,05. Los resultados mostrados en el panel D
corresponden al promedio de los cultivares Facón y Viper.
Figure 1. Plant
variables and yield components per pot at harvest (103 days after planting) as
dry matter.
Figura 1. Variables
de la planta y componentes del rendimiento por maceta a la cosecha (103 días
después de la siembra).
Regarding BiomGrain,
D156, D70 and D193 had higher values (1.7, 1.6, and 1.5 g pot-1,
respectively) than D191 and D192 strains (figure 1B).
Regarding BiomAerial, D191 (3.6 g pot-1) showed significantly lower
values (22%) than other strains (figure 1C). Considering
yield components, strain D156, followed by D70, threw the best results compared
to D191, except in BiomGrain-I where D191 had the second-best weight
after D156. In addition, D156 stran had 6.5 pods pot-1, followed by
D70 strain (5.8 pods pot-1), both with Facón cultivar having more
pod number per pot (N°Pod) than the rest (figure 1D).
Regarding grain number per pod (N°Grain-pod), D156 also performed
better with Facón (4.0 grains pod-1) and D70 with Viper (3.8 grains
pod-1) (figure 1E). Likewise, D156 followed by
D191, had the highest individual grain biomasses (BiomGrain-I) with
136 and 132 mg grain-1 respectively, with Facón (figure
1F). D156 with Facón had higher N°Grain (18.8 grains pot-1)
than the rest (44%) (figure 1G).
Total N (%Nupt)
and δ15N (‰) contents of whole plants were determined at 92 DAP for Facón and
Viper inoculated with D70, D156, and D191 (Additional
material 3). D156 (2.2%) and D70 (2.1%) strains had higher biomass upt%Nupt
than D191 (p˂0.01). The mean β value was -0.66‰, where D70 had lower depletion
of δ15N (-0.45‰), without differences with D156 (-0.55‰), being both over D191
(-0.88‰) with a significance level of p=0.06 (Additional
material 3).
Field
experiment
In the field
experiment, only nodule number had significant interaction between factors.
Regarding strain behavior significant differences were found for BiomVeg,
BiomAerial, and BiomNod-I.
Strain D191
behaved differently than in the greenhouse regarding BiomVeg, YieldGrain,
BiomAerial and Nupt. Likewise, strain D191 strain had
better behavior in BiomVeg (3.3 Mg ha-1) and BiomAerial
(6.7 Mg ha-1) than D156 (15% in both variables) and D70 (33 and 25%,
respectively, table 1).
Table
1. Nodulation at 60 DAP, nitrogen plant
variables at 95 DAP and plant harvest at 108 DAP.
Tabla 1. Nodulación
a los 60 DDP, de las variables de nitrógeno en planta (95 DDS) y rendimiento de
granos a cosecha (108 DDS).
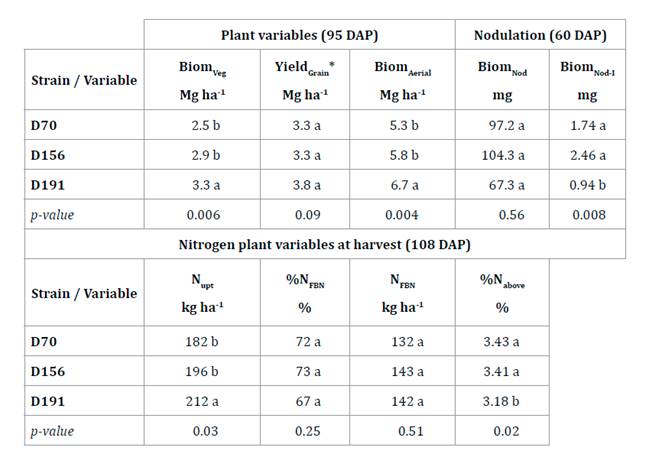
*Adjusted to 0.135 kg H2O kg grain−1
grain moisture. Values followed by different letters in columns indicate
significant differences at p ≤ 0.05.
