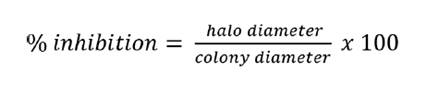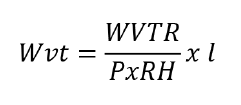Revista de la Facultad de Ciencias
Agrarias. Universidad Nacional de Cuyo. Tomo 56(2). ISSN (en línea) 1853-8665.
Año 2024.
Original article
Antibacterial
activity and physicochemical characterization of bioplastic films based on
cassava (Manihot esculenta Crantz) starch and rosemary (Salvia
rosmarinus) essential oil
Actividad
antibacteriana y caracterización fisicoquímica de láminas bioplásticas basadas
en almidón de yuca (Manihot esculenta Crantz) y aceite esencial de
romero (Salvia rosmarinus)
Luis Gabriel Poveda
Perdomo1,
Raul Alberto Cuervo
Mulet1,
Jessica Esparza
Estrada1,
Joaquin Hernández
Umaña1
1Universidad de San Buenaventura Cali. Facultad de Ingeniería.
Grupo de Investigación Biotecnología. Carrera 122 # 6-65. Santiago de Cali
76001. Colombia.
*dpnavia@usbcali.edu.co
Abstract
Bioplastics
composed of renewable sources and antimicrobial components are desirable in
food packaging. This study prepared bioplastic films with cassava starch and
rosemary essential oil using a casting methodology. Film antibacterial
activity, water vapour transmission (Wvt), mechanical resistance, and
microstructure were measured after exposure to pathogenic bacteria such as Salmonella
enterica, Escherichia coli, Staphylococcus aureus, and Bacillus cereus. Antibacterial
activity was evidenced against the pathogens evaluated except for B. cereus.
The films showed average values of Wvt 3.6988 (10-14 g/Pa s m), tensile
strength 8.90 MPa, young modulus 1679.72 MPa, and elongation at break 4.33%.
Film microstructure showed good adhesion to bioplastic components in the
matrix. Bioplastics of cassava starch and rosemary oil constitute potential
food packaging solutions mainly for fruits, egg-based products or chicken.
Keywords: polymers, packaging,
bacteria, water vapour
Resumen
Los bioplásticos
elaborados a partir de fuentes renovables y componentes antimicrobianos son
deseables en el empacado de alimentos. Por tanto, se prepararon láminas
bioplásticas con almidón de yuca y aceite esencial de romero usando el método
de vaciado en placa. Se midió la actividad antibacteriana, transmisión de vapor
de agua (Tva), resistencia mecánica y microestructura de láminas
bioplásticas. Las láminas fueron expuestas a bacterias patógenas como Salmonella
enterica, Escherichia coli, Staphylococcus aureus y Bacillus cereus. Se
evidenció actividad antibacteriana para los patógenos evaluados excepto para B.
cereus. Las láminas evidenciaron valores promedio de Tva 3,6988 (10-14
g/Pa s m), esfuerzo a tensión 8,90 MPa, módulo de young 1679,72
MPa y deformación a la rotura 4,33%. Su microestructura evidenció buena
adhesión entre los componentes de la matriz bioplástica. Estos resultados
muestran el potencial de los bioplásticos de almidón de yuca y aceite esencial
de romero para el empacado de alimentos, principalmente de frutas o productos
elaborados con huevo o pollo.
Palabras clave: polímeros, empaques,
bacterias, vapor de agua
Originales: Recepción: 27/07/2023 - Aceptación: 13/06/2024
Introduction
The production of
bioplastics from renewable sources is a field of research, development, and
innovation of great interest worldwide (58).
Bioplastics have increased from 2.4 million tons in 2021 to 7.5 million tons in
2023 (21). Applications include the packaging
industry, agriculture/horticulture, consumer electronics, automobile, consumer
goods, and household appliances. Package manufacturing, where rigid and
flexible materials are required, is the most representative market segment (12, 23).
Bioplastics can be
totally or partially obtained from natural sources (32).
