Revista de la Facultad de Ciencias
Agrarias. Universidad Nacional de Cuyo. Tomo 56(1). ISSN (en línea) 1853-8665.
Año 2024.
Original article
Bio-efficacy
of entomopathogenic fungi and vegetable oils against the pink pineapple
mealybug: Dysmicoccus brevipes (Cockerell)
Bioeficacia
de hongos entomopatógenos y aceites vegetales contra el piojo harinoso rosado
de la piña: Dysmicoccus brevipes (Cockerell)
Omara Pérez
Panti1,
Héctor González
Hernández2,
Saúl Sánchez
Soto1,
Pedro Antonio
Moscoso Ramírez1,
Francisco
Izquierdo Reyes1
1Colegio
de Postgraduados Campus Tabasco. C. P. 86500. México.
2Colegio
de Postgraduados Campus Montecillos. Institución de adscripción. Dirección
Postal: 56230. México.
*rubeng@colpos.mx
Abstract
Dysmicoccus
brevipes (Cockerell)
(Hemiptera: Pseudococcidae) is an important insect pest of pineapple worldwide
due to the direct damage it causes and because it is a vector of mealybug
pineapple wilt. Entomopathogenic fungi are an alternative management tool for
this pest. A preliminary experiment evaluated the lethal effects of two
isolates of Beauveria bassiana (BbCT, BbCa) and one isolate of Metarhizium
anisopliae (Ma) against adult female D. brevipes. Subsequently, the
efficacy of the most virulent isolates and a commercial strain of Paecilomyces
fumosoroceus (PAE-SIN) were evaluated under laboratory and greenhouse
conditions, either alone or in combination with soybean oil or neem oil.
Results showed variation amongst isolates and that B. bassiana was the
most effective. Isolate BbCa at 1×107 mL-1 conidia, was
the most effective against D. brevipes nymphs and adults at 26 ± 1°C,
causing 66% ± 6% mortality 8 days after inoculation. BbCa was the most virulent
with an LC50 of 3.45x107 mL-1 conidia and a LC95
of 2.29x108 mL-1 conidia, under controlled conditions.
Efficacy of BbCa increased when combined with neem oil, causing 100% mortality
6 days after inoculation. In conclusion, a combination of B. bassiana isolate
BbCa and neem oil achieved 100% mortality in D. brevipes under the
experimental conditions reported in this study.
Keywords: entomopathogenic
fungi, biological control, pineapple, Dysmicoccus, vegetable oils
Resumen
Dismiccoccus
brevipes (Cockerell)
(Hemiptera: Pseudococcidae) es una de las plagas de la piña de mayor
importancia a nivel mundial, no solo por los daños directos que ocasiona, sino
por ser trasmisor del virus marchitez roja de la piña. Los hongos
entomopatógenos son una alternativa para el manejo de este insecto. En un
experimento preliminar se evaluó la patogenicidad y la virulencia de dos
aislamientos de Beauveria bassiana (BbCT, BbCa) y uno de Metarhizium
anisopliae (Ma), sobre hembras adultas de D. brevipes. Posteriormente,
los aislamientos más virulentos y una cepa comercial de Paecelomyces
fumosoroceus (PAE-SIN), fueron evaluados en otro experimento en condiciones
de invernadero, solos o en combinación con aceite de soya y aceite de neem. Los
aislamientos evaluados presentaron diferente grado de virulencia; sin embargo, B.
bassiana resultó ser el más virulento. El aislamiento BbCa a una
concentración inicial de 1×107 conidios mL-1 fue más
efectivo contra adultos de D. brevipes comparado con el control,
causando mortalidad del 66% ± 6% a los 8 días pos inoculación a 26 ± 1°C. BbCa
presentó la mayor virulencia con una CL50, de 3.45x107
conidios mL-1 y una CL95 de 2.29x108 conidios
mL-1, bajo condiciones controladas. Sin embargo, la eficacia se
incrementó para BbCa, cuando se combinó con aceite de neem, al causar el 100 %
de mortalidad a los 6 días pos inoculación. En conclusión, la combinación B.
bassiana (BbCa) y aceite de neem fue el mejor tratamiento, con una
mortalidad de 100% de D. brevipes bajo las condiciones experimentales
reportadas en este estudio.
Palabras clave: entomopatógenos,
control biológico, piña, Dysmicoccus, aceite vegetal
Originales: Recepción: 06/06/2023 - Aceptación: 11/03/2024
Introduction
Pineapple
production generates significant economic resources worldwide. Mexico's main
pineapple exports are destined for the United States market, with an annual
value in 2020 of $30,602,000 USD (24).