*Ajustado a una humedad de grano de 0,135 kg H2O
kg grano−1. Los valores seguidos de letras diferentes en la columna
indican diferencias significativas en p ≤ 0,05.
In addition,
D191 strain had higher N content in aerial biomass (212 kg N ha-1)
than D156 (8%) and D70 (16%). Average YieldGrain was 3.5 Mg ha-1,
%NFBN was 70.5% and N°FBN was 139 kg N ha-1 (table 1). Instead, regarding %Nupt, D156 (3.43%)
and D70 (3.41%) showed higher values than D191 (8 and 7%, respectively),
maintaining the same behavior as in the greenhouse (table 1).
Strain D191 had the highest N°Nod per plant (10.5 nod pl-1)
differing 105% with the rest. Instead, D156 and D70 had highly significant
differences (p≤0.01) in BiomNod-I (2.5 and 1.7 mg nod-1,
respectively) with respect to D191 (162 and 85%, respectively), but with no
differences in BiomNod (table 1).
Molecular
characterization
Molecular
identification of D70, D156, and D191 strains via partial sequencing of the 16S
rRNA gene showed 100% identity with Rhizobium leguminosarum, and
deposited in the same database under accession numbers KU933357, KX 066064, and
KX346599, respectively. Sequences were then processed to obtain multiple
alignments then concatenated for the construction of a phylogenetic tree. In
turn, strains Az39 (Azospirillum argentinense) and E109 (Bradyrhizobium
japonicum) were incorporated as members of other groups. Results of this
analysis showed that D70 and D156 are more closely related than D191.
Discussion
Our results
showed that nodulation ability of both native and naturalized isolates of
rhizobia in field pea was similar to that observed by other authors (3, 8, 23, 38). For example, Ballard et al. (2004) found that 67% (22 of 33)
of naturalized populations of rhizobia (applied to pea plants as soil
suspensions) caused early and abundant nodulation in one cultivar of field pea,
while our work found that 44-55% of native isolates of Argentina or introduced
strains kept at the IMYZA-INTA collection were infective in four field pea
cultivars. Likewise, Boivin et al. (2020b)
observed that Rhizobium leguminosarum sv viciae are all potential
symbionts of Fabaceae hosts (field pea, vetch, and lens) but display
variable competitiveness to form root nodules. However, when Fabaceae legumes
are exposed to natural soil bacterial populations, symbiotic efficiency is
often suboptimal. In a recent study, Boivin et al. (2020a)
determined that small genetic differences in nodD genes allowed
observing a higher specificity degree among R. leguminosarun sv viciae
in pea, faba bean, and lentil. In the present study, we observed that rhizobia
nodule isolates from Lathyrus sp and Lens sp were the most
infective, followed by Vicia sp. Instead, isolates from pea plants did
not ensure infection in all cultivars of the same species (Pisum sativum),
since only 1 of 12 isolates/strains caused nodules in the four cultivars
evaluated. Therefore, field pea cultivars could have genetic differences (2, 36), which, associated with the rhizobial
genome, may influence competitiveness for nodulation as well as rhizosphere
colonization, decisive steps in the competition for nodule occupancy by R.
leguminosarum in soil populations (23).
The elite strain
D70, isolated from Vicia sp, is most used in inoculant formulations.
This strain has high nodulation ability and BNF contribution. Enrico et al. (2020) determined an average of
134 kg ha-1 of NFBN in field pea (8 experiments,
average). In this work, D70 had a contribution of 131 kg N ha-1,
while D156, also isolated from Vicia sp, showed a similar and better BNF
behavior in both greenhouse and field experiments (i.e. 143 kg N ha-1).