Fossil raw materials are generally not biodegradable. However, exceptions such
as polycaprolactone can be used to make bioplastics. Polysaccharides, proteins,
and fatty acids are renewable raw materials commonly used to manufacture
bioplastics. Cellulose, starch, pectin, alginate, soy, wheat gluten, and
gelatin are used alone or mixed with fossil polymers such as polyethylene or
polypropylene (15, 46). Starch is a
polysaccharide frequently used in bioplastics due to availability, costs, and
biodegradable and renewable characteristics (58).
Among bioplastics, starch-based bioplastics are the most widely traded (21). However, some disadvantages, mainly related
to polarity, limit some applications (36, 58).
Bioplastic food packaging must overcome the “polarity challenge” that implies
high deterioration risks (48).
Active compounds
increase biopolymers functionality for active food packaging (25). Food packaging with antimicrobial components
has a positive impact on shelf life of packaged products (43, 46). These components are generally
compatible with the natural raw materials used to produce bioplastic. Many
studies have incorporated essential oils and plant extracts in polymer matrices
to obtain bioplastics (10, 22, 25, 29, 42, 52, 57).
Nevertheless, very few studies measure antimicrobial effects of rosemary oil
incorporated in bioplastic films on more than three strains of bacteria or
fungi. In fact, the composition of the essential oil may vary according to the
place of origin affecting both bioplastic antimicrobial and physicochemical
properties. To the best of our knowledge, no study has simultaneously evaluated
the influence against Gram-negative bacteria (E. coli and Salmonella
sp.) and, Gram-positive bacteria (S. aureus and B. cereus).
Bioplastic mechanical properties, stability against moisture and
antimicrobial characteristics determine their applications. This study aimed to
determine the antibacterial activity against E. coli, S. enterica,
S. aureus, and B. cereus, physical-chemical and mechanical
properties of a bioplastic film made with cassava starch and rosemary essential
oil.
Materials
and methods
Materials
Cassava starch (Manihot
esculenta Crantz) was purchased from Tecnas S. A. (Cali, Colombia).
Rosemary (Salvia rosmarinus) oil was purchased at the local market
(Cali, Colombia). Food-grade glycerol was purchased from Merck (Burlington, MA,
USA). All chemicals were reagent grade and purchased from Merck (Burlington,
MA, USA). The American Type Culture Collection (ATCC) of the Universidad de San
Buenaventura Cali (Colombia) provided bacteria. Two Gram-positive bacteria, Staphylococcus
aureus ATCC 25923, Bacillus cereus ATCC 15579 and two Gram-negative
bacteria, Salmonella enterica ATCC 13314 and Escherichia coli ATCC
10798, were evaluated. This study was conducted at the University of San
Buenaventura Cali, in Cali, Colombia.
Rosemary
essential oil extraction
Rosemary leaves
were placed in distilled water (mass/volume ratio 1/12). The essential oil was
extracted in a hydrodistillation system for 4 hours at 100°C and stored
refrigerated.
Film
preparation
Cassava starch (CS)
films were produced by the casting method (47)
from forming suspensions (FSs). The FSs were prepared by dissolving 3 g of CS,
120 mg of rosemary essential oil, 83 mg of tween-80, and 0.75 g of glycerol in
100 mL of distilled water with heating (75 ± 5°C) and magnetic stirring. The
FSs were dehydrated by convective drying at 40°C until obtaining films with 10%
humidity, optimized formulation from a previous study (39) with a central composite design. The
optimized formulation was validated with error values ranging from - 3.31 to
10.61%.
Antibacterial
properties
Film antimicrobial
activity was evaluated against Gram-negative bacteria (E. coli and
S. enterica) and Gram-positive bacteria (S. aureus and B.
cereus) using the disc diffusion method (54).
Mueller-Hinton agar
(Sigma-Aldrich) was used to inoculate the bacteria. Then, a foil disc was
placed in the center of the Petri dishes, and incubated at 37 ± 2°C for 24
hours. A calibrator (Mitutoyo, Japan) was used to measure the halo around the
disc, determining inhibition percentage with Equation 1:
Five repetitions
were made for each bacteria. Chloramphenicol (Colmed, International) was used
as a positive control at 100 ppn (parts-per notation).