Unfortunately, the pineapple industry is affected by various phytosanitary
problems. Since pineapple is grown intensively and in monoculture, pesticides
are commonly applied for pest management, causing problems for human health,
the environment, and agroecosystems. The mealybugs Dysmicoccus brevipes (Cockerell)
(Hemiptera; Pseudococcidae) and D. neobrevipes Beardsley (Hemiptera:
Pseudococcidae) are major pests of commercial pineapple cultivation (29) causing
significant damage throughout the crop growth cycle; they are also vectors of
Pineapple Mealybug Wilt associated Virus (PMWaV) which can cause up to 100% of
export crop losses due to rejection of fruit (19). Recent
management strategies for D. brevipes in pineapple are largely based on
synthetic organophosphate insecticides. However, efficacy of chemical control
is limited by the cryptic location and behavior of these insects on plants, and
their waxy surface layer which is a barrier to the action of contact
insecticides, even protecting eggs from residual effects. There is also
increasing concern in general about the toxic risks of excessive pesticide use
in agriculture. Therefore, exploration of economically viable and
environmentally safe strategies is necessary. We hypothesize that commercial
pineapple production could benefit from the use of botanical extracts and
biological pest control agents, such as entomopathogenic fungi, within
integrated pest management (33).
Entomopathogenic
fungi can infect directly without the need for ingestion and so are effective
against sucking pests such as aphids, mealybugs, whiteflies, and mosquitoes (4). Some
entomopathogenic fungi have a combination of modes of action against
arthropods: toxins; nutrient depletion; physiological disruption; and
mechanical damage to internal tissues due to mycelium development (12). Efficacy of
entomopathogenic fungi has been widely documented, particularly against
mealybugs. For example, Beauveria bassiana (Bals.) Vuill. (Ascomycota:
Hypocreales), Lecanicillium lecanii (Zimm.) and Metarhizium
anisopliae (Metschnikoff) Sorokin (Ascomycota: Hypocreales) infect and kill
Paracoccus marginatus Williams & Granara de Willinks (2). Despite this,
there are few laboratory and field studies on management of D. brevipes using
entomopathogenic fungi in pineapple. One study by Miranda Vindas
and Blanco Getzler (2013) has evaluated a range of options in the laboratory
that included both entomopathogenic fungi and
botanical oil extracts that are known for their repellent, anti-feeding, and
growth inhibition properties; high degradability; and environmental safety (18).
Specific evaluations included: B. bassiana (4.0 x1010
conidia/g); M. anisopliae (1.0 x1010 conidia/g); a mixture of
both fungi (0.5 g + 0.5 g/L distilled water, 4.0 x1010 conidia/g +
1.0 x1010 conidia/g); potassium salts; fatty acids (7 mL/L); and
botanical extracts (a mixture of hot chili, garlic, onion, mustard and jackass
bitters) (7 mL/L). Results showed high efficiency of entomopathogenic
fungi and that the botanical extract achieved the fastest mortality. In the
same publication, the botanical extract was also evaluated in a commercial
pineapple plantation in comparison with the typical chemical control options
Diazinon® 60 EC (diazinon) (0.5 ml/L) and Sevin® 80 WP (carbaryl) (1 kg/ha); the
lowest incidence of mealybugs was achieved in the botanical extract treatment (16).
These
results suggest that combinations of entomopathogenic fungi and vegetable oil
extracts have potential as control agents that may increase mortality of adult D.
brevipes females. For this reason, the potential of two native fungal
isolates was evaluated in comparison with a commercial product based on Paecelomyces
fumosoroseus. Our specific objectives were to determine the pathogenicity and
virulence of the entomopathogenic fungi, alone and in combination with neem oil
or soybean oil, against the pineapple mealybug under laboratory and greenhouse
conditions.
Material
and Methods
Experiments were
done at the Biological Control Laboratory, Postgraduate College, Tabasco
Campus, Cárdenas, Tabasco, Mexico, between January and December 2021.
Collection
and mass rearing of D. brevipes
Dysmicoccus
brevipes adults
were collected from two varieties of pineapple (MD2 and bighead) on commercial
plantations in Huimanguillo, Tabasco. Insects were taken to the Biological
Control laboratory of the Postgraduate College, Tabasco Campus, for laboratory
breeding, following the methods of Pandey &
Johnson (2006).
For the breeding stock, 50 eight-month-old pineapple cloves (25 bighead and 25
MD2 variety) were transplanted from a commercial pineapple plantation in
Huimanguillo, Tabasco, into plastic pots and kept in a greenhouse at 30-35°C.
Twenty days after potting, they were infested with adult female D. brevipes (20
per plant) in the greenhouse.
Entomopathogenic
fungal isolates
Pathogenicity
and virulence evaluations were made on two Beauveria bassiana (BbCa,
BbCT) isolates and one isolate of Metarhizium anisopliae (Ma); all were
native entomopathogenic isolates from Tabasco, Mexico held in the collection of
the Biological Control Laboratory of the Colegio de Postgraduados, Tabasco
Campus (table
1).
Table
1. Reference of the fungi used in the
evaluation of pathogenicity against D. brevipes.
Tabla 1. Referencia
de los hongos usados en la evaluación de patogenicidad contra D. brevipes.

Subsequent bio-efficacy
experiments included a commercial product based on Paecilomyces fumosoroceus
(PAE-SIN®).
Mycelia from
each isolate was grown on sterile Sabouraud Dextrose Agar (ADS, Bioxon, Mexico)
in Petri dishes, 90 x 15 mm for 3 weeks at 26±1°C in darkness. Conidia were
then scraped from the surface and suspended in 0.03% Tween 80. Conidial concentration
was determined using a Neubauer chamber, following the method of Inglis
et al. (2012).