Instead, the high nodulation ability observed in D191 (obtained from Lens sp)
in the growth chamber, then resulted the least effective regarding yield
variables in the greenhouse experiment. However, this strain performed better
in the field experiment regarding biomass production (6.7 Mg ha-1),
grain yield (3.8 Mg ha-1) and N absorbed (212 kg ha-1),
considering the other strains. Table 2 shows BNF
contributions from different studies, indicating the selected strains had high
N contribution.
Table
2. Contribution of biological nitrogen
fixation (BNF) on field pea.
Tabla 2.
Contribución de la fijación biológica de nitrógeno (FBN) en arveja sobre
experimentos de campo.
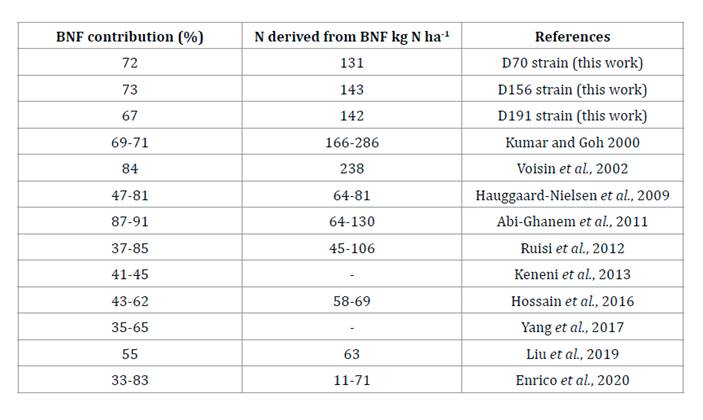
Likewise, D156
and D70 had better N content in biomass (%Nupt), which, according to
Laguerre et al. (2007), depends on BNF
efficiency of rhizobia derived from a robust nodular system.
In the present
study, strains D70 and D191 had high nodulation ability in controlled
conditions (growth chamber and greenhouse). In contrast, both D156 and D70 had
higher individual nodule biomass than D191, similar to controlled conditions.
This behavior may be due to differences observed by the 16S genes concerning
D70 or the larger number of specific genes that control nodulation competitiveness
(8). This behavior can also be due to
plant energetic regulation in association since, according to Rainbird et al. (1984), sustaining nodular
system involves 22% of total energy, and nitrogenase activity (BNF) consumes an
additional 52% (in soybean). Likewise, Ryle et al.
(1986) determined that the energy consumption of white clover nodules was
22-27%. Therefore, nodule numbers adapt to plant possibility to maintain a
symbiotic system benefiting yield.
Early growth
effects in the growth chamber (D156) and greenhouse experiment (D156, D192, and
D193 strains) could be associated with strain-dependant phytohormone effects, a
fact not addressed in this study. Therefore, strains tending to increase auxins
would be associated with stem and root elongation during post-emerging growth (6, 27, 33). In addition, cytokine balance in the
early nodulation stages may be associated with a larger number of nodules.
Also, final number of nodules may depend on ethylene production (4, 15). Strains D192, D193 and D156 stood out in
stem and root elongation and nodule number and could have an adequate profile
of phytohormones helping plant implantation. Then, at an advanced stage (78
DAP), the most relevant aspect would be symbiotic functionality, where the
contribution of biological N translates into greater biomass (11, 34). Both strains D156 and D70 showed
differences with D191, possibly due to symbiotic effectiveness. Differences
were also observed in grain yield when the only N source was biological.
Likewise, the only source of 15N abundance in biomass resulted to be the
biological source (β value), adjusting the contribution of BNF in field conditions.
These results are consistent with those obtained by Unkovich
et al. (1994) in clovers under different environments and with those
by López-Bellido et al. (2010) in chickpea
and faba beans between selected and natural soil strains. For non-different
nodule structures (i.e. the same cultivar), differences in 15N abundance
in the plant would be associated with strain genotype, generating a distinctive
behavior (23). This study confirms the
importance of Rhizobium strains in 15N isotopic discrimination in field
pea, due to isolate origin, where adaptive or microevolutionary changes would
be expected after genetic divergences (20, 28).