Statistical
Analysis
ANOVA and Fisher’s
LSD determined significant differences among treatments. Minitab 19 software
was used to analyze variance with a significance level of 5%.
Rosemary
essential oil
A Gas Chromatograph (AT 6890 Series Plus, Agilent Technologies,
Palo Alto, California, USA) coupled to a mass selective detector (Agilent
Technologies, MSD 5975 Inert XL) determined the chemical composition of
rosemary essential oil operated in the full radio frequency sweep. The column
was DB-5MS (J & M Scientific, Folsom, CA, and USA) [5% -phenyl-poly
(dimethylsiloxane), 60mm x 0.25mm x 0.25μm]. Injection was done in Split mode
(30:1) with a volume of 2μL.
Water
vapour transmission
The water vapour transmission
Wvt was measured gravimetrically following the ASTM E96-05 standard
methodology (16). We used glass
permeation cells filled with silica gel (0% RH). Films with a diameter of 80 mm
were bonded with liquid silicone in the circular mouth of each cell. Cells were
stored in airtight containers with a saturated sodium chloride solution (73 ± 2%
RH) at 25°C. Weight variation in the permeation cell was plotted against time.
Slopes were calculated by linear regression. The Wvt (g/Pa s m) was
calculated by equation 2:
where:
WVTR = water vapour
transmission rate, calculated as the ratio between the slope of the straight
line (g/s) and the permeation cell area (m2)
P = saturation vapour
pressure of water (Pa)
RH = relative humidity
in the airtight container
l = mean film
thickness (m). Analyses were conducted in triplicate.
Mechanical
properties
A texturometer
(EZ-Test L, Shimadzu, Japan) equipped with Trapezium X software conducted the
test following the ASTM D882-10 standard (55).
The films were cut in a rectangular shape of 20 mm wide and 100 mm long and
stored for a week at 50% RH. The initial gauge was 65 mm long and test speed
was 50 mm/min, using a load cell of 500 N. Tensile strength Ts, young
modulus Ym, and elongation at break Eb were measured. Tests were
performed ten times and the average was reported.
Scanning
electron microscopy
Film surface
morphology was analyzed at 20Kv scanning electron microscopy (SEM) (Jeol
JSM-6490LV, USA) with backscattered electrons obtaining surface and
cross-section images. Samples 5 mm wide and 5 mm long were coated with gold in
a vacuum chamber (Denton Vacuum, Desk IV, USA). Images were captured at 500 and
2000 increases.
Results
and discussion
Antibacterial
properties
Inhibition
percentages shown in table 1 indicate bioplastic films showed
higher inhibition against E. coli, S. aureus than against S. enterica.
Table 1. Antibacterial
inhibition percentages of bioplastic films.
Tabla
1. Porcentaje de inihibición antibacteriana de las
láminas bioplásticas.
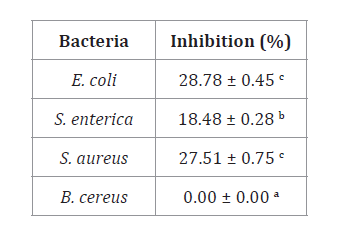
Different
letters in the same column indicate significant differences (p<0.05).
Letras
diferentes en la misma columna indican diferencias significativas (p<0,05).
The antibacterial activity of rosemary essential oil depends on
ketones and monoterpene hydrocarbons that affect cell membrane permeability (5). This oil has proven antibacterial effects
against E. coli (5, 18, 27, 30, 31),
Salmonella (2, 33), and S.
aureus strains (5, 6, 18, 27).
As shown in table 1, B. Cereus was not inhibited, probably given to
bacterial rapid mutation and adaptation to different media, reaching quick
resistance against antimicrobial agents (14, 53).
Unlike E. Coli, S. enterica, and S. aureus, B. cereus is a
sporulated bacterium, a mechanism that reinforces cell wall protection via
environmental isolation and prevention of inhibitory interactions (40). It also generates highly resistant biofilms
hindering its elimination (19).
Table
2 shows how rosemary oil incorporated in the film is mainly composed of β-Mircene
(27.8 g/100 g), Camphor (23.9 g/100 g), and 1.8-Cineol (16.2 g/100 g).