Pathogenicity
and virulence of D. brevipes
Pathogenicity
and virulence of B. bassiana (BbCa, BbCT) and M. anisopliae (Ma)
against D. brevipes were determined experimentally using a completely
randomized design with four replicates of each treatment and control; the
entire experiment was repeated on three occasions. Groups of ten adult females
were each placed on two basal pieces of MD2 pineapple leaf (8 x 8 cm) inside a
plastic box (20 cm x 10 cm x 10 cm) with openings covered with organdy mesh for
ventilation. Wet filter paper was placed in each box to provide moisture. Each
group of adults was sprayed (from a spray bottle) with 1.5 ml of conidia
(either 106, 107 or 108 mL-1)
suspended in 0.05% aqueous Tween 80; the control was sprayed with 0.05% aqueous
Tween 80 only. Applications were made following the methodology of Ramírez-Sánchez
et al. (2019).
Boxes containing treated insects were incubated at 26 ± 1°C, 65 -70% RH and a
14:10 h light: dark regime). Mortality was assessed daily for 8 days. Dead
insects were incubated to determine cause of death (mycosis), following the methodology
of Butt and Goettel (2000). Abbott´s
formula was used to correct data for control mortality (1).
Bio-efficacy
of B. bassiana and P. fumosoroseus, either alone or in combination with
vegetable oils, against D. brevipes under greenhouse conditions
An experiment
was set up under greenhouse conditions based on the results of the
aforementioned bioassays. Pineapple suckers (variety MD2 [40 cm in size]) were
planted individually in replicate pots, each containing 2 kg of sandy soil
collected from a comercial pineapple plantation in Huimanguillo, Tabasco,
Mexico. To each pot twenty D. brevipes adults were inoculated at the
base of the pineapple sucker and incubated for one month before experimental
treatments were added.
A total of eight
treatments were compared including the highly virulent B. bassiana isolate,
BbCa, and a formulated strain of P. fumosoroseus (PAE-SIN®), either
alone or in combination with soybean oil (CARRIER)® or
neem extract oil (Nimicide 80®) (table 2).
Table
2. Treatments evaluated in the greenhouse
assay.
Tabla 2. Tratamientos
evaluados en el experimento de invernadero.
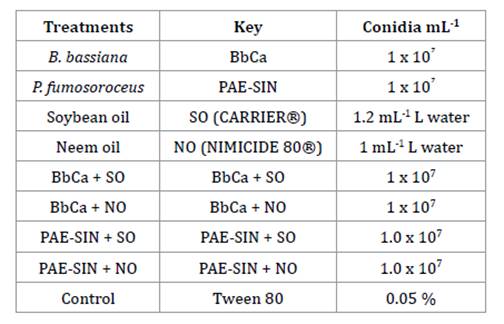
All treatments
were applied as 20 ml solutions/ suspensions; all fungal treatments contained 1
x 107 conidia mL-1. There were four replicates of each
treatment arranged in a completely randomized design. After inoculation, insect
mortality was assessed daily for 8 days.
Statistical
analysis
Probit analysis
was used to estimate the LC50 and LC95 of each isolate
with a 95% confidence limit. ANOVA and multiple comparisons of means for both
isolates and their concentrations were also done with the Tukey test (p≤ 0.05)
in SAS software (25). The probit
regression model Φ-1 [Π(x)]=α+βx and the formula LC (P) = (qnorm (P)
- α) / β were used to estimate lethal concentrations. Virulence graphs were
constructed using R software v. 1.0.143 (22).
Results
Pathogenicity
and virulence
Both isolates of
B. bassiana (BbCa and BbCT) caused higher levels of D. brevipes mortality
at all conidia concentrations evaluated compared with M. anisopliae at
the same concentrations. There were highly significant differences amongst
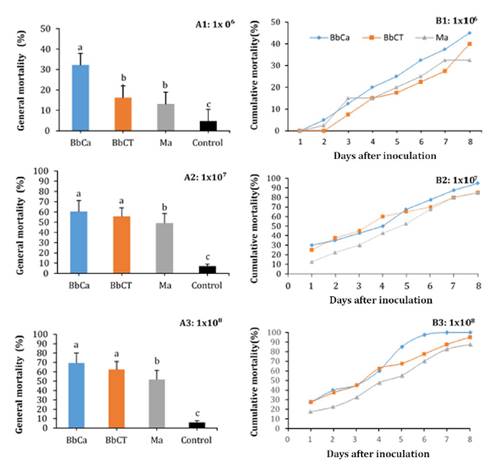
treatments (p < 0.0001) in the mean daily mortality after 8 days at the 1x106
conidia concentration. The highest daily mean % mortality was achieved by
isolate BbCa (32.2%), followed by isolate BbCT (16.3%) and then Ma (15.8%); in
the control mortality was 4.7% (figure 1 A1). However,
cumulative mortality at day 8 after inoculation was 45, 40, 32.5, and 4.5% for
isolates BbCa, BbCT, Ma and the control treatment, respectively (figure
1
B1).
Bars with different letters are significantly
different to each other (Tukey p ≤ 0.05). Error bars represent ±1 standard
error of the mean (SEM), n= 4.