In our field
experiment, aerial biomass (BiomAerial) and yield (YieldGrain)
had no interaction (same as in the greenhouse). Yields obtained from the field
experiment (3.2 Mg ha-1 for D70, 3.3 for D156 Mg ha-1,
and 3.8 Mg ha-1 for D191; 3.5 Mg ha-1 on average) were
higher than those obtained in the 2014 crop cycle by Prieto
et al. (2015) (2.7 Mg ha-1) and Enrico
et al. (2020) (1.3 Mg ha-1) in eight experiments
performed in different environments of Argentina between 2012 and 2017. Unlike
that observed in the greenhouse, inoculation with D191 showed the best behavior
in aerial and vegetative biomass and nodule number per plant. In the field
experiment, D156 and D70 had the same behavior on the specific variables
derived from the Rhizobium strain, such as %NBNF and/or the
contribution of NBNF, and maintained the efficiency demonstrated in
the greenhouse (23). In this study, in
the field experiment, we observed 70.5% (range 67 to 73%) of %NBNF
on average with 67% for D91, 72% for D70, and 73% for D156, all higher values
than those established by Enrico et al. (2020),
who found 59% inoculated with D70, but within the interquartile range obtained
for field pea (33 to 83%).
The
incorporation of a winter legume such as field pea (inoculated with efficient
N-fixing strains) preceding a summer crop (e.g. maize) may constitute a
valid agronomic management. Enrico et al. (2020)
observed a positive effect on maize yield when the previous crop was inoculated
(field pea with strain D70). Having rich in N and low C:N
ratio stubble allowed rapid soil N availability. This higher availability of N
considerably reduced the need for chemical nitrogen fertilization.
Conclusions
Most of the
isolates that were infective and effective for Pisum sativum cultivars
here evaluated came from species other than field pea.
Strain D70
maintained good behavior in the growth chamber, greenhouse, and field
experiments concerning nodule, nitrogen, biomass, and yield variables, whereas,
D156 had similar or better behavior in the greenhouse and field experiments. In
contrast, D191 had only better behavior in the field experiment.
The β value
calculation of 15N natural abundance model to determine %NFBN
was key to adjusting NFBN contribution. Determining differences in
strain behavior results an essential requirement and would allow a more precise
adjustment of N input.
Finally, strains
selected for BNF efficiency should implicitly carry infectivity ability in all
commercial cultivars. This will allow certainty in their recommendation for the
formulation of inoculants. If new evaluations are required in different
agroecological environments, strain D156 could be a good candidate for
bacterial single-strain inoculants, as well as for a microbial consortium for Pisum
sativum.
1. Abi-Ghanem,
R.; Carpenter-Boggs, L.; Smith, J. L. 2011. Cultivar effects on nitrogen
fixation in peas and lentils. Biology and Fertility of Soils. 47(1): 115-120.
2. Abi-Ghanem,
R.; Bodah, E. T.; Wood, M.; Braunwart, K. 2013. Potential breeding for high
nitrogen fixation in Pisum sativum L.: germplasm phenotypic
characterization and genetic investigation. American Journal of Plant Sciences.
4(08): 1597.
3. Ballard, R.
A.; Charman, N.; McInnes, A.; Davidson, J. A. 2004. Size, symbiotic
effectiveness and genetic diversity of field pea rhizobia (Rhizobium
leguminosarum bv. viciae) populations in South Australian soils. Soil
Biology and Biochemistry. 36(8): 1347-1355.
4. Bartoli, C.;
Boivin, S.; Marchetti, M.; Gris, C.; Gasciolli, V.; Gaston, M.; Lepetit, M.