Table 2. Rosemary
oil composition.
Tabla 2. Composición
del aceite de romero.
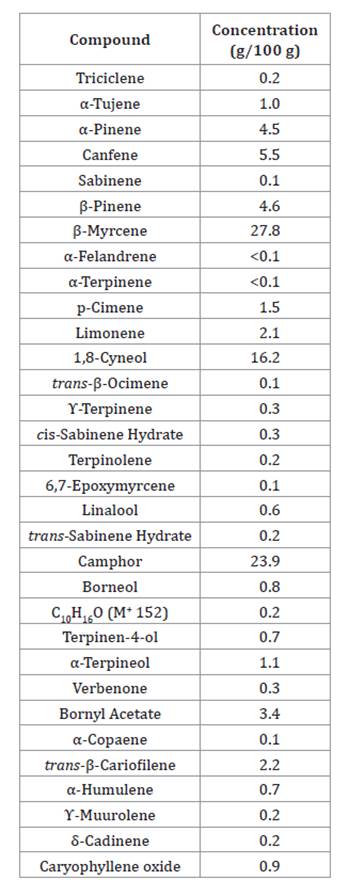
β-Mircene is an
antibacterial monoterpene against S. aureus, E. coli, Pseudomonas
aeruginosa, and Proteus vulgaris (3).
Camphor is a terpenoid that affects lipoproteins and lipopolysaccharides
present in bacteria cell walls, particularly gram-negative ones, generating
lysis and subsequent cell death (6, 59).
The third main component, 1,8-Cineol (9),
is an oxygenated monoterpene (26, 35)
widely used as inhibitory agent of food pathogens (9,
37). Even though many compounds have antimicrobial capacity (3), microorganisms develop defence and resistance
mechanisms such as biofilms, a conglomeration of different cells allowing group
protection from external factors (38).
However, 1.8-Cineol inhibits biofilm formation in S. aureus through
inhibitory agents affecting cell wall (34).
Water
vapour transmission
Table 3 shows an experimental average Wvt of
3.6988 (10-14 g/Pa s m), lower than for
other studies under similar manufacturing conditions. Considering that minimum Wvt
values allow low vapour exchange between the food and the surrounding
atmosphere, bioplastics for the food packaging industry should have low Wvt values
for a longer shelf life (17).
Table 3. Physiochemical
and mechanical characteristics of bioplastic films.
Tabla 3. Caracterización
fisicoquímica y mecánica de las láminas bioplásticas.
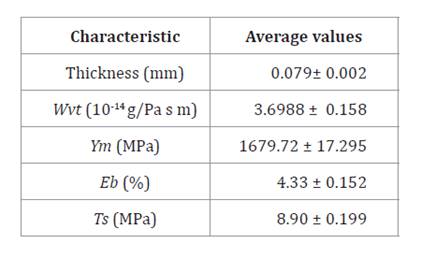
Wvt values of 3.11 to
8.72 (10-11 g Pa s m) were reported
in anchovy (Coccinia abyssinica) starch films with cellulose
nanocrystals and rosemary essential oil (27);
from 2.95 to 2.7 (10-10 g/Pa s m) in films of
polyvinyl alcohol, corn starch and cardanol oil (56);
from 5.8 to 11 (10-10 g/Pa s m) in cassava
starch films with rosemary extract (47);
4.16 to 5.27 (10-11 g/Pa s m) in modified
cassava starch films (13); 5.8 to 12.5
(10-10 g/Pa s m) in cassava
starch films with rosemary nanoparticles (20)
and 3.9 to 8.2 (10-11 g/Pa s m) in
biodegradable films of cassava starch with nanoclays (50). In the food industry, cellophane polymer
derived from cellulose is used as wrapping film in the confectionery industry
with a Wvt of 8.44 (10-11 g/Pa s m) (20). Considering conventional films, our
bioplastic obtained good values.
The Wvt values
obtained are related to film composition. The starch/tween 80 ratio constitutes
a relevant factor since when its concentration allows for a continuous network,
this polysorbate acts as water vapour transmission barrier (7). The network keeps the surfactant molecules
dispersed, promoting a balance between the hydrophobic and hydrophilic phases
and reducing Wvt. An excessive concentration of tween 80 will enhance
the plasticizer effect, increasing the free volume inside the bioplastic
structure and increasing Wvt (8).