Barras con diferentes letras son significativamente
diferentes unas de otras (Tukey p ≤ 0,05). Barras de error representan ±1 el
estándar error de la media (SEM), n= 4.
Figure 1.
Dynamics of A) general mean mortality for the period 1 - 8 days post
inoculation and B) daily cumulative mortality of adult female Dysmicoccus
brevipes after treatment with 1 x 106 (A), 1 x 107
(B) or 1 x 108 (C) conidia mL-1, of B. bassiana (BbCa,
BbCT) and M. anisopliae (Ma).
Figura 1.
Dinámica de A) mortalidad media general del periodo de 1 - 8 días post inoculación
y B) mortalidad acumulada diaria de hembras adultas de Dysmicoccus brevipes después
de los tratamientos con 1 x 106 (A), 1 x 107 (B) o 1 x 108
(C) conidios mL-1, de B. bassiana (BbCa, BbCT) y M.
anisopliae (Ma).
Mortality data
for the 1 x 107 conidia mL-1 concentration showed an
increase in % mortality compared with the 1 x 106 conidia mL-1
concentration. There were highly significant differences amongst treatments (p
< 0.0001); the highest mean daily % mortality over 8 days was achieved by
isolates BbCa and BbCT, with 60% and 58.43% mortality, respectively, followed
by isolate Ma with 49.06% and the control with 7.18% (figure 1 A2). Cumulative
mortality on day 8 was 95, 85, 82, and 7.18% for isolates BbCa, BbCT, Ma and
the control, respectively (figure 1 B2).
Mortality data
for the 1x108 conidia mL-1 concentration showed an even
greater increase in % mortality compared with the 1 x 106 and 1 x 107
conidia mL-1. Highly significant differences among treatments (p
< 0.0001) were detected, with the highest mean daily % mortality achieved by
isolates BbCa and BbCT being 69.4 and 62.5%, respectively, followed by Ma (51.9
%), while in the control mortality was 5.9 % (figure 1 A3). Cumulative
% mortality on day 8 was 100, 95, 87.5 and 7.1% for isolates BbCa, BbCT, Ma and
the control, respectively (figure 1 B3).
Concerning cumulative
mortality, B. bassiana isolate BbCa was more effective from day 5
post-inoculation than isolate BbCT. However, both isolates of B. bassiana,
at each concentration evaluated, showed high efficacy against pineapple
mealybug with increasing cumulative mortality over time after inoculation (figure
1B).
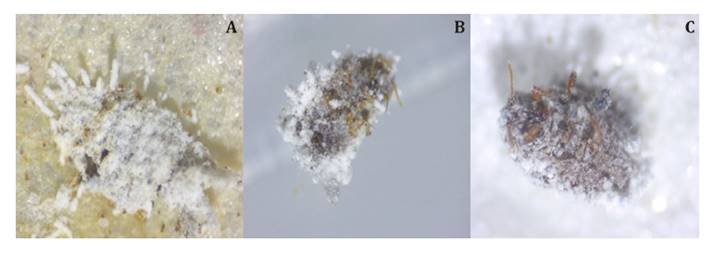
Proliferation of
mycelium and conidial structures was observed on cadavers of D. brevipes produced
during the first 4 days after inoculation. Abundant sporulation was detected
from day 8 after inoculation, particularly from cadavers killed by B.
bassiana isolates (figure 2).
Figure 2. Beauveria
bassiana isolates A)
BbCa, B) BbCT and C) Metarhizium anisopliae (Ma) infecting adult female
pineapple mealybug Dysmicoccus brevipes at 4 x magnification with an
optical microscope.
Figura 2. Aislamientos
de Beauveria bassiana A) BbCa, B) BbCT y C) Metarhizium anisopliae (Ma)
infectando a hembras adultas del piojo harinoso de la piña Dysmicoccus
brevipes a una magnificación de 4 x con un microscopio óptico.
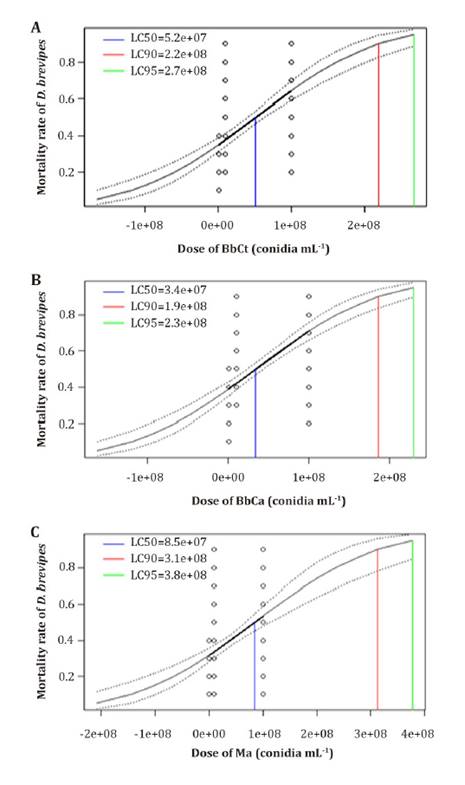
Determination
of virulence
LC₅₀ values were
3.4 x 107, 5.2 x 107 and 8.5 x 107 mL-1
conidia for isolates BbCa, BbCT, and Ma, respectively. LC95 values
were 2.29 x 108, 2.67 x 108 and 3.77 x 108
conidia mL-1 for isolates BbCa, BbCT and Ma, respectively (figure
3).