2020. Rhizobium leguminosarum symbiovar viciae strains are natural wheat
endophytes and can stimulate root development and colonization by arbuscular
mycorrhizal fungi. hal-02967159.
https://hal.inrae.fr/hal-02967159
5. Boddey, R.
M.; Peoples, M. B.; Palmer, B.; Dart, P. J. 2000. Use of the 15N natural
abundance technique to quantify biological nitrogen fixation by woody
perennials. Nutrient Cycling in Agroecosystems. 57: 235-270.
6. Boenel, M.;
Fontenla, S.; Solans, M.; Mestre, M. C. 2023. Effect of yeast and mycorrhizae
inoculation on tomato (Solanum lycopersicum) production under normal and
water stress conditions. Revista de la Facultad de Ciencias Agrarias.
Universidad Nacional de Cuyo. Mendoza. Argentina. 55(2): 141-151. DOI:
https://doi.org/10.48162/rev.39.116
7. Boivin, S.;
Mahé, F.; Tancelin, M.; Tauzin, M.; Wielbo, J.; Mazurier, S.; Lepetit, M.
2020a. Genetic variation in host-specific competitiveness of the symbiont Rhizobium
leguminosarum symbiovar viciae. DOI:10.22541/au.159237007.72934061
8. Boivin, S.;
Ait Lahmidi, N.; Sherlock, D.; Bonhomme, M.; Dijon, D.; Heulin-Gotty, K.;
Jensen, E. 2020b. Host‐specific competitiveness to form nodules in Rhizobium
leguminosarum symbiovar viciae. New Phytologist. 226(2): 555-568.
9. Collino, D.
J.; Salvagiotti, F.; Perticari, A.; Piccinetti, C.; Ovando, G.; Urquiaga, S.;
Racca, R. W. 2015. Biological nitrogen fixation in soybean in Argentina:
relationships with crop, soil, and meteorological factors. Plant and Soil. 392:
239-252.
10. De Bortolli
Pagnoncelli Jr., F.; Muzell Trezzi, M.; Bortolanza Pereira, P.; Rader, D. R.;
Biedacha, R.; Galon, L.; Bresciani Machado, A. 2023. Volunteer soybean (Glycine
max) interference in bean (Phaseolus vulgaris) crops: ethoxysulfuron
and halosulfuron critical level of damage and selectivity. Revista de la
Facultad de Ciencias Agrarias. Universidad Nacional de Cuyo. Mendoza.
Argentina. 55(2): 108-119. DOI: https://doi.org/10.48162/rev.39.113
11. Dejong, T.
M.; Phillips, D. A. 1981. Nitrogen stress and apparent photosynthesis in
symbiotically grown Pisum sativum L. Plant physiology. 68(2): 309-313.
12. Di Rienzo J.
A.; Casanoves F.; Balzarini M. G.; Gonzalez L.; Tablada M.; Robledo C. W.;
InfoStat versión 2018. Centro de Transferencia InfoStat, FCA. Universidad
Nacional de Córdoba. Argentina. URL http://www.infostat.com.ar
13. Enrico, J.
M.; Piccinetti, C. F.; Barraco, M. R.; Agosti, M. B.; Eclesia, R. P.; Salvagiotti,
F. 2020. Biological nitrogen fixation in field pea and vetch: Response to
inoculation and residual effect on maize in the Pampean region. European
Journal of Agronomy. 115: 126016.
14. Galon, L.;
do Amarante, L.; Favretto, E., L.; Cavaletti, D. C.; Henz Neto, O. D.;
Brandler, D.; Senhori, V. M.; Concenço, G.; Stradioto Melo, T.; Aspiazú, I.;
Muzzel Trezzi, M. 2022. Competitive ability of bean Phaseolus vulgaris cultivars
with Urochloa plantaginea. Revista de la Facultad de Ciencias Agrarias.