In addition, when starch and glycerol proportions increase, Wvt values
may as well increase. Both starch and glycerol behave as polar components
stimulating OH bonds with water molecules. Instead, the interaction between
starch and rosemary oil limits the amount of water absorbed by the film (27) with covalent bonds that reduce OH groups and
consequently decrease Wvt (49).
The equilibrium among bioplastic components promoted low water values for food
packaging.
Mechanical
properties
Mechanical
properties define bioplastic usage in food packaging. Tensile strengths and
Young’s modulus relate to mechanical tensile strength, while elongation at
break defines ductility.
Table 3 shows an average tensile strength of
8.9 MPa, and Young’s modulus of 1679.72 MPa, both higher than those reported in
similar studies. Biofilms made from anchovy starch (Coccinia abyssinica)
with cellulose nanocrystals and rosemary essential oil evidenced Ts values of
9.42 to 23.44 MPa (27). Bioplastic films
made of modified starch with soybean oil oligomers reported Ts values of
3.35 MPa (58), while other ones made from
cassava starch showed Ts values from 0.1 to 1.07 MPa and Ym values from
0.07 to 0.50 MPa (11). Films with
essential oils had Ts values from 3 to 14 MPa (20), and bioplastic films of cassava starch with
cinnamon essential oil showed Ts values ranging from 1.05 to 3.75 MPa (51). Plantain starch films had Ts values from 2.4
to 12.4 MPa and Ym values from 55.6 to 1482.2 MPa (41).
Bioplastic
components define final mechanical properties while their concentration affects
moisture gain. Starch is the major film component affecting mechanical
resistance, forming hydrogen bridges with water and promoting adsorption. Water
acts as a plasticizer agent, increasing mobility of polymer structure and,
thus, decreasing mechanical resistance. On the other hand, the oil-starch bonds
promote structural stiffness and increase polymer mechanical strength (41). However, excessive apolar components could
reduce cohesion of starch binding forces and consequently, mechanical strength (24, 27).
Table
3, shows average elongation at break (Eb) of 4.33%. In other
studies, Eb values were higher, indicating low flexibility of our films.
Biofilms made from anchovy (Coccinia abyssinica) starch with cellulose
nanocrystals and rosemary essential oil reported Eb values between 27.71
and 73.91% (27). Others made of starch
with soybean oil showed Eb of 58.32% (58);
while films made of Dioscorea hispida Dennst starch and natural antimicrobial
agents from turmeric extract showed Eb of 30.24% (28). Films made of cassava starch with cinnamon
essential oil had Eb values between 128 and 264%; others made of corn
starch with essential oils had Eb values from 30 to 170% (20). In bioplastic films made of cassava starch
with cinnamon, cloves, and oregano essential oils, Eb values ranged
between 8 and 17% (1), while films of
rice starch with oregano essential oil, showed Eb values between 83.5%
and 108.8% (45).
Bioplastic low
flexibility is related to intra-structure free volume. Molecular movement of
the polymer is directly proportional to intern free volume. Based on the above,
we state that molecular adhesion in the assessed bioplastic matrix was high,
and films had low free volume. Components promoting molecular mobility are
glycerol, behaving as a plasticizer, and tween 80, a surfactant. Surfactants
increase free volume into adjacent starch chains generating a flexible
structure (44).