Figure 3.
Corrected probit plot mortality of adult female D. brevipes treated with
different conidia concentrations (log dose) of B. bassiana (BbCa [A],
BbCT [B]) and M. anisopliae (Ma[C]) visualized
using R software v. 1.0.143 (22).
Figura 3.
Mortalidad probit corregida de hembras adultas de D. brevipes tratadas
con diferentes concentraciones de conidios (log dosis) de B. bassiana (BbCa
[A], BbCT [B]) y M. anisopliae (Ma[C])
visualizadas con el uso del programa R v. 1.0.143 (22).
Probit
regression lines for B. bassiana were Y = - 0.2864+8.401-09
(x) and Y= - 0.3915+7.602-09(x), for BbCa, BbCT, respectively,
whereas for M. anisopliae, it was Y= - 0.4795+5.626-09(x),
where ‘Y’ was the probit mortality and ‘x’ was the fungal concentration (figure
3).
Data fitted well with the model and there was a positive correlation between
conidial concentration evaluated and bioinsecticidal activity of both fungi.
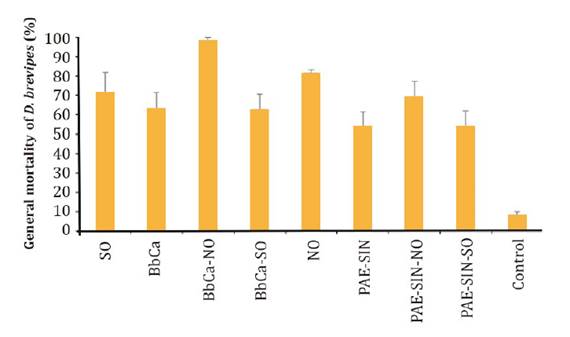
Bio-efficacy
of entomopathogenic fungi and vegetable oils against D. brevipes
Mortality of
adult D. brevipes females following treatment with isolate BbCa (at 1 x
108 conidia mL-1) or P. fumosoroseus alone or in
combination with vegetable oils varied significantly amongst treatments (p <
0.0001). Mean mortality in the untreated control was 5.5%. The combination BbCa
+ neem oil achieved the highest mortality, 98.4 %, (p < 0.0001) (figure
4).
Bars with different letters are significantly
different to each other (Tukey p ≤ 0.05). Error bars represent ±1 standard
error of the mean (SEM), n= 4.
Barras con diferentes letras son significativamente
diferentes unas de otras (Tukey p ≤ 0,05). Barras de error representan ±1 el
estándar error de la media (SEM), n= 4.
Figure 4. Overall
mortality of D. brevipes after treatment with B. bassiana (isolate
BbCa; 1 x 107 conidia mL-1) or P. fumosoroseus at
1 x 107 conidia mL-1 (PAE-SIN®) either alone or in
combination with soybean oil (SO) or neem oil (NO).
Figura 4. Mortalidad
total de D. brevipes después de los tratamientos con B. bassiana (aislamiento
BbCa; 1 x 107 conidios mL-1) y P. fumosoroseus a 1
x 107 conidios mL-1 (PAE-SIN®) solos o en combinación con
aceite de soya (SO) o aceite de neem (NO).
Neem oil
treatment alone caused 81.4 % mortality. The conidial concentrations of the
commercial formulation of P. fumosoroseus used in this experiment,
caused only 55.1% mortality. However, when P. fumosoroseus was combined
with neem oil mortality was 66.1%.
Cumulative
mortality data also showed that the combination of isolate BbCa and neem oil
was sufficient to achieve high efficacy by day 6 after inoculation, which
shortened the time to kill by 100 % compared with neem oil alone.
Discussion
In the present
study isolates of both local native entomopathogenic fungi, B. bassiana and
M. anisopliae, were pathogenic. They caused mortality and variable
mycosis in adult female D. brevipes. The BbCa isolate was significantly
more virulent than the M. anisopliae isolate under laboratory
conditions. B. bassiana isolates are widely used as biological control
agents of a wide variety of insect pests, including sucking pests such as
mealybugs (21, 26, 32). For example,
previous research showed that two isolates of B. bassiana (FF and
PPRC-56) caused 97% and 100% mortality in adults of the mealybug Paraputo
ensete (Williams and Ferrero) (Hemiptera: Pseudococcidae) twenty days after
inoculation (14). In another
study, two isolates of B. bassiana (GAR 17 B3, GB AR 23133) caused 67.5%
and 64% mortality in the citrus mealybug Planococcus citri (Risso)
(Hemiptera: Pseudococcidae) (5). Another
isolate of B. bassiana caused 93% mortality in Pseudococcus
jackbeardsleyi Gimpel & Miller (Hemiptera: Pseudococcidae) nymphs 5
days after treatment (10). The results
of our research are in agreement with Mohamed (2016), who evaluated
the virulence of B. bassiana, M. anisopliae and Lecanicillium
lecanii isolates against adults of the mealybug, Planococcus ficus (Signoret)
under laboratory conditions; they reported that virulence levels of B.
bassiana were higher than those of M. anisopliae and L. lecanii,
resulting in up to 98% mortality at a concentration of 5 x 108 mL-1
conidia. Moreover, Manjushree and Chellapan (2019) reported that
isolates of B. bassiana caused higher mortality in D. brevipes (Cockerell)
(Hemiptera: Pseudococcidae) at a concentration of 109 conidia mL-1
than isolates of M. anisopliae and L. lecanii. Like Surulivelu
et al. (2012),
Manjushree
and Chellapan (2019)
also reported that the same fungi were also effective against papaya mealybug Paracoccus
marginatus (Williams) (Hemiptera: Pseudococcidae) under field conditions.
Concentration of
inoculum (conidia) is a very important aspect of fungal pathogenicity and
virulence. Results of our research suggest that isolate BbCa was the most
virulent of the evaluated isolates with an LC50 of 3.4x107
conidia mL-1, and that the higher the concentration of conidia the
greater the mortality of D. brevipes.; overall 98% mortality was
achieved 6 days after inoculation at 1 x107 conidia mL-1.
These results are consistent with other studies that report B. bassiana caused
higher mortality of various mealybug species than M. anisopliae (11,
13).
It has been reported that foliar applications of V. lecanii and B.
bassiana (2×108 conidia mL-1) in approximately 5 g/mL
per L water is sufficient to reduce mealybug populations during months when the
relative humidity is high (28). High
effectiveness of B. bassiana and P. fumosoroseus against adult D.
brevipes females was recorded from the 6th day after inoculation. In a
field-level study, Ugalde-Trejos (2010) found no
differences in the efficacy of B. bassiana, M. anisopliae, Trichoderma
spp., and Bacillus thuringiensis treatments against populations of D.
brevipes infesting pineapple.
Results of the
bio-efficacy assay showed that mortality of D. brevipes increased when B.
bassiana (isolate BbCa) and neem oil were combined, making it possible to
consider this treatment for future field trials. Fernández and
Juncosa (2002)
reported that the use of adjuvant oils improved effectiveness of
entomopathogenic fungi. In the same way, Elósegui and
Elizondo (2010)
found that mixtures of entomopathogenic fungi and adjuvants increased efficacy
and tolerance of the product to wider ranges of temperatures. Vásquez,
(2000)
evaluated in vitro effectiveness of B. bassiana, M. anisopliae,
Entomophthora, soap, hydrated lime, garlic extract (Allium sativum)
and neem extract for control of D. brevipes in an organic pineapple
plantation, where the greatest efficacy was achieved with mixtures of
entomopathogenic fungi and extracts of soap, garlic, neem, and hydrated lime.
Results of the present study agree with Miranda Vindas
& Blanco Getzler (2013), who found that botanical extracts were highly
efficient in causing D. brevipes mortality under field conditions. Gopal
et al. (2021),
found that the maximum cumulative mortality of Maconellicoccus
hirsutus (Green) (Hemiptera: Pseudococcidae) was achieved when
entomopathogenic fungi B. bassiana + L. lecanii (6 g/L + 6 g/L)
were applied together rather than individually, resulting in 57.6% mortality,
while neem and pongamia vegetable oils at 15 mL/L caused cumulative mortality
of 81.4%, compared with the standard dose of neem oil (10 g/L) which caused
78.1% mortality. Our results showed that neem oil in combination with
entomopathogenic fungi such as B. bassiana was the most efficient in
killing adult female D. brevipes in greenhouse tests with up to 100%
mortality by day 8 post-inoculation.
Conclusion
Both local
isolates of B. bassiana and M. anisopliae were pathogenic to
adult female D. brevipes. When B. bassiana and P. fumosoroseus
were combined with neem oil under greenhouse conditions bio-efficiency
increased by 20 % and 11%, respectively. B. bassiana (BbCa) combined
with neem oil resulted in the highest mortality of D. brevipes reaching
up to 100 % by 8 days after inoculation.
We suggest that
more research is needed to evaluate effectiveness of entomopathogenic fungi in
combination with vegetable oils under field conditions. Design of biocontrol
programs against pineapple mealybug is recommended in pineapple-growing regions
of Mexico, as a strategy within integrated pest management programs. This
option could reduce the use of toxic insecticides, which are harmful to the
environment and human health.
Acknowledgments
The authors
would like to thank CONACYT (México) for the scholarship granted to the first
author.
1. Abbott, W. S.
1925 A method of computing the effectiveness of an insecticide. Journal of
Economic Entomology. 18: 265-267.
2. Amutha;
Gulsar Banu 2017. Variation in mycosis of entomopathogenic fungi on mealybug, Paracoccus
marginatus (Homoptera: Pseudococcidae). Proceedings of the National Academy
of Sciences, India Section B: Biological Sciences. 87: 343-349.
3. Butt, T. M.;
Goettel, M. S. 2000. Bioassays of Entomogenous Fungi. In: Navon, N., and &
Ascher, K.R.S. (eds.). Bioassays of Entomopathogenic Microbes and Nematodes.