Universidad Nacional de Cuyo. Mendoza. Argentina. 54(1): 117-131. DOI:
https://doi.org/10.48162/rev.39.071
15. Gresshoff,
P. M.; Lohar, D.; Chan, P. K.; Biswas, B.; Jiang, Q.; Reid, D.; Ferguson, B.;
Stacey, G. 2009. Genetic of ethylene regulation of legume nodulation. Plant
signaling & behavior. 4(9): 818-823.
16.
Hauggaard-Nielsen, H.; Gooding, M.; Ambus, P.; Corre-Hellou, G.; Crozat, Y.;
Dahlmann, C.; Dibet, A.; von Fragstein, P.; Pristeri, A.; Monti, M.; Jensen, E.
S. 2009. Pea–barley intercropping for efficient symbiotic N2-fixation, soil N
acquisition and use of other nutrients in European organic cropping systems.
Field crops research. 113(1): 64-71.
17. Hoagland, D.
R.; Arnon, D. I. 1938. The water culture method for growing plants without
soil. Circular 347. Agricultural Experimental Station, University of
California.
18. Hossain, Z.;
Wang, X.; Hamel, C.; Knight, J. D.; Morrison, M. J.; Gan, Y. 2016. Biological
nitrogen fixation by pulse crops on semiarid Canadian prairies. Canadian
journal of plant science. 97(1): 119-131.
19. Jantalia C.
P.; Dos Santos, H. P.; Urquiaga, S.; Boddey, R. M.; Alves, B. J. R. 2008.
Fluxes of nitrous oxide from soil under different crop rotations and tillage
systems in the South of Brazil. Nutr Cycl Agroecosyst. 82: 161-173.
20. Jozefkowicz,
C.; Brambilla, S.; Frare, R.; Stritzler, M.; Puente, M.; Piccinetti, C.; Ayub,
N. 2017. Microevolution rather than large genome divergence determines the
effectiveness of legume-rhizobia symbiotic interaction under field conditions.
Journal of Molecular Evolution. 85: 79-83.
21. Keneni, G.;
Assefa, F.; Imtiaz, M.; Bekele, E.; Regassa, H. 2011. Genetic diversity for
attributes of biological nitrogen fixation in Abyssinian field pea (Pisum
sativum var. Abyssinicum) germplasm accessions. Journal of Genetics and
Breeding (under review).
22. Kumar, K.;
Goh, K. M. 2000. Biological nitrogen fixation, accumulation of soil nitrogen
and nitrogen balance for white clover (Trifolium repens L.) and field
pea (Pisum sativum L.) grown for seed. Field Crops Research. 68(1):
49-59.
23. Laguerre,
G.; Depret, G.; Bourion, V.; Duc, G. 2007. Rhizobium leguminosarum bv. viciae genotypes interact with pea plants in developmental
responses of nodules, roots and shoots. New Phytologist. 176(3): 680-690.
24. Liu, L.; Knight,
J. D.; Lemke, R. L.; Farrell, R. E. 2019. A side-by-side comparison of
biological nitrogen fixation and yield of four legume crops. Plant and Soil.
442(1-2): 169-182.
25.
López-Bellido, F. J.; López-Bellido, R. J.; Redondo, R.; López-Bellido, L. 2010.
B value and isotopic fractionation in N 2 fixation by chickpea (Cicer
arietinum L.) and faba bean (Vicia faba L.). Plant and soil.
337(1-2): 425-434.
26.
Masson-Boivin, C.; Giraud, E.; Perret, X.; Batut, J. 2009. Establishing
nitrogen-fixing symbiosis with legumes: how many rhizobium recipes? Trends in
microbiology. 17(10): 458-466.
27. Mazur, A.;
De Meyer, S. E.; Tian, R.; Wielbo, J.; Zebracki, K.; Seshadri, R.; Reddy, T. B.
K.; Markowitz,V.; I vanova, N.; Pati, A.; Woyke, T.;
C. Kyrpides, N.; Wayne, T. 2015. High-quality permanent draft genome sequence
of Rhizobium leguminosarum bv viciae strain GB30; an effective
microsymbiont of Pisum sativum growing in Poland. Standards in genomic
sciences. 10(1): 36.
28. Mutch, L.
A.; Young, J. P. W. 2004. Diversity and specificity of Rhizobium
leguminosarum biovar viciae on wild and cultivated legumes. Molecular
Ecology. 13(8): 2435-2444.
29. Prieto, G.;
Amado, R.; Apella, C.; Avila, F.; Brassesco, R.; Buschittari, D.; Coletta, M.;
Depaolo, J.; Espósito, A.; Fariña, L.; Figueroa, E.; Gordo, L.; López Tessore,
M.; Maggio, J.; Manso, L.; Martínez, S.; Martínez, G.; Martins, L.; Pérez, G.;
Vallejos, M.; Vita, E.; Vizgarra, O. 2015. Rendimiento de cultivares de Arveja
(Pisum sativum L) en diferentes ambientes de la República Argentina.
Campaña 2014/2015. Agricultores Federados Argentinos. N° 23: 71.
30. Rainbird, R.
M.; Hitz, W. D.; Hardy, R. W. 1984. Experimental determination of the
respiration associated with soybean/Rhizobium nitrogenase function, nodule
maintenance, and total nodule nitrogen fixation. Plant physiology. 75(1):
49-53.
31. Ruisi, P.;
Giambalvo, D.; Di Miceli, G.; Frenda, A. S.; Saia, S.; Amato, G. 2012. Tillage
effects on yield and nitrogen fixation of legumes in Mediterranean conditions.
Agronomy journal. 104(5):1459-1466.
32. Ryle, G. J.
A.; Powell, C. E.; Gordon, A. J. 1986. Defoliation in white clover: nodule
metabolism, nodule growth and maintenance, and nitrogenase functioning during
growth and regrowth. Annals of botany. 57(2): 263-271.
33. Subramanian,
S. 2013. Distinct hormone regulation of determinate and indeterminate nodule
development in legumes. J Plant Biochem Physiol. 1(110): 2.
34. Unkovich, M.
J.; Pate, J. S.; Sanford, P.; Armstrong, E. L. 1994. Potential precision of the
δ15N natural abundance method in field estimates of nitrogen fixation by crop
and pasture legumes in south-west Australia. Australian Journal of Agricultural
Research. 45(1): 119-132.
35. Vincent, J.
M. 1970. A manual for the practical study of the root nodule bacteria. In: IBP
Handbook 15. Blackwell Scientific Publications. Oxford. UK.
36. Voisin, A.
S.; Salon, C.; Munier-Jolain, N. G.; Ney, B. 2002. Quantitative effects of soil
nitrate, growth potential and phenology on symbiotic nitrogen fixation of pea (Pisum
sativum L.). Plant and soil. 243(1): 31-42.
37. Weisburg, W.
G.; Barns, S. M.; Pelletier, D. A.; Lane, D. J. 1991. 16S ribosomal DNA
amplification for phylogenetic study. Journal of bacteriology. 173(2): 697-703.
38. Wielbo, J.;
Podleśna, A.; Kidaj, D.; Podleśny, J.; Skorupska, A. 2015. The diversity of pea
microsymbionts in various types of soils and their effects on plant host
productivity. Microbes and environments. ME14141.
39. Yang, C.;
Bueckert, R.; Schoenau, J.; Diederichsen, A.; Zakeri, H.; Warkentin, T. 2017.
Symbiosis of selected Rhizobium leguminosarum bv viciae strains with
diverse pea genotypes: effects on biological nitrogen fixation. Canadian
journal of microbiology. 63(11): 909-919.
Squematic figure
of the dual-choice olfatometer utilized in choice test with stored grain pests:
https://drive.google.com/drive/folders/17vBzpdAKT_WP2l_SdnicLF_EH-puPzCy?usp=sharing