Scanning
electron microscopy
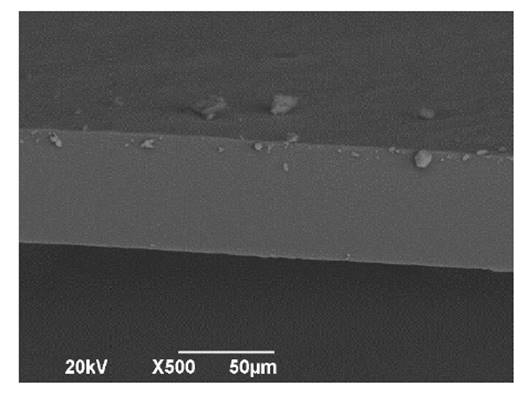
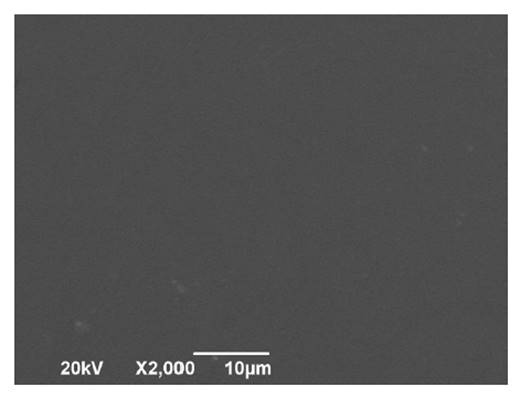
Figure 1, and figure 2, show
flm cross-section and surface micrographs obtained by scanning electron
microscopy.
Figure
1. Scanning electron microscopic image of a
cross-section of bioplastic film.
Figura 1. Imagen
de microscopía electrónica de barrido de la sección transversal de la lámina
bioplástica.
Figure
2. Scanning electron microscopic image of the surface
of bioplastic film.
Figura 2. Imagen
de microscopía electrónica de barrido de la superficie de la lámina
bioplástica.
A smooth and homogeneous surface on both sides of the film
indicate mixing and forming processes that allow whole matrix integration and
adhesion. This indicates good bioplastic functionality for food packaging. The
appropriate linkage of matrix components directly affects mechanical strength
and stability against moisture. In the first case, a more compact structure
could have high resistance and lower deformation or breakage capacity.
Researchers stated that the finely distributed structure shown in the cross-section
of corn starch films through SEM justified a better physical-mechanical
behaviour and even better antibacterial response than other films without this
characteristic (20).
Structural
integrity leads to good mechanical properties such as tensile strength and
deformation, given by a high intermolecular interaction, components
entanglement, and a continuous phase in the polymer matrix (45). Thus, interfacial interactions between
mixture components and the essential oil are improved. On the other hand, when
intermolecular linkage is high, the film has less porosity and empty spaces.
Thus, bioplastics could have better stability against moisture.
In addition, no oil droplets were observed on film surface. In
this regard, oil droplets may cause discontinuity, resulting in a cracked
structure (4).
Conclusions
Bioplastic films based on cassava starch showed antimicrobial
activity against S. enterica, E. coli, and S. aureus, low permeability
to water vapour, good mechanical resistance, and high homogeneity in the
surface and internal structure, indicating appropriate component linkage. These
bioplastics constitute alternatives for packaging of susceptible foods. In this
regard, these films could be used in packaging of fresh fruits or dairy
products such as cheeses, where a high vapour barrier is required.
Acknowledgments
The authors thank Universidad de San Buenaventura Cali
(Colombia).
1. Acosta, S.;
Chiralt, A.; Santamarina, P.; Rosello, J. 2016. Food Hydrocolloids Antifungal
films based on starch-gelatin blend, containing essential oils. 61.
2. Alibi, S.;
Selma, W.; Mansour, H.; Navas, J. 2022. Activity of essential oils against
multidrug-resistant Salmonella enteritidis. Curr Microbiol. 79(9).
3. AL-Jabri, N.;
Hossain, M. 2018. Chemical composition and antimicrobial potency of locally
grown lemon essential oil against selected bacterial strains. J King Saud Univ
Sci. 30(1): 14-20.
4. Atarés, L.;
Pérez-Masiá, R.; Chiralt, A. 2011. The role of some antioxidants in the HPMC
film properties and lipid protection in coated toasted almonds. J Food Eng.
104(4): 649-656.
5. Bajalan, I.;
Rouzbahani, R.; Pirbalouti, A.; Maggi, F. 2017. Antioxidant and antibacterial
activities of the essential oils obtained from seven Iranian populations of Rosmarinus
officinalis. Ind Crops Prod. (107): 305-311.
6. Bezerra, K.;
Iukava, L.; Ono, J.; de Souza, S.; dos Santos, I.; Barbosa, L. 2022. Resistance
profile and biofilm production capacity of Staphylococcus spp. beef slaughterhouse isolates and their sensitivity to Rosmarinus
officinalis essential oil. Vet Res Commun. (47): 911-919.