CAB International. Wallingford. Oxon. UK. 141-195.
4. Cabanillas,
H. E.; Jones, W. A. 2009. Pathogenicity of Isaria sp. (Hypocreales:
Clavicipitaceae) against the sweet potato whitefly B biotype, Bemisia tabaci
(Hemiptera: Aleyrodidae). Crop Protection. 28(4): 333-337.
5. Chartier, V,
C.; Hill, M. P.; Moore, S. D.; Dames, J. F. 2016. Screening of entomopathogenic
fungi against citrus mealybug, Planococcus citri (Hemiptera:
Pseudococcidae). African Entomology. 24(2): 343-351.
6. Elósegui. C.
O.; Elizondo Silva, A. I. 2010. Evaluación microbiológica in vitro de
mezclas de especies de hongos entomopatógenos ingredientes activos de
bioplaguicidas cubanos. Fitosanidad. 14(2): 103-109.
7. Fernández,
C.; Juncosa, R. 2002. Biopesticidas: la agricultura del futuro. Phytoma.
141: 14-19.
8. Ginting, S.;
Djamilah, D.; Pamekas, T.; Bustaman, H.; Priyatiningsih, P.; Sipriyadi, S.;
Wibowo, R. H. 2020. Pathogenicity of entomopathogenic fungi Lecanicillium
lecanii and Beauveria bassiana against Pseudococcus
jackbeardsleyi (Pseudococcidae) infecting rambutan. Serangga. 25: 1-11.
9. Gopal, G. S.;
Venkateshalu, B.; Nadaf, A. M.; Guru, P. N.; Pattepur, S. 2021. Management of
the grape mealy bug, Maconellicoccus hirsutus (Green), using
entomopathogenic fungi and botanical oils: a laboratory study. Egyptian Journal
of Biological Pest Control. 31(1): 1-8.
10. Inglis, G.
D.; Enkerli, J.; Goettel, M. S. 2012. Laboratory techniques used for
entomopathogenic fungi: Hypocreales. Manual of techniques in invertebrate
pathology. 2: 18-53.
11. Lacey, L.
A.; Frutos, R.; Kaya, H. K.; Vail, P. 2001. Insect pathogens as biological
control agents: do they have a future? Biological Control. 21(3): 230-248.
12. Lacey, L.
A.; Grzywacz, D.; Shapiro-Ilan, D. I.; Frutos, R.; Brownbridge, M.; Goettel, M.
S. 2015. Insect pathogens as biological control agents: back to the future.
Journal of Invertebrate Pathology. 132: 1-41.
13. Lemawork, S.
2008. Evaluation of entomopathogenic fungi and hot water treatment against root
mealybug, Cataenococcus ensete, Williams and Matile-Ferrero (Homoptera:
Pseudococcidae) Doctoral dissertation thesis, Department of Plant Sciences.
Awassa College of Agriculture, School of Graduate Studies Hawassa University.
Awassa. Ethiopia.
14. Lemawork,
S.; Azerefegne, F.; Alemu, T.; Addis, T.; Blomme, G. 2011. Evaluation of entomopathogenic
fungi against Cataenococcus ensete [Williams and Matile-Ferrero, (Homoptera:
Pseudococcidae)]. Crop Protection. 30(4): 401-404.
15. Manjushree,
G.; Chellappan, M. 2019. Evaluation of entomopathogenic fungus for the
management of pink mealybug, Dysmicoccus brevipes (Cockerell)
(Hemiptera: Pseudococcidae) on pineapple in Kerala. Journal of Entomology and
Zoology Studies. 7: 1215-1222.
16. Miranda
Vindas, A.; Blanco Metzler, H. 2013. Control de Dysmicoccus brevipes (Hemiptera:
Pseudococcidae), en el fruto de piña, San Carlos, Costa Rica. Agronomía Costarricense.
37(1): 103-111.
17. Mohamed, G.
S. 2016. Virulence of entomopathogenic fungi against the vine mealy bug, Planococcus
ficus (Signoret) (Hemiptera: Pseudococcidae). Egyptian Journal of Biological
Pest Control. 26(1): 47.
18. Oparaeke, A.
M.; Dike, M. C.; Amatobi, C. I. 2005. Evaluation of botanical mixtures for
insect pest management on cowpea plants. Journal of Agriculture and Rural
Development in the Tropics and Subtropics. 106(1): 41-48.
19.
Palma-Jiménez, M.; Blanco-Meneses, M.; Guillén-Sánchez, C. 2019. Las
cochinillas harinosas (Hemiptera: Pseudococcidae) y su impacto en el cultivo de
Musáceas. Agronomía Mesoamericana. 30(1): 281-298.
20. Pandey, R.
R.; Johnson, M. W. 2006. Enhanced production of pink pineapple mealybug, Dysmicoccus
brevipes (Hemiptera: Pseudococcidae). Biocontrol Science and Technology.
16(4): 389-401.
21. Pelizza, S.;
Mancini, M.; Russo, L.; Vianna, F.; Scorsetti, A. C. 2023. Control capacity of
the LPSc 1067 strain of Beauveria bassiana (Ascomycota: Hypocreales) on
different species of grasshoppers (Orthoptera: Acrididae: Melanoplinae),
agricultural pests in Argentina. Revista de la Facultad de Ciencias Agrarias.
Universidad Nacional de Cuyo. Mendoza. Argentina. 55(1): 98-103. DOI:
https://doi.org/10.48162/rev.39.099
22. R Core Team.
2020. R: A Language and Environment for Statistical Computing. R Foundation for
Statistical Computing, Vienna, Austria: Available at: https://www. R-project. org/
23.
Ramírez-Sánchez, C. J.; Morales-Flores, F. J.; Alatorre-Rosas, R.;
Mena-Covarrubias, J.; Méndez-Gallegos, S. D. J. 2019. Efectividad de hongos
entomopatógenos sobre la mortalidad de Dactylopius opuntiae (Hemiptera:
Dactylopiidae) en condiciones de laboratorio. Revista mexicana de ciencias
agrícolas. 10(SPE22): 1-14.
24. SADER. 2021.
Crece 16.2% producción de piña en México durante 2020. Secretaría de
Agricultura y Desarrollo Rural(SADER). Lastaccess:
August 8th, 2021. https://www.gob.mx/agricultura/prensa/ crece-16-2-produccion-de-pina-en-mexicodurante2020?idiom=es#:~:text=esquera%20 (SIAP).
La%20Secretar%C3%ADa%20de%20Agricultura%20y%20Desarrollo%20Rural%20
report%C3%B3%20que%20M%C3%A9xico,comparaci%C3%B3n%20con%20el%20
a%C3%B1o%20previo
25. SAS. 2012.
Statistical Analysis System, User’s Guide. Statistical. Ver. 9. SAS
Inst. Inc. Cary. N.C. USA.
26. Shah, P. A.;
Pell, J. K. 2003. Entomopathogenic fungi as biological control agents. Applied
Microbiology and Biotechnology. 61(5): 413-423.
27. Surulivelu,
T.; Banu, J. G.; Rajan, T. S.; Dharajothi, B.; Amutha, M. 2012. Evaluation of
fungal pathogens for the management of mealybugs in Bt cotton. Journal of
Biological Control. 26(1): 92-96.
28. Tanwar, R.
K.; Jeyakumar, P.; Monga, D. 2007. Mealybugs and their management Technical Bulletin
19. September 2007. National Centre for Integrated Pest Management LBS
Building, Pusa Campus. New Delhi. 110(12): 1-10.
29. Torres, Á.
A.; Aguilar Ávila, J.; Santoyo, C. V. H.; Uriza Á. D. E.; Zetina, L.;
Rebolledo, M. A. 2018. La piña mexicana frente al reto de la innovación.
Avances y retos en la gestión de la innovación. Colección Trópico
Húmedo. Universidad Autónoma Chapingo. Chapingo, Estado de México.
México.
http://ciestaam.edu.mx/publicaciones2018/libros/piniamexicana-frente-alreto-de-la-innovacion.pdf.
30.
Ugalde-Trejos, R. 2010. Evaluación a nivel de campo de la patogenicidad de
microorganismos benéficos sobre poblaciones de cochinilla harinosa Dysmicoccus
brevipes (Hemiptera: Pseudococcidae), en el periodo posterior a la
inducción floral del cultivo de piña (Ananas comosus (L.) MERR), en
Finca Indaco Horquetas SA. Trabajo final de Graduación presentado a la Escuela
de Agronomía como requisito parcial para optar el grado de Licenciatura en
Ingeniería en Agronomía. Instituto Tecnológico de Costa Rica, Sede Regional San
Carlos. Costa Rica.
31. Vásquez, A.
O. L. 2000. Manejo de cochinilla (Dysmicoccus brevipes) en el cultivo de
piña orgánica en la zona del Lago de Yojoa, Honduras. Proyecto especial
presentado como requisito parcial para optar al título de Ingeniero Agrónomo en
el Grado Académico de Licenciatura. Zamorano, Honduras.
32. Vianna, F.;
Russo, L.; Troncozo, I.; Ferreri, N.; de Abajo, J. M.; Scorsetti, A. C.;
Pelizza, S. 2023. Susceptibility of Rhyzopertha dominica (Coleoptera:
Bostrichidae) and Sitophilus oryzae (Coleoptera: Curculionidae) to the
fungal entomopathogen Beauveria bassiana (Balsamo-Crivelli) Vuillemin s.l.
(Hypocreales: Clavicipitaceae). Revista de la Facultad de Ciencias Agrarias.
Universidad Nacional de Cuyo. Mendoza. Argentina. 55(2): 76-84. DOI:
https://doi.org/10.48162/rev.39.110
33. Zart, M.;
Ferracim de Macedo, M.; Santos, R. J. S.; Souza, D. G.; Pereira Brito, C.; de
Souza Poletto, R.; Alves, V. S. 2021. Performance of entomopathogenic nematodes
on the mealybug, Dysmicoccus brevipes (Hemiptera: Pseudococcidae) and
the compatibility of control agents with nematodes. Journal of Nematology.
20(53): 1-10.