Revista de la Facultad de Ciencias
Agrarias. Universidad Nacional de Cuyo. Tomo 56(1). ISSN (en línea) 1853-8665.
Año 2024.
Original article
A
polyphasic study of non-aflatoxigenic Aspergillus flavus Link, isolates
from maize in the Chaco semi-arid region of Argentina
Estudio
polifásico de aislados no aflatoxigénicos de Aspergillus flavus Link, en
maices de la región del Chaco semiárido argentino
María Silvina
Alaniz Zanon6,
Ada Karina
Torrico5,
Marcelo Druetta7,
Ignacio Martín
Luna7,
Agustina Ruiz Posse8,
Sofía Noemí
Chulze6,
María de la Paz
Giménez Pecci5
1Universidad
Nacional de Cuyo. Facultad de Ciencias Agrarias. Cátedra de Fitopatología.
Almirante Brown 500. Chacras de Coria. Mendoza. Argentina. M5528AHB.
2Instituto
Nacional de Tecnología Agropecuaria (INTA). Estación Experimental Mendoza. San
Martín 3853. Luján de Cuyo. Mendoza. Argentina. 5507
3Instituto
de Biología Agrícola de Mendoza (IBAM). Almirante Brown 500. Chacras de Coria.
Mendoza. Argentina. M5528AHB.
4Consejo
Nacional de Investigaciones Científicas y Técnicas (CONICET). Godoy Cruz 2290.
Ciudad Autónoma de Buenos Aires. Argentina. C1425FQB.
5INTA.
Instituto de Patología Vegetal (IPAVE). Centro de Investigaciones Agropecuarias
(CIAP). Av. 11 de Septiembre 4755. Córdoba. Córdoba. Argentina. X5014MGO.
6Instituto
de Investigación en Micología y Micotoxicología (IMICO). CONICET. Universidad
Nacional de Río Cuarto (UNRC). Ruta Nac. 36 km 601. Río Cuarto. Córdoba.
Argentina. X5804BYA.
7Estación
Experimental Agropecuaria. Este de Santiago del Estero. Instituto Nacional de
Tecnología Agropecuaria (EEA - ESE - INTA). Ruta Prov. N° 6 Km 9. Quimilí.
Santiago del Estero. Argentina. G3740CDA.
8Unidad
de Fitopatología y Modelización Agrícola (UFyMA). INTA. CONICET. Av. 11 de
Septiembre 4755. Córdoba. Córdoba. Argentina. X5014MGO.
*jbarontini@fca.uncu.edu.ar
Abstract
Maize (Zea
mays L.) is one of the most widely planted crops globally with Argentina
leading world production and exportation. Santiago del Estero province, east of
Tucumán and north of Córdoba encompasses eight agro-climatic zones in the Chaco
Semi-arid region, agro-ecologically characterized by a wide temperature range
and frequent drought periods that expose the crop to pathogens, particularly Aspergillus
flavus. This pathogen is responsible for ear rot and grain contamination
with mycotoxins such as aflatoxin B1 and cyclopiazonic acid. This study
obtained fungal isolates from ears of maize and characterized them according to
toxigenic capability and morphotype of sclerotia (S < 400 μm, associated
with high levels of aflatoxins and L > 400 μm, related to variable levels of
aflatoxins). In addition, those not producing aflatoxins were studied to
determine phylogenetic relationships based on sequences of a segment of the CaM
gene. Fifty-eight isolates were obtained in eight localities representing
each agro-climatic zone, 30 of which were non-aflatoxigenic, 28 aflatoxigenic,
and all producers of ciclopiazonic acid. Six isolates did not produce
sclerotia, 51 were L and only one was S, the latter being a non-producer of
aflatoxins. The number of sclerotia was positively correlated with the
production of aflatoxin B1, while size was negatively correlated. The CaM gene
sequences corroborated that the isolates belonged to the A. flavus clade
and the high nucleotide similarity among them (99.4% to 100%) revealed almost
zero genetic diversity in this geographic region. No significant differences
were observed in the proportion of isolates between growing seasons or among
agroclimatic districts. This research revealed characteristics of fungus
populations in this agricultural region of north Argentina.
Keywords: non-aflatoxigenic
isolate, cyclopiazonic acid, sclerotia production, phylogenetic relationships
Resumen
El maíz (Zea
mays L.) es uno de los cultivos más sembrados en el mundo, siendo Argentina
líder mundial en producción y exportación. La Provincia de Santiago del Estero,
el este de Tucumán y el norte de Córdoba abarcan ocho zonas agroclimáticas en
la región Chaco semiárida, la cual se caracteriza por un amplio rango de
temperatura y frecuentes sequías que exponen al cultivo a patógenos,
particularmente Aspergillus flavus, responsable de la podredumbre de la
espiga y la contaminación de granos con micotoxinas como aflatoxina B1 y ácido
ciclopiazónico. Se obtuvieron aislados del hongo desde espigas de maíz y se
caracterizaron según capacidad toxigénica y morfotipos de esclerocios (S <
400 μm, asociados con elevados niveles de aflatoxinas y L > 400 μm,
relacionados con niveles variables de aflatoxinas). Además, aquellos que no
producían aflatoxinas se estudiaron para determinar sus relaciones
filogenéticas sobre la base de un segmento del gen CaM. Cincuenta y ocho
aislados fueron obtenidos en ocho localidades representativa de cada zona
agroclimática, 30 de los cuales resultaron no aflatoxigénicos, 28
aflatoxigénicos y todos productores de ácido ciclopiazónico. Seis aislados no
produjeron esclerocios, 51 fueron L y solo uno fue S, siendo este último no
aflatoxigénico. El número de esclerocios correlacionó positivamente con la
producción de aflatoxina B1, mientras que el tamaño lo hizo de manera negativa.
Las secuencias del gen CaM corroboraron que los aislados pertenecen al
clado A. flavus y la alta similitud nucleotídica (99,4% a 100%) reveló
casi nula diversidad genética en esta región. No se observaron diferencias en
la proporción de aislados entre campañas agrícolas estudiadas ni distritos
agroclimáticos. Esta investigación reveló características de las poblaciones de
hongos en esta región agrícola del norte argentino.
Palabras clave: aislado no
aflatoxigénico, ácido ciclopiazónico, producción de esclerocios, relaciones
filogenéticas
Originales:
Recepción: 02/08/2023 - Aceptación: 07/05/2024
Introduction
Argentina ranks
sixth in maize production, with approximately 60 million tons a year. The
region encompassing Santiago del Estero province, east of Tucumán and north of
Córdoba, in the Chaco Semi-arid region, produces about 6 million tons (58). This
geographical area is a critical climatic zone, where climatic factors are
highly variable (57). Thus, maize
crop development in these agroecosystems is constrained by stressful
environmental factors that expose the crop to different pathogens, such as
those responsible for ear rot (27, 67). The most
dangerous of these pathogens is Aspergillus flavus Link, a globally
distributed filamentous, cosmopolitan pathogen that causes opportunistic
infections in animals and plants (12, 42). This pathogen
is commonly present in air and soil mycobiota. Under certain water and thermal
stress conditions, some isolates produce mycotoxins like aflatoxins and
cyclopiazonic acid (CPA). Aflatoxins are secondary metabolites with strong
hepatotoxic, teratogenic, immunosuppressive and mutagenic activity when
inhaled, ingested or absorbed through animal and human skin (51); A. flavus is
the main driver of aflatoxin contamination in maize in the world (45). While CPA
produces liver necrosis, convulsions myocardium lesions in animals (62,
63),
the specific exposure response to this toxin differs among species, without
sufficient evidence about natural contamination of foods (81).
The maximum
safety limit of aflatoxins for maize commercialization varies between
countries, being 20 ng. g-1 in Argentina,
Brazil and USA, 4 ng. g-1 in the European
Union, and 15 ng. g-1 in African countries
like Ghana (4, 9, 16, 34, 77).
Species of the
genus Aspergillus produce sclerotia, resistance structures consisting of
darkly pigmented and hardened hyphal mats (50) conferring
different phenotypic characteristics (1). Thus, Aspergillus
species can be classified into two morphotypes: S isolates, which produce
numerous sclerotia under 400 μm in diameter, associated with high levels of
aflatoxins, and L isolates, with fewer sclerotia, diameter over 400 μm, and
related to variable levels of aflatoxins (75). A third type
of isolate not producing sclerotia under laboratory conditions, has been
identified in Argentina and Italy (13, 38).
The variability
among A. flavus populations can be estimated by establishing
phylogenetic relationships based on morphological and molecular markers (51), as in the
case of the calmodulin gene (CaM) (36). Calmodulin (CaM)
is a highly conserved polypeptide present in eukaryotic cells. In the genus Aspergillus,
CaM is important in phosphorylation and dephosphorylation of proteins
involved in the biosynthesis of aflatoxins (47).
Aflatoxin
contamination usually starts in the field, during harvest of infected grains,
becoming more severe during storage (35). In the field,
spores of A. flavus are transported by biotic agents (insects, birds)
and/or abiotic agents (wind, rain splash) to the maize ear. Spores enter
through the silk during flowering and through the wounds of the ear cover or of
developing kernels (66). Water stress,
high temperatures, insect attack, certain sowing dates and high crop density
favor contamination with the pathogen and the possible production of aflatoxins
(48).
Some members of A.
flavus were found unable to biosynthesize aflatoxins being, therefore,
plausible biocontrol tools (23). Biological
control applied in maize and peanut (6, 73), among other
crops, is based on the competition for infection sites and essential nutrients
between non-aflatoxigenic and aflatoxigenic isolates. Thus, non-aflatoxigenic
isolates displace aflatoxigenic ones and reduce the latter’s capacity to
produce aflatoxins (70, 79).
Non-aflatoxin
producing strains selected as biocontrol agents must meet some requirements,
such as being native to the area of application, being able to displace the
toxicogenic isolates in the ecosystem and thereby reduce infections (23) and be
genetically incompatible with the remaining fungal population. Therefore,
studies of the native fungal population are crucial (42).
This work aimed
to study the diversity of A. flavus populations in the Chaco Semi-arid
region of Argentina through the morphological, toxicological and molecular
characterization of isolates obtained from maize ears.
Materials
and methods
Sampling
Sampling was
conducted during the 2015/16 and 2016/17 growing seasons in representative
localities of eight agro-climatic zones: Sachayoj (Zone 4: Río Muerto), Quimilí
(Zone 5: Hickmann), Bandera (Zone 17: Bandera), Santiago del Estero (Zone 18:
Monteagudo), Sumampa (Zone 37: Soto), Santa Rosa de Leales (Zone 18A:
Monteagudo), Rayo Cortado (Zone 37C: Soto-north of Córdoba) and Villa de
Tulumba (Zone 61B: Deán Funes), located between -30.38 and -26.55 S and -61.82
and -65.27 W (25) (figure
1).
Sampling localities / Localidades evaluadas:
Sachayoj. Quimilí. Santiago del Estero. Bandera. Sumampa (Santiago del Estero province). Santa Rosa de Leales (Tucumán province). Rayo Cortado. Villa de Tulumba (Córdoba
province).
Figure 1. Sampling
localities in the Chaco Semi-arid region of Argentina.
Figura 1. Localidades
evaluadas en la región del Chaco semiárido argentino.
Ten ears were
randomly collected along a diagonal line in the plot, at plant physiological
maturity before harvest. They were threshed to form a composite sample and
oven-dried at 38°C for 72 hours until final humidity was under 12 %, therefore,
minimizing fungus development (26).
Isolation
and morphological and molecular identification
From each
sample, 100 kernels were taken, surface-desinfested with a 1% sodium
hypochlorite wash followed by three washes with distilled water and plated in
DG-18 medium. Kernels were incubated for 7 days at 25°C and cultures with
characteristics similar to those of A. flavus were transferred to MEA
medium. They were incubated for 7 days at 25°C (65) and then
identified through taxonomic keys (48). Spores of
each isolate were serially diluted, and the most diluted concentration was
cultured in 2 % Water - Agar (WA) medium and incubated for 18 hours at 27°C (65). The
germinated conidia were identified under Nikon SMZ-10 stereoscopic microscope
(15X). Two conidia were extracted from each cultured Petri dish, along with a
portion of culture medium, and transferred to Petri dishes containing MEA
medium. They were incubated for 7 days at 25°C. Then, one of them was stored to
obtain the single spore cultures corresponding to each isolate.
The identity of A.
flavus was confirmed by PCR using the primers FLA1,
5’-GTAGGGTTCCTAGCGAGCC-3’; FLA2 5’-GGAAAAAGATTGATTTGCGTTC-3’ (41), with the ITS
region as target, using the isolate A. flavus CCC 101-92 < NRRL 3251
as positive control, and the isolate A. parasiticus NRRL 2999 as
negative control. Each single-spore isolate was cultured in MEA and incubated
for 7 days at 25°C. Then, an aliquot of mycelium was taken, and DNA was
extracted (55). For each
reaction, 1 μL (final concentration of 20-100 ng of DNA.μL-1,
quantified in Nano Drop, Thermo Fisher Scientific, USA) of DNA solution was
mixed with 24 μL of a solution composed of 5 μL 5x Green GoTaq® reaction buffer
(Promega®, Madison, WI, USA), 0.5 μL of a mixture of dNTP (10 mM of dATP, dCTP,
dGTP and dTTP), 1 μL of the primers (20 μM), 1 μL of polymerase enzyme GoTaq®
(5U.μL-1, Promega®, Madison, WI, USA) and 16.5 μL DEPC-treated
water. The PCR reaction was performed following González Salgado
et al. (2011).
PCR products were revealed by agarose gel electrophoresis, visualized in UV
transilluminator, with previous staining in GelRedTM Biotium solution (2.5 ng.
μL-1) and confirmed by the expected band size of 500 pb. Molecular
size of the DNA fragments was estimated using the “qLadder 100 pb precision”
marker (PB-L®, Bs As, Argentina).
Sclerotia
production
Each isolate was
cultured in 6-cm Petri dishes containing Czapeck dox (Cz) medium. They were
inoculated in the center of the dish with 10 μL of a spore suspension and
incubated for 14 days at 30°C (64). Sclerotia
were recovered, their diameters measured under a microscope (Nikon eclipse Cs1
spectral) and characterized following Cotty (1989) into L or S morphotypes or
isolates not producing (NP) sclerotia (60). Isolates not
producing sclerotia after an incubation period were cultured in 5/2 medium for
5 to 7 days at 31°C to induce their production (38).
Toxigenic
capability
Considering
Aflatoxin B1 (AFB1) was one of the greatest frequency and
toxicity of the four most important aflatoxins identified, its production was
quantified (23, 82). A. flavus isolates
were inoculated in duplicate on MEA slants for 7 days at 28°C. These cultures
were used to prepare spore suspensions, following the method described by Alaniz
Zanon et al. (2013). Spore concentration was measured in a Neubauer
chamber and adjusted to 105 spores. mL-1.
Four-milliliter vials containing 1 mL broth from a medium containing 150 g
sucrose, 20 g yeast extract, 10 g soytone, and 1 L distilled water, were
inoculated with 100 μL of each spore suspension. Medium pH was adjusted to 5.9
with HCl. Cultures were incubated for 7 days at 30°C. Vial cultures were
extracted according to Horn et al. (1996), by adding 1 mL
of chloroform to each vial and vortexing for 30 s.
The first group
of dried extracts was evaluated in their capacity to produce aflatoxins by HPLC
according to Horn et al. (1996). Aflatoxins
were analyzed by injecting 50 μL of extract from each vial into an HPLC system
consisting of a Hewlett Packard model 1100 pump (Palo Alto, CA) connected to a
Hewlett Packard model 1046A programmable fluorescence detector and a data
module Hewlett Packard Kayak XA (HP ChemStation Rev.A.06.01). Chromatographic
separations were performed on a stainless steel, C18 reversed-phase column (150
mm × 4.6 mm i.d., 5 μm particle size; Luna Phenomenex, Torrance, CA, USA)
connected to a precolumn Security Guard (20 mm × 4.6 mm i.d., 5 μm particle
sizes, Phenomenex). The mobile phase was water:methanol:
acetonitrile (4:1:1, v/v/v) at a flow rate of 1.5 mL. min-1.
Pure aflatoxin solutions were used as external standards (Sigma-Aldrich, St.
Louis, MO, USA).
The second group
of dried extracts was resuspended in 500 μL of mobile phase of acetonitrile:
ZnSO4 4 mM buffer solution (65:35, V/V) and CPA was determined in
the HPLC system. Chromatographic separations were performed in Agilent ZORBAX
RX-SIL column (250 mm x 4.6 mm i.d., 5 μm particle size) connected to a
pre-column Security Guard (20 mm x 4.6 mm i.d., 5 μm particle sizes,
Phenomenex) and 50 μl of each simple were analyzed at a flow velocity of 0.8
mL. min-1.
The quantitative
analysis was performed by normalization of peak areas. A calibration curve was
elaborated from the areas obtained for the different concentrations of
standards of aflatoxins and CPA, as applicable (Sigma Aldrich, St. Louis, MO,
USA). The AFB1 and CPA detection limit was 1 ng. g-1 (24,
32).
Sequencing
and Phylogenetic relationships
Molecular
variability of the non-aflatoxigenic isolates was explored using a 688-pb fragment
of the CaM gene. We studied only this type of isolate given their
potential for biocontrol strategies. Spores of the isolates cultivated in MEA
medium were suspended for 7 days at 25°C. Conidia (1x106 conidia. mL-1)
were inoculated in 100 mL of lixiviated medium of potato glucose agar (10% of
potato infusion from 200 g potato, 2% glucose, 4.5 pH). They were incubated in
orbital shaker at 150 rpm for 48 hours at 25°C. Mycelium was collected by
filtration and powdered with liquid N2 (28). This culture
medium allows one to obtain enough amount of mycelium. DNA was extracted using
the cetyl-trimethylammonium bromide (CTAB) method (54), quantified by
spectrophotometry and a segment of the CaM was amplified with the
primers CL1, 5’- GA(GA)T(AT)CAAGGAGGCCTTCTC -3’; CL2A,
5’- TTTTTGCATCATGAGTTGGAC -3’ (61). The obtained
fragments of the expected size (688 pb) were purified using DNA columns of
Wizard® SV Gel columns and PCR clean - Up system (Promega, Madison, WI, USA),
following manufacturer instructions. Then, they were quantified by
spectrophotometry. Both DNA chains were sent for sequencing by Sanger method
(Macrogen, Seoul, South Korea). The obtained sequences were aligned using the
software Clustal X2 (54). The software
MEGA version 7 (53) was used to
select the best nucleotide substitution model and to obtain the phylogenetic
trees using Neighbor-Joining (NJ) (74) and
Maximum-Likelihood (ML) methods (39, 76), both with
10,000 replicates bootstrap. The sequences were compared with the reference
NW_002477238.1 and A. niger MH645004.1 was used as outgroup, both from
the GenBank.
Statistical
analysis
Data on
sclerotia morphotypes, toxigenic capacity and variability of the sequences of
the CaM genes were analyzed using InfoStat statistical software as well
as correlation analyses (29). Mean values
were obtained using analysis of variance (ANOVA) and differences between means
were compared using Fisher’s LSD test (P< 0.05).
Results
and discussion
Morphological
and molecular identification
A total of 58
isolates were obtained in the sampling localities from the eight agro-climatic
zones (table
1)
with A. flavus morphological characteristics, according to Klich
(2002)
(figure
2).
Table
1. Isolates of Aspergillus flavus from
maize ears collected from representative localities of eight agro-climatic
zones of the Chaco Semi-arid region of Argentina. Production of aflatoxin B1,
cyclopiazonic acid and sclerotia morphotype during the 2015/16 and 2016/17
growing seasons.
Tabla 1. Aislados
de Aspergillus flavus de espigas de maíz colectadas en localidades
representativas de ocho zonas agroclimáticas del Chaco semiárido argentino.
Producción de aflatoxina B1, ácido ciclopiazónico y morfotipo de esclerocios
durante las campañas agrícolas 2015/16 y 2016/17.
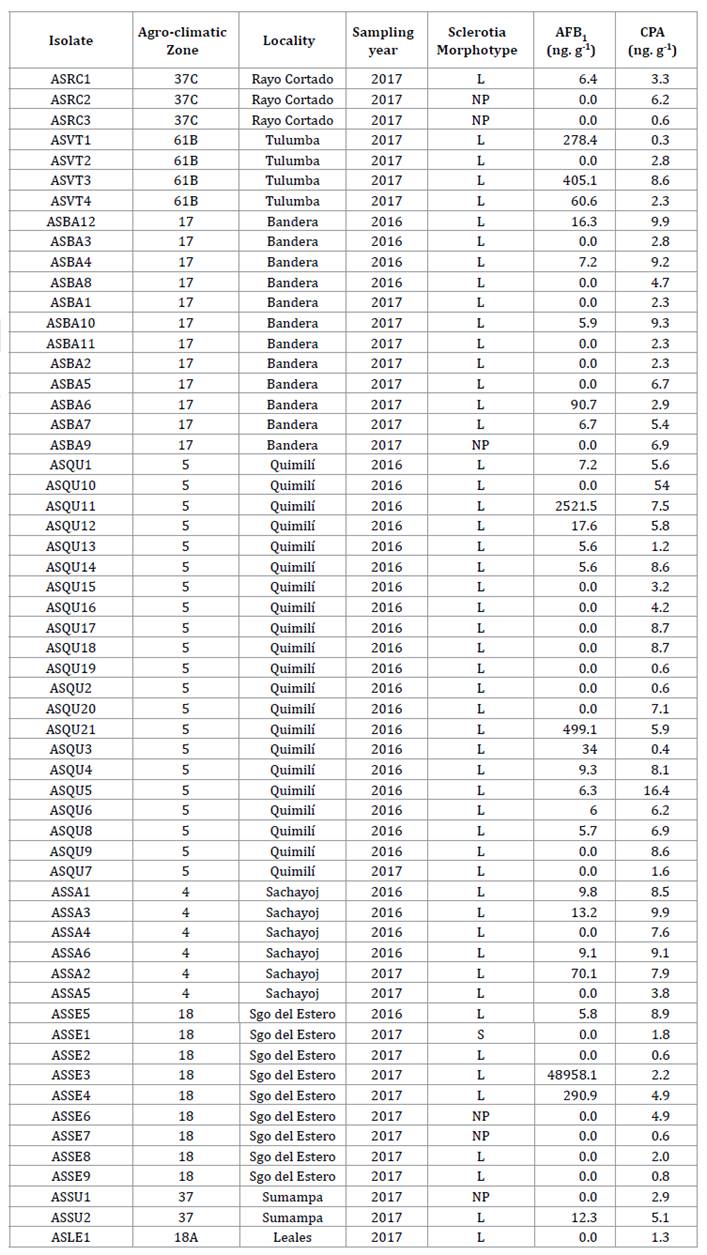
Agro-climatic zones: 4: Río Muerto, 5: Hickmann, 17:
Bandera, 18 and 18A: Monteagudo, 37: Soto, 37C: Soto - North of Córdoba, 61B:
Dean Funes. L: sclerotia of > 400 μm; S: sclerotia of < 400 μm; NP:
non-producer of sclerotia. AFB1: Aflatoxin B; CPA: cyclopiazonic acid.
Zonas agroclimáticas: 4: Río Muerto, 5: Hickmann,
17: Bandera, 18 y 18A: Monteagudo, 37: Soto, 37C: Soto - Norte de Córdoba, 61B:
Deán Funes. L: esclerocios de tamaño > 400 μm; S: esclerocios de tamaño <
400 μm; NP: no productor de esclerocios. AFB1: Aflatoxina B; CPA: ácido
ciclopiazónico.
Figure 2. Aspergillus
flavus Link colony on malt extract agar
(MEA) culture medium.
Figura 2. Colonia
de Aspergillus flavus Link en medio de cultivo agar extracto de malta
(MEA).
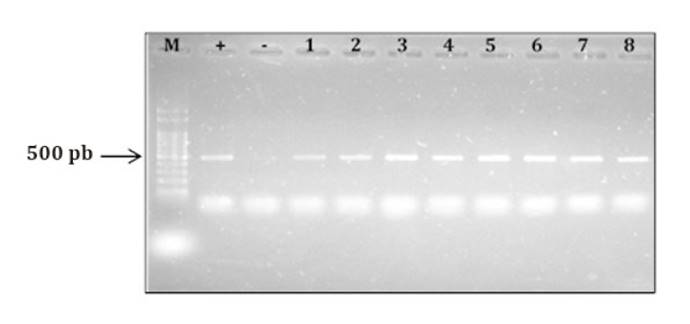
A. flavus identity was
confirmed using the specific primers FLA1 and FLA2 through amplification of a
500-pb band (41) (figure
3).
M: molecular marker (100-pb DNA Ladder); +: Aspergillus
flavus NRRL 3251 (positive control); -: A. parasiticus NRRL2999
(negative control); 1: ASSA2; 2: ASQU7; 3: ASQU21; 4: ASQU10; 5: ASQU15; 6:
ASRC2; 7: ASQU12; 8: ASQU6; 9: ASBA3; 10: ASVT1.
M: marcador molecular (100-pb DNA Ladder); +:
Aspergillus flavus NRRL 3251 (control positivo); -: A. parasiticus NRRL2999
(control negativo); 1: ASSA2; 2: ASQU7; 3: ASQU21; 4: ASQU10; 5: ASQU15; 6:
ASRC2; 7: ASQU12; 8: ASQU6; 9: ASBA3; 10: ASVT1.
Figure 3. Electrophoresis
gel showing PRC products obtained using the specific primers FLA1 and FLA2 (González Salgado et al., 2011).
Figura 3. Gel
de electroforesis mostrando productos de PCR obtenidos utilizando los
iniciadores específicos FLA1 y FLA2 (González
Salgado et al., 2011).
The presence of isolates in the eight zones,
each one being considered a different agroecological environment given different
meteorological scenarios at each growing season shows the broad distribution of
the fungus in the study region, as reported for other regions of the country (8,
17)
and the world (11, 30, 32).
Sclerotia
production
Of the 58 A.
flavus isolates identified, 6 (10%) produced no sclerotia, whereas 52 (90%)
produced sclerotia. Of the latter, 51 (98%) were identified as L morphotype (figure
4),
with ˃ 400 μm in diameter, and only isolate, ASSE1, (2%) corresponded to S
morphotype, with ˂ 400 μm in diameter (table 1).
Figure 4. Sclerotia
L morphotype in aflatoxigenic isolate of Aspergillus flavus. Observation
under stereo microscope Nikon SMZ-10 (15X).
Figura 4. Esclerocios
morfotipo L en aislado aflatoxigénico de Aspergillus flavus. Observación
bajo lupa estereoscópica Nikon SMZ-10 (15X).
Similar
proportions between L, S and NP morphotypes in maize kernels were reported by Moreno
(2004)
in Mexico and by Pildain et al. (2005) in Argentina in
peanut isolates. The low or null presence of S morphotype in maize was also
reported by Giorni et al. (2007) in Italy. These
authors identified one S isolate out of 70 isolates, with the remaining ones
being NP. Similarly, Alaniz Zanon et al. (2018) reported a high
proportion of L morphotype in Argentina with respect to NP, with absent S morphotype.
Sclerotia
production was reported in isolates from all the agro-climatic zones and in
both evaluated growing seasons. Dominance of L morphotype among isolates
obtained in the Chaco Semi-arid region agrees with previous records in
Argentina (7, 18) and in other
countries, like Brazil, Portugal, Nigeria and Sub-Saharan Africa regions (5,
20, 33, 68, 72).
The high
incidence of S morphotype is associated with regions of low precipitation and
high temperatures, where high number of small sclerotia can be a survival trait
facing rapid temperature and humidity fluctuations (19). However, and
even though the study region is dry and hot, this was not observed.
Toxigenic
capability
Based on the
production of AFB1 and CPA of the 58 isolates obtained, we
identified 30 (52%) non-aflatoxigenic and 28 (48%) aflatoxigenic. This
similarity of proportions was indicated by Martins et
al. (2017)
in peanut crop in Brazil and Camiletti et
al. (2018)
in maize ears in Argentina.
Aflatoxin-producer
and non-producer isolates were obtained in all the agro-climatic zones and
growing seasons, except Santa Rosa de Leales, where the only isolate found was
non-producer.
Of the 28
isolates producing AFB1, 26 (93%) had concentrations below 500 ng. g-1,
whereas ASQU11 and ASSE3 were higher aflatoxin producers of 2,521.5 ng. g-1
and 48,958.1 ng. g-1 respectively (table 1). Although the
capacity to produce mycotoxins may depend on geographical origin and
environmental conditions (62), our results
do not show any pattern associated with zones or growing seasons. This agrees
with Bayman and Cotty (1993), who did not
detect differential patterns between nearby zones.
All isolates
were CPA producers, indicating toxicity importance (78) and plausible
use as aflatoxin biocontrol strategies. This is the case of the AF36 agent used
in competitive exclusion strategies and found responsible for CPA increase in
maize kernels inoculated in the field, and peanuts (2,
31).
By contrast, Camiletti et al. (2018) detected 19% of
CPA non-producer isolates in maize kernels in Argentina. On the other hand, Vaamonde
et al. (2003)
studied wheat, soybean and peanut in Argentina and found between 6% and 27% of
isolates not producing this mycotoxin. In Italy, Giorni et al.
(2007)
reported 39% CPA non-producer isolates in maize kernels, whereas, in organic
nut plantations in Brazil, Reis et al.
(2014)
found that 34% of the A. flavus isolates were non-producers of that
mycotoxin. In addition, Jamali et al. (2012) identified 19 %
of the isolates as CPA non-producers in soil samples of pistachio plantations
in Czech Republic.
The presence of
isolates producing both CPA and aflatoxins was reported in Argentina and other
parts of the world (8, 18, 37, 71). The isolates
with the highest AFB1 production obtained in the Chaco Semi-arid
region do not correspond to those with the highest CPA production.
Correlations
between AFB1 production and the number (r= 062) and size of
sclerotia (r= -0.38) indicate that the produced amount of AFB1
increases as sclerotia size decreases. These results are similar to those
reported by Arrúa Alvarenga et al. (2012) and Pildain
et al. (2005),
but differ from Bouti et al. (2020), who did not
find any correlation between sclerotia and aflatoxin production. No correlation
was observed between CPA production and sclerotia morphotype.
The only S
morphotype identified in this study (ASSE1), collected in 2016/17 growing
season, did not produce AFB1. While the presence of
non-aflatoxin-producing isolates was also reported for USA, Ghana and Brazil (3,
22, 40, 44),
in general, S morphotype isolates produce higher aflatoxin concentrations than
L or NP morphotypes and are identified as major causal agents of severe
contamination in maize and of most human deaths due to aflatoxicosis (13,
23).
The S isolates are worldwide distributed, associated with aflatoxins B
production in the USA and Africa (68), and of both B
and G aflatoxins (3, 38).
Sequencing
and phylogenetic analysis
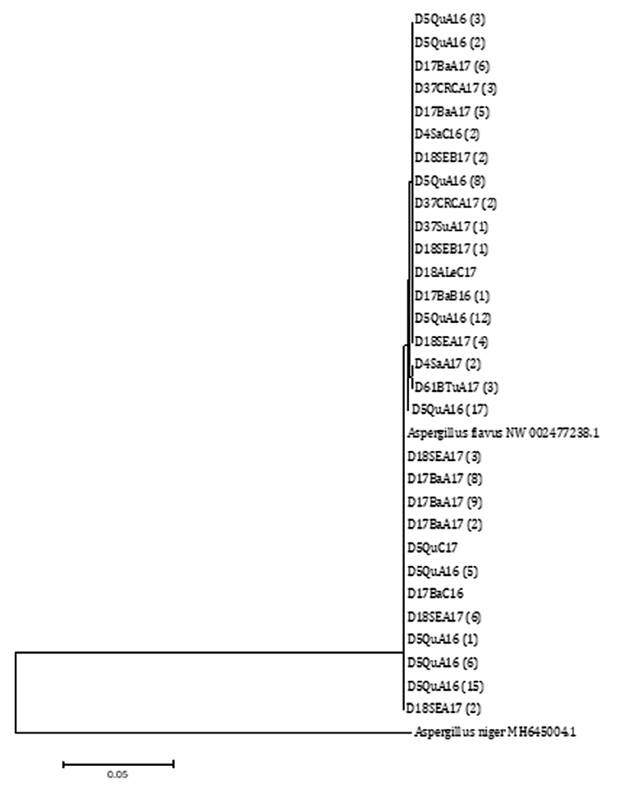
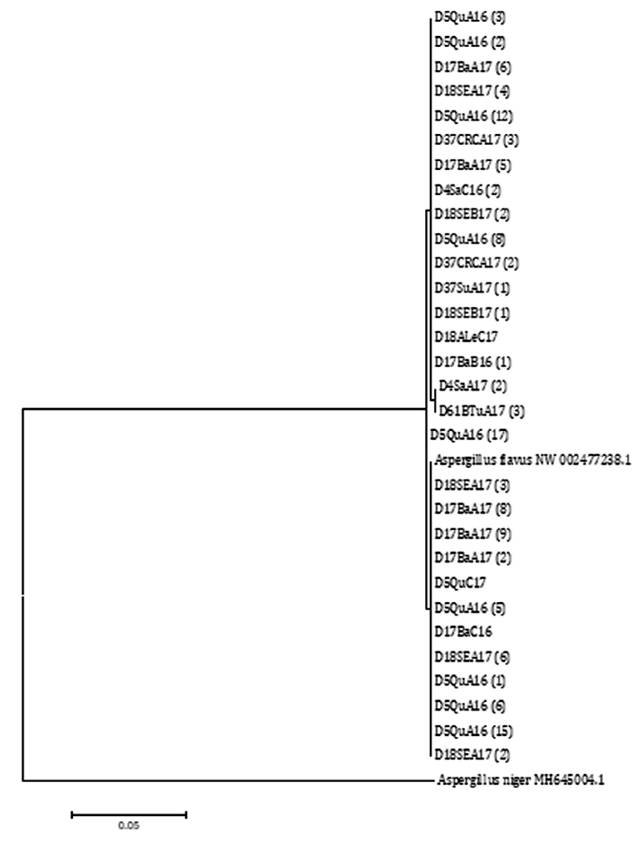
All the CaM gene
sequences studied in this work, using both NJ and ML, belong to the A.
flavus clade (figure 5 and figure
6).
Node number corresponds to bootstrap support values
(10000 replicates). A. flavus NW 002477238.1 was selected as reference
sequence and A. niger MH645004.1 as outgroup sequence.
El número de nodos corresponde a un valor de
bootstrap de 10000 réplicas. A. flavus NW 002477238.1 se utilizó como
secuencia de referencia y A. niger MH645004.1 como secuencia externa al
grupo.
Figure 5. Phylogenetic
tree built using the Neighbor-Joining (NJ) statistic method based on the
relationship among segments of the sequence of the CaM gene from A.
flavus isolates from agro-climatic zones of the Chaco Semi-arid region in
Argentina.
Figura 5. Árbol
filogenético realizado con el método estadístico Neighbor-Joining (NJ) basado
en las relaciones entre las secuencias de segmentos del gen CaM de
aislados de A. flavus provenientes de zonas agroclimáticas del Chaco
semiárido argentino.
Node number corresponds to bootstrap support values
(10000 replicates). A. flavus NW 002477238.1 was selected as reference
sequence and A. niger MH645004.1 as outgroup sequence.
El número de nodos corresponde a un valor de
bootstrap de 10000 réplicas. A. flavus NW 002477238.1 se utilizó como
secuencia de referencia y A. niger MH645004.1 como secuencia externa al
grupo.
Figure 6. Phylogenetic
tree built using the Maximum-Likelihood (ML) statistical method based on the
relationship among segments of sequences of the CaM gene from A.
flavus isolates from agro-climatic zones in the Chaco Semi-arid region in
Argentina.
Figura 6. Árbol
filogenético realizado con el método estadístico Maximum-Likelihood (ML) basado
en las relaciones entre las secuencias de segmentos del gen CaM de
aislados de A. flavus provenientes de zonas agroclimáticas del Chaco
semiárido argentino.
The high similarity
among isolates for that character showed limited genetic diversity in this
geographic region. In addition, the only S morphotype is located together with
L morphotype isolates, all of which are aflatoxin non-producers. This finding
may be attributed to the fact that these are young populations and, therefore,
may have not undergone sufficient mutations or recombination events leading to
variability among isolates from the region (42, 62). However, only
the northern zones of the region (4 and 5) correspond to land recently
converted to agriculture, whereas in the remaining districts, both commercial
hybrids and maize for self-consumption have been cultivated for several
decades.
Conclusions
A. flavus is present in
all localities of the sampled agro-climatic zones and both growing seasons,
evidencing the wide distribution of the pathogen in the Chaco Semi-arid region.
The population of A. flavus exhibits diversity in terms of sclerotia
morphotypes and production of AFB1 and CPA.
Low genetic
variability among the isolates was observed, all belonging to the A. flavus clade.
In the future,
genes from more variable regions will be used to perform phylogenetic analyses
among A. flavus isolates.
Acknowledgments
The authors
thank PhD. Ricardo Comerio (EEA INTA Anguil, La Pampa, Argentina) and PhD.
Boris Camiletti (FCA Universidad Nacional de Córdoba, Córdoba, Argentina), for
the fungal reference isolates used in this study and PhD Verónica Trucco (IPAVE
- CIAP - INTA, Córdoba, Argentina) for computer assistance with phylogenetic
analysis.
Funding sources
This work was
supported by a grant from the PE I 074 (Integrated Pest Management) and PE I
147 (Food Safety) of the Instituto Nacional de Tecnología Agropecuaria (INTA).
1. Abbas, H. K.;
Weaver, M. A.; Zablotowicz, R. M.; Horn, B. W.; Shier, W. T. 2005.
Relationships between aflatoxin production and sclerotia formation among
isolates of Aspergillus section Flavi from the Mississippi Delta.
European Journal of Plant Pathology. 112(3): 283-287.
https://doi.org/10.1007/s10658-004-4888-8
2. Abbas, H. K.;
Weaver, M. A.; Horn, B. W.; Carbone, I.; Monacell, J. T.; Shier, W. T. 2011.
Selection of Aspergillus flavus isolates for biological control of
aflatoxins in corn. Toxin Reviews. 30(2-3): 59-70.
https://doi.org/10.3109/15569543.2011.591539
3. Agbetiameh,
D.; Ortega Beltran, A.; Awuah, R. T.; Atehnkeng, J.; Cotty, P. J.;
Bandyopadhyay, R. 2018. Prevalence of aflatoxin contamination in maize and
groundnut in Ghana: Population structure, distribution, and toxigenicity of the
causal agents. Plant Disease. 102(4): 764-772.
https://doi.org/10.1094/PDIS-05-17-0749-RE
4. Agbetiameh,
D.; Ortega Beltran, A.; Awuah, R. T.; Atehnkeng, J.; Islam, M. S.; Callicott,
K. A.; Cotty, P. J.; Bandyopadhyay, R. 2019. Potential of atoxigenic Aspergillus
flavus vegetative compatibility groups associated with maize and groundnut
in Ghana as biocontrol agents for aflatoxin management. Frontiers in
Microbiology. 10: 1-15. https://doi.org/10.3389/fmicb.2019.02069
5. Ait Mimoune,
N.; Arroyo Manzanares, N.; Gámiz Gracia, L.; García Campaña, A. M.; Bouti, K.;
Sabaou, N.; Riba, A. 2018. Aspergillus section Flavi and aflatoxins in
dried figs and nuts in Algeria. Food Additives & Contaminants: Part B.
11(2): 119-125. https://doi.org/10.1080/19393210.2018.1438524
6. Alaniz Zanon,
M. S.; Chiotta, M.; Giaj Merlera, G.; Barros, G.; Chulze, S. 2013. Evaluation
of potential biocontrol agent for aflatoxin in Argentinean peanuts. International
Journal of Food Microbiology. 162(3): 220-225.
https://doi.org/10.1016/j.ijfoodmicro.2013.01.017
7. Alaniz Zanon,
M. S.; Barros, G. G.; Chulze, S. N. 2016. Non-aflatoxigenic Aspergillus
flavus as potential biocontrol agents to reduce aflatoxin contamination in
peanuts harvested in Northern Argentina. International Journal of Food
Microbiology. 231: 63-68. https://doi.org/10.1016/j.ijfoodmicro.2016.05.016
8. Alaniz Zanon,
M. S.; Clemente, M. P.; Chulze, S. N. 2018. Characterization and competitive
ability of non-aflatoxigenic Aspergillus flavus isolated from the maize
agro-ecosystem in Argentina as potential aflatoxin biocontrol agents.
International Journal of Food Microbiology. 277(March). 58-63.
https://doi.org/10.1016/j.ijfoodmicro.2018.04.020
9. ANVISA.
Agencia Nacional de Vigilancia Sanitaria. 2011. Resolución RDC N° 7.
Disposición sobre límites máximos tolerados para micotosinas en alimentos.
Diario oficial de la Unión, Poder Ejecutivo, Brasilia. DF. 1: 66-67.
10. Arrúa
Alvarenga, A. A.; Moreno Martínez, E.; Quezada Viay, M. Y.; Moreno Lara, J.;
Vázquez Badillo, E.; Flores Olivas, A. 2012. Aspergillus aflatoxigénicos:
enfoque taxonómico actual. Revista Mexicana de Ciencias Agrícolas. 3(4):
1047-1052. https://doi.org/10.29312/remexca.v3i5.1414
11.
Bandyopadhyay, R.; Ortega Beltran, A.; Akande, A.; Mutegi, C.; Atehnkeng, J.;
Kaptoge, L.; Senghor, A. L.; Adhikari, B. N.; Cotty, P. J. 2016. Biological
control of aflatoxins in Africa: current status and potential challenges in the
face of climate change. World Mycotoxin Journal. 9(5): 771-789.
https://doi.org/10.3920/WMJ2016.2130
12. Baranyi, N.;
Kocsubé, S.; Jakšić Despot, D.; Šegvić Klarić, M.; Szekeres, A.; Bencsik, O.;
Kecskeméti, A.; Manikandan, P.; Tóth, B.; Kredics, L.; Khaled, J. M.; Alharbi,
N. S.; Vágvölgyi, C.; Varga, J. 2017. Combined genotyping strategy reveals
structural differences between Aspergillus flavus lineages from
different habitats impacting human health. Journal of Basic Microbiology.
57(11): 899-909. https://doi.org/10.1002/jobm.201700243
13. Barros, G.
G.; Torres, A.; Rodriguez, M.; Chulze, S. 2006. Genetic diversity within Aspergillus
flavus strains isolated from peanut-cropped soils in Argentina. Soil
Biology and Biochemistry. 38(1): 145-152. https://doi.org/10.1016/j.soilbio.2005.04.028
14. Bayman, P.;
Cotty, P. J. 1993. Genetic diversity in Aspergillus flavus: association
with aflatoxin production and morphology. Canadian Journal of Botany. 71(1):
23-31. https://doi.org/10.1139/b93-003
15. Bouti, K.;
Verheecke Vaessen, C.; Mokrane, S.; Meklat, A.; Djemouai, N.; Sabaou, N.;
Mathieu, F.; Riba, A. 2020. Polyphasic characterization of Aspergillus section
Flavi isolated from animal feeds in Algeria. Journal of Food Safety. 40(1):
1-9. https://doi.org/10.1111/jfs.12743
16. CAA. Código
Alimentario Argentino. 2019. Secretaría de Alimentos, Bieoconomía y Desarrollo
Regional Resolución Conjunta 22/ 2019 and 9/2021.
17. Camiletti,
B. X.; Torrico, A. K.; Maurino, M. F.; Cristos, D.; Magnoli, C.; Lucini, E. I.;
Giménez Pecci, M. P. 2017. Fungal screening and aflatoxin production by Aspergillus
section Flavi isolated from pre-harvest maize ears grown in two Argentine
regions. Crop Protection. 92: 41-48.
https://doi.org/10.1016/j.cropro.2016.10.012
18. Camiletti,
B. X.; Moral, J.; Asensio, C. M.; Torrico, A. K.; Lucini, E. I.; Giménez Pecci,
M. P.; Michailides, T. J. 2018. Characterization of argentinian endemic Aspergillus
flavus isolates and their potential use as biocontrol agents for mycotoxins
in maize. Phytopathology. 108(7): 818-828. https://doi.org/10.1094/PHYTO-07-17-0255-R
19. Cardwell, K.
F.; Cotty, P. J. 2002. Distribution of Aspergillus section Flavi among
field soils from the four agroecological zones of the Republic of Bénin, West
Africa. Plant Disease. 86(4): 434-439. https://doi.org/10.1094/PDIS.2002.86.4.434
20. Costa
Baquião, A.; Martins Melo de Oliveira, M.; Alves Reis, T.; Zorzete, P.; Diniz
Atayde, D.; Correa, B. 2013. Polyphasic approach to the identification of Aspergillus
section Flavi isolated from Brazil nuts. Food Chemistry. 139(1-4):
1127-1132. https://doi.org/10.1016/j.foodchem.2013.01.007
21. Cotty, P. J.
1989. Virulence and cultural characteristics of two Aspergillus flavus strains
pathogenic on cotton. Phytopathology. 79(7): 808-814.
22. Cotty, P. J.
1997. Aflatoxin-producing potential of communities of Aspergillus section
Flavi from cotton producing areas in the United States. Mycological Research.
101(6): 698-704. https://doi.org/10.1017/S0953756296003139
23. Cotty, P.
J.; Probst, C.; Jaime García, R. 2008. Etiology and management of aflatoxin
contamination. In Mycotoxins: detection methods, management, public health and
agricultural trade (Issue May: p. 287-299). CABI. Oxfordshire. UK.
https://doi.org/10.1079/9781845930820.0287
24. Da Motta,
S.; Valente Soares, L. M. 2000. Simultaneous determination of tenuazonic and
cyclopiazonic acids in tomato products. Food Chemistry. 71(1): 111-116.
https://doi.org/10.1016/S0308-8146(00)00040-6
25. De Fina, A.
1973. Mapa nacional de los distritos agroclimáticos argentinos. IDIA. 21-28.
26. De Oliveira
Rocha, L.; Reis, G. M.; Braghini, R.; Kobashigawa, E.; de Araújo, J.; Corrêa,
B. 2012. Characterization of aflatoxigenic and non-aflatoxigenic strains of Aspergillus
section Flavi isolated from corn grains of different geographic origins in
Brazil. European Journal of Plant Pathology. 132(3): 353-366.
https://doi.org/10.1007/s10658-011-9881-4
27. De Rossi, R.
L.; Guerra, F. A.; Brücher, E.; Torrico, A. K.; Maurino, M. F.; Lucini, E.;
Gimenéz Pecci, M. P.; Plaza, M. C.; Guerra, G. D.; Camiletti, B. X.; Ferrer,
M.; Laguna, I. G. 2017. Enfermedades del maíz de siembra tardía causada por
hongos. In L. Borras; S. Uhart (Eds.), El mismo maíz, un nuevo desafío:
Compendio primer congreso de maíz tardío. Dow Agrosciences. 109-127.
28. Devi Prameela,
T.; Prabhakaran, N.; Kamil, D.; Borah, J.; Alemayehu, G. 2013. Development of
SCAR marker for specific detection of Aspergillus flavus. African
Journal of Microbiology Research. 7(9): 783-790.
https://doi.org/10.5897/AJMR12.1552
29. Di Rienzo,
J. A.; Casanoves, F.; Balzarini, M. G.; Gonzalez, L.; Tablada, M.; Robledo, C.
W. 2021. InfoStatversión 2020. Centro de Transferencia InfoStat. FCA.
Universidad Nacional de Córdoba. Argentina. www.infostat.com.ar
30. Donner, M.;
Lichtemberg, P. S. F.; Doster, M.; Picot, A.; Cotty, P. J.; Puckett, R. D.;
Michailides, T. J. 2015. Community structure of Aspergillus flavus and A.
parasiticus in major almondproducing areas of California, United States.
Plant Disease. 99(8): 1161-1169. https://doi.org/10.1094/PDIS-05-14-0450-RE
31. Dorner, J.
W.; Horn, B. W.; Cole, R. J. 2000. Non-toxigenic strain of Aspergillus
oryzae and Aspergillus sojae for biocontrol of toxigenic fungi.
United States Department of Agriculture Patents N°6,027,724.
https://patentimages.storage.googleapis.com/2b/7e/3e/f35408d90a6b8b/US6027724.pdf
32. Doster, M.
A.; Cotty, P. J.; Michailides, T. J. 2014. Evaluation of the atoxigenic Aspergillus
flavus strain AF36 in Pistachio orchards. Plant Disease. 98(7): 948-956.
https://doi.org/10.1094/PDIS-10-13-1053-RE
33. Ezekiel, C.
N.; Atehnkeng, J.; Odebode, A. C.; Bandyopadhyay, R. 2014. Distribution of
aflatoxigenic Aspergillus section Flavi in commercial poultry feed in
Nigeria. International Journal of Food Microbiology. 189: 18-25.
https://doi.org/10.1016/j.ijfoodmicro.2014.07.026
34. FDA. Food
and Drug Administration. 2011. Guidance for Industry: Action Levels for
Poisonous or Deleterious Substances in Human Food and Animal Feed. p. 1-15.
https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industryactionlevels-poisonous-or-deleterious-substances-human-food-and-animal-feed#afla
35. Folcher, L.;
Delos, M.; Marengue, E.; Jarry, M.; Weissenberger, A.; Eychenne, N.; Regnault
Roger, C. 2010. Lower mycotoxin levels in Bt maize grain. Agronomy for
Sustainable Development. 30(4): 711-719. https://doi.org/10.1051/agro/2010005
36. Frisvad, J.
C.; Hubka, V.; Ezekiel, C. N.; Hong, S. B.; Nováková, A.; Chen, A. J.;
Arzanlou, M.; Larsen, T. O.; Sklenář, F.; Mahakarnchanakul, W.; Samson, R. A.;
Houbraken, J. 2019. Taxonomy of Aspergillus section Flavi and their
production of aflatoxins, ochratoxins and other mycotoxins. Studies in
Mycology. 93: 1-63. https://doi.org/10.1016/j.simyco.2018.06.001
37. Garcia Cela,
E.; Gari Sanchez, F. J.; Sulyok, M.; Verheecke Vaessen, C.; Medina, A.; Krska,
R.; Magan, N. 2020. Carbon dioxide production as an indicator of Aspergillus
flavus colonization and aflatoxins/cyclopiazonic acid contamination in
shelled peanuts stored under different interacting abiotic factors. Fungal
Biology. 124(1): 1-7. https://doi.org/10.1016/j.funbio.2019.10.003
38. Giorni, P.;
Magan, N.; Pietri, A.; Bertuzzi, T.; Battilani, P. 2007. Studies on Aspergillus
section Flavi isolated from maize in northern Italy. International Journal
of Food Microbiology. 113(3): 330-338.
https://doi.org/10.1016/j.ijfoodmicro.2006.09.007
39. Gomez
Talquenca, G.; Lanza Volpe, M.; Setien, N.; Gracia, O.; Grau, O. 2023.
Serological relationships among strains of grapevine leafroll-associated virus
4 reflect the evolutive behavior of its coat protein gene. Revista de la
Facultad de Ciencias Agrarias. Universidad Nacional de Cuyo. Mendoza.
Argentina. 55(1): 104-114. DOI: https://doi.org/10.48162/rev.39.100
40. Gonçalves,
S. S.; Cano, J. F.; Stchigel, A. M.; Melo, A. S.; Godoy Martinez, P. C.;
Correa, B.; Guarro, J. 2012. Molecular phylogeny and phenotypic variability of
clinical and environmental strains of Aspergillus flavus. Fungal
Biology. 116(11): 1146-1155. https://doi.org/10.1016/j.funbio.2012.08.006
41. González
Salgado, A.; González Jaén, T.; Vázquez, T.; Patiño, B. 2011. Microbial toxins.
In O. Holst (Ed.), Microbial Toxins: Methods and Protocols, Methods in
Molecular Biology. 739(19). Humana Press.
https://doi.org/10.1007/978-1-61779-102-4
42. Grubisha, L.
C.; Cotty, P. J. 2015. Genetic analysis of the Aspergillus flavus vegetative
compatibility group to which a biological control agent that limits aflatoxin
contamination in U.S. crops belongs. Applied and Environmental Microbiology.
81(17): 5889-5899. https://doi.org/10.1128/AEM.00738-15
43. Horn, B. W.;
Greene, R. L.; Sobolev, V. S.; Dorner, J. W.; Powell, J. H.; Layton, R. C.
1996. Association of morphology and mycotoxin production with vegetative
compatibility groups in Aspergillus flavus, A. parasiticus, and A.
tamarii. Mycologia. 88(4): 574. https://doi.org/10.2307/3761151
44. Horn, B. W.;
Dorner, J. W. 1999. Regional differences in production of aflatoxin B1 and
cyclopiazonic acid by soil isolates of Aspergillus flavus along a
transect within the United States. Applied and Environmental Microbiology.
65(4): 1444-1449.
45. Hoyos Ossa,
D. E.; Hincapié, D. A.; Peñuela, G. A. 2015. Determination of aflatoxin M1 in
ice cream samples using immunoaffinity columns and ultra-high performance
liquid chromatography coupled to tandem mass spectrometry. Food Control. 56:
34-40. https://doi.org/10.1016/j.foodcont.2015.03.011
46. Jamali, M.;
Ebrahimi, M.; Karimipour, M.; Shams Ghahfarokhi, M.; Dinparas Djadid, N.;
Kalantari, S.; Pilehvar Soltanahmadi, Y.; Amani, A.; Razzaghi Abyaneh, M. 2012.
An insight into the distribution, genetic diversity, and mycotoxin production
of Aspergillus section Flavi in soils of pistachio orchards. Folia
Microbiologica. 57(1): 27-36. https://doi.org/10.1007/s12223-011-0090-5
47. Juvvadi, P.
R.; Chivukula, S. 2006. Putative calmodulin-binding domains in aflatoxin
biosynthesisregulatory proteins. Current Microbiology. 52(6): 493-496.
https://doi.org/10.1007/s00284-005-0389-z
48. Kebede, H.;
Abbas, H. K.; Fisher, D.; Bellaloui, N. 2012. Relationship between aflatoxin
contamination and physiological responses of corn plants under drought and heat
stress. Toxins. 4(11): 1385-1403. https://doi.org/10.3390/toxins4111385
49. Klich, M. A.
2002. Identification of common Aspergillus species. Centraalbureau voor
Shimmelcultures. Utrecht. The Netherlands. p.116.
50. Klich, M. A.
2007. Environmental and developmental factors influencing aflatoxin production
by Aspergillus flavus and Aspergillus parasiticus. Mycoscience.
48(2): 71-80. https://doi.org/10.1007/S10267-006-0336-2
51.
Klingelhöfer, D.; Zhu, Y.; Braun, M.; Bendels, M. H. K.; Brüggmann, D.;
Groneberg, D. A. 2018. Aflatoxin-Publication analysis of a global health
threat. Food Control. 89: 280-290.
https://doi.org/10.1016/J.FOODCONT.2018.02.017
52. Kocsubé, S.;
Perrone, G.; Magistà, D.; Houbraken, J.; Varga, J.; Szigeti, G.; Hubka, V.;
Hong, S. B.; Frisvad, J. C.; Samson, R. A. 2016. Aspergillus is
monophyletic: Evidence from multiple gene phylogenies and extrolites profiles.
Studies in Mycology. 85: 199-213. https://doi.org/10.1016/j.simyco.2016.11.006
53. Kumar, S.;
Stecher, G.; Tamura, K. 2016. MEGA7: Molecular Evolutionary Genetics Analysis
versión 7.0 for Bigger Datasets. Molecular Biology and Evolution. 33(7):
1870-1874. https://doi.org/10.1093/molbev/msw054
54. Larkin, M.
A.; Blackshields, G.; Brown, N. P.; Chenna, R.; Mcgettigan, P. A.; McWilliam,
H.; Valentin, F.; Wallace, I. M.; Wilm, A.; Lopez, R.; Thompson, J. D.; Gibson,
T. J.; Higgins, D. G. 2007. Clustal W and Clustal X version 2.0.
Bioinformatics. 23(21): 2947-2948. https://doi.org/10.1093/bioinformatics/btm404
55. Leslie, J.
F.; Summerell, B. 2006. The Fusarium laboratory manual. Blackwell
Publishing Professionall. 387 p.
56. Liu, D.;
Coloe, S.; Baird, R.; Pedersen, J. 2000. Rapid mini-preparation of fungal DNA
for PCR. Journal of Clinical Microbiology. 38(1): 471.
57. MAGyP -
Agroindustria. Ministerio de Agricultura Ganadería y Pesca. 2020.
Características de la región Parque Chaqueño.
http://forestoindustria.magyp.gob.ar/archivos/informacionpor-region/parque-chaqueno.pdf
58. MAGyP.
Ministerio de Agricultura Ganadería y Pesca. 2021. Estimaciones agrícolas.
http://datosestimaciones.magyp.gob.ar/reportes.php?reporte=Estimaciones
59. Martins, L.
M.; Sant’Ana, A. S.; Pelegrinelli Fungaro, M. H.; Silva, J. J.; Da Silva do
Nascimento, M.; Frisvad, J. C.; Taniwaki, M. H. 2017. The biodiversity of Aspergillus
section Flavi and aflatoxins in the Brazilian peanut production chain. Food
Research International. 94: 101-107.
https://doi.org/10.1016/j.foodres.2017.02.006
60. Moreno, J.
2004. Estudio comparativo de Aspergillus flavus y Aspergillus
parasiticus en la producción de aflatoxinas bajo diferentes condiciones de
humedad y temperatura. UNAM. Facultad de Estudios Superiores de Posgrado.
Cuautitlán Izcali, Estado de México.
61. O’Donnell,
K.; Nirenberg, H.; Aoki, T.; Cigelnik, E. 2000. A multigene phylogeny of the Gibberella
fujikuroi species complex: Detection of additional phylogenetically
distinct species. Mycoscience. 41(1): 61-78. https://doi.org/10.1007/BF02464387
62. Perrone, G.;
Gallo, A.; Logrieco, A. F. 2014a. Biodiversity of Aspergillus section
Flavi in Europe in relation to the management of aflatoxin risk. Frontiers in
Microbiology. 5(377): 1-5. https://doi.org/10.3389/fmicb.2014.00377
63. Perrone, G.;
Haidukowski, M.; Stea, G.; Epifani, F.; Bandyopadhyay, R.; Leslie, J. F.;
Logrieco, A. 2014b. Population structure and aflatoxin production by Aspergillus
Sect. Flavi from maize in Nigeria and Ghana. Food Microbiology. 41: 52-59.
https://doi.org/10.1016/j.fm.2013.12.005
64. Pildain, M.
B.; Cabral, D.; Vaamonde, G. 2005. Poblaciones de Aspergillus flavus en
maní cultivados en diferentes zonas agroecológicas de la Argentina,
caracterización morfológica y toxigénica. RIA. 34(3): 3-19.
65. Pitt, J.;
Hocking, A. 2009. Fungi and Food Spoilage. Springer US.
https://doi.org/10.1007/978-0-387-92207-2
66. Presello, D.
A.; Botta, G.; Iglesias, J. 2004. Podredumbres de espiga de maíz y micotoxinas
asociadas. Idia Xxi. 4(6): 152-157. http://www.biblioteca.org.ar/libros/210728.pdf
67. Presello, D.
A.; Oviedo, M.; Fernandez, M.; Iglesias, J.; Copia, P. 2016. Resistencia a
podredumbres. RTA. 10(32): 29-32.
68. Probst, C.;
Bandyopadhyay, R.; Cotty, P. J. 2014. Diversity of aflatoxin-producing fungi
and their impact on food safety in sub-Saharan Africa. International Journal of
Food Microbiology. 174: 113-122.
https://doi.org/10.1016/j.ijfoodmicro.2013.12.010
69. Reis, T. A.;
Baquião, A. C.; Atayde, D. D.; Grabarz, F.; Corrêa, B. 2014. Characterization
of Aspergillus section Flavi isolated from organic Brazil nuts using a
polyphasic approach. Food Microbiology. 42: 34-39.
https://doi.org/10.1016/j.fm.2014.02.012
70. Ren, Y.;
Jin, J.; Zheng, M.; Yang, Q.; Xing, F. 2020. Ethanol inhibits Aflatoxin B1
biosynthesis in Aspergillus flavus by up-regulating oxidative
stress-related genes. Frontiers in Microbiology. 10.
https://doi.org/10.3389/fmicb.2019.02946
71. Riba, A.;
Bouras, N.; Mokrane, S.; Mathieu, F.; Lebrihi, A.; Sabaou, N. 2010. Aspergillus
section Flavi and aflatoxins in Algerian wheat and derived products. Food
and Chemical Toxicology. 48(10): 2772-2777.
https://doi.org/10.1016/j.fct.2010.07.005
72. Rodrigues,
P.; Santos, C.; Venâncio, A.; Lima, N. 2011. Species identification of Aspergillus
section Flavi isolates from Portuguese almonds using phenotypic, including
MALDI-TOF ICMS, and molecular approaches. Journal of Applied Microbiology.
111(4): 877-892. https://doi.org/10.1111/j.1365-2672.2011.05116.x
73. Rosada, L.;
Sant’anna, J.; Franco, C.; Esquissato, G.; Santos, P.; Yajima, J.; Ferreira,
F.; Machinski, M.; Corrêa, B.; Castro-Prado, M. 2013. Identification of Aspergillus
flavus isolates as potential biocontrol agents of aflatoxin contamination
in crops. Journal of Food Protection. 76(6): 1051-1055. https://doi.org/10.4315/0362-028X.JFP-12-436
74. Saitou, N.;
Nei, M. 1987. The neighbor-joining method: a new method for reconstructing
phylogenetic trees. Molecular Biology and Evolution. 4(4): 406-425.
https://doi.org/10.1093/oxfordjournals.molbev.a040454
75. Singh, P.;
Cotty, P. J. 2019. Characterization of Aspergilli from dried red chilies
(Capsicum spp.): Insights into the etiology of aflatoxin contamination.
International Journal of Food Microbiology. 289: 145-153.
https://doi.org/10.1016/j.ijfoodmicro.2018.08.025
76. Tamura, K.;
Nei, M. 1993. Estimation of the number of nucleotide substitutions in the
control region of mitochondrial DNA in humans and chimpanzees. Molecular
Biology and Evolution. 10(3): 512-526.
https://doi.org/10.1093/oxfordjournals.molbev.a040023
77. Toso, R. E.;
Toribio, M. S.; Diesser, M.; Borello, A. B.; Ardoino, S. M. 2018. Affections in
animals and humans due to ingestion or exposure to aflatoxins. Preventive
measures to avoid toxic effects. Ciencia Veterinaria. 20(1): 51-67. https://doi.org/10.19137/cienvet-20182013
78. Uka, V.;
Moore, G. G.; Arroyo Manzanares, N.; Nebija, D.; De Saeger, S.; Di Mavungu, J.
D. 2017. Unravelling the diversity of the cyclopiazonic acid family of
mycotoxins in Aspergillus flavus by UHPLC triple-TOF HRMS. Toxins. 9(1):
1-21. https://doi.org/10.3390/toxins9010035
79. Ul Hassan,
Z.; Al Thani, R.; Atia, F. A.; Alsafran, M.; Migheli, Q.; Jaoua, S. 2021.
Application of yeasts and yeast derivatives for the biological control of
toxigenic fungi and their toxic metabolites. Environmental Technology &
Innovation. 22. 101447. https://doi.org/10.1016/j.eti.2021.101447
80. Vaamonde,
G.; Patriarca, A.; Fernández Pinto, V.; Comerio, R.; Degrossi, C. 2003.
Variability of aflatoxin and cyclopiazonic acid production by Aspergillus section
flavi from different substrates in Argentina. International Journal of Food
Microbiology. 88(1): 79-84. https://doi.org/10.1016/S0168-1605(03)00101-6
81. Vaamonde, G.
2008. Micotoxinas emergentes y re-emergentes: Ácido ciclopiazónico. XI Congreso
Argentino de Micología.
82. Wu, F.;
Stacy, S. L.; Kensler, T. W. 2013. Global risk assessment of aflatoxins in
maize and peanuts: are regulatory standards adequately protective?
Toxicological Sciences. 135(1): 251-259. https://doi.org/10.1093/toxsci/kft132