7. Brandelero, R.; Yamashita, F.; Grossmann, M. 2010. The effect
of surfactant Tween 80 on the hydrophilicity, water vapour permeation, and the
mechanical properties of cassava starch and poly (butylene
adipate-co-terephthalate) (PBAT) blend films. Carbohydr Polym. 82(4):
1102-1109.
8. Brandelero, R.;
Grossmann, M.; Yamashita, F. 2011. Effect of the method of production of the
blends on mechanical and structural properties of biodegradable starch films
produced by blown extrusion. Carbohydr Polym. 86(3): 1344-1350.
9. Brożyna, M.;
Paleczny, J.; Kozłowska, W.; Ciecholewska-Juśko, D.; Parfieńczyk, A.;
Chodaczek, G.; Junka, A. 2022. Chemical composition and antibacterial activity
of liquid and volatile phase of essential oils against planktonic and
biofilm-forming cells of Pseudomonas aeruginosa. Molecules. 27(13).
10. Chiralt, A.;
Atar, L. 2016. Trends in food science & technology essential oils as
additives in biodegradable films and coatings for active food packaging. (48).
11. Chiumarelli,
M.; Hubinger, M. 2014. Evaluation of edible films and coatings formulated with
cassava starch, glycerol, carnauba wax and stearic acid. Food Hydrocoll. 38:
20-27.
12. Chowdhury, M.
A.; Nayem, H.; Badrudduza, M. D.; Rana, M. M. 2023. Development and
characterization of natural sourced bioplastic for food packaging applications.
Heliyon. 9(2).
13. Colivet, J.;
Carvalho, R. 2017. Hydrophilicity and physicochemical properties of chemically
modified cassava starch films. Ind Crops Prod. (95): 599-607.
14. Diao, M.; Qi,
D.; Xu, M.; Lu, Z.; Lv, F.; Bie, X.; Zhang, C.; Zhao, H. 2018. Antibacterial
activity and mechanism of monolauroyl-galactosylglycerol against Bacillus
cereus. Food Control. (85): 339-344.
15. Emadian, S. M.;
Onay, T.; Demirel, B. 2017. Biodegradation of bioplastics in natural
environments. Waste Management. (36): 59: 526.
16. Factors, C.;
Europeias, D. A. S. C.; Comissão Europeia, A. S. T. M. 2002. Standard test
methods for water vapour transmission of materials 1. Astm. 14: 1-10.
17. Fakhreddin, S.;
Rezaei, M.; Zandi, M.; Ghavi, F. 2013. Preparation and functional properties of
fish gelatin-chitosan blend edible films. Food Chem. 136(3-4): 1490-1495.
18. Fawal, G.;
Omer, A.; Tamer, T. 2019. Evaluation of antimicrobial and antioxidant
activities for cellulose acetate films incorporated with Rosemary and Aloe Vera
essential oils. J Food Sci Technol. 56(3): 1510-1518.
19. Fink, R.; Oder,
M.; Stražar, E.; Filip, S. 2017. Efficacy of cleaning methods for the removal
of Bacillus cereus biofilm from polyurethane conveyor belts in bakeries.
Food Control. 80: 267-272.
20. Ghasemlou, M.;
Aliheidari, N.; Fahmi, R.; Shojaee-aliabadi, S. 2013. Physical, mechanical and
barrier properties of corn starch films incorporated with plant essential oils.
Carbohydr Polym. 98(1): 1117-1126.
21. Grossule, V.;
Zanatta, S.; Modesti, M.; Lavagnolo, M. C. 2023. Treatment of food waste
contaminated by bioplastics using BSF larvae: Impact and fate of starch-based
bioplastic films. J Environ Manage. 15(330).
22. Guzman-Puyol,
S.; Hierrezuelo, J.; Benítez, J.; Tedeschi, G.; Porras-Vázquez, J.; Heredia,
A.; Athanassiou, A.; Romero, D.; Heredia-Guerrero, J. A. 2022. Transparent,
UV-blocking, and high barrier cellulose-based bioplastics with naringin as
active food packaging materials. Int J Biol Macromol. (94): 209-1985.
23. Herbes, C.;
Beuthner, C.; Ramme, I. 2018. Consumer attitudes towards biobased packaging - A
cross-cultural comparative study. J Clean Prod. (18): 194-203.
24. Jiménez, A.;
Fabra, M.; Talens, P.; Chiralt, A. 2013. Phase transitions in starch based
films containing fatty acids. Effect on water sorption and mechanical behavior.
Food Hydrocoll. 30(1): 408-418.
25. Kaewpetch, T.;
Pratummang, A.; Suwarak, S.; Wongphan, P.; Promhuad, K.; Leelaphiwat, P.;
Bumbudsanpharoke, N.; Lorenzo, J.; Harnkarnsujarit, N. 2023. Ylang-ylang (Cananga
odorata) essential oils with flora odorants enhanced active function of
biodegradable polyester films produced by extrusion. Food Biosci. 1: 51.
26. Karaca, N.;
Demirci, B.; Gavahian, M.; Demirci, F. 2023. Enhanced bioactivity of rosemary,
sage, lavender, and chamomile essential oils by fractionation, combination, and
emulsification. ACS Omega. 8: 10941-10953.
27. Kassa, H.; Jabasingh,
S.; Mohammed, S.; Park, S.; Baek, S. 2023. Mechanical, barrier, and
antimicrobial properties of anchote (Coccinia abyssinica) starch films
containing cellulose nanocrystals and rosemary essential oil. Biomass Convers
Biorefin. (13): 7333-7347.
28. Khairani, D.;
Marlina, S.; Helwati, H. 2019. Effect of natural ingredients addition as
antimicrobial agents in Dioscorea hispida Dennst starch-based biofilm.
In: IOP Conference Series: Earth and Environmental Science. Institute of
Physics Publishing. 364. doi:10.1088/17551315
/364/1/012006
29. Klinmalai, P.;
Srisa, A.; Laorenza, Y.; Katekhong, W.; Harnkarnsujarit, N. 2021. Antifungal
and plasticization effects of carvacrol in biodegradable poly (lactic acid) and
poly (butylene adipate terephthalate) blend films for bakery packaging. LWT.
152.
30. Lagha, R.;
Abdallah, B. A. L.; Sarhan, B. O.; Al-Sodany, Y. 2019. Antibacterial and
biofilm inhibitory activity of medicinal plant essential oils against Escherichia
coli isolated from UTI patients. Molecules. 24(6).
31. Lakehal, S.; Chaouia, C.; Benrebiha, F. 2018. Antibacterial
and antioxidant activities of rosemary (Rosmarinus officinalis L.)
essential oil growing in djelfa (algeria). In: Advances in Science, Technology
and Innovation. Springer Nature. 1253-4.
32. Li, H.; Zhou,
M.; Mohammed, A.; Chen, L.; Zhou, C. 2022. From fruit and vegetable waste to
degradable bioplastic films and advanced materials: A review. Sustainable
Chemistry and Pharmacy. Elsevier B.V. (30)
33. Lira, M.;
Rodrigues, J.; Almeida, E.; Ritter, A.; Tondo, E.; Torres, S.; Schaffner, D.;
de Souza, E.; Magnani, M. 2020. Efficacy of oregano and rosemary essential oils
to affect morphology and membrane functions of noncultivable sessile cells of Salmonella
enteritidis 86 in biofilms formed on stainless steel. Journal of Applied
Microbiology. 128: 376-386. https:// doi.org/10.1111/jam.14423
34. Merghni, A.;
Noumi, E.; Hadded, O.; Dridi, N.; Panwar, H.; Ceylan, O.; Mastouri, M.;
Snoussi, M. 2018. Assessment of the antibiofilm and antiquorum sensing
activities of Eucalyptus globulus essential oil and its main component
1,8-cineole against methicillin-resistant Staphylococcus aureus strains.
Microb Pathog. 118: 74-80.
35. Mezza, G.;
Borgarello, A.; Grosso, N.; Fernandez, H.; Pramparo, M.; Gayol, M. 2018.
Antioxidant activity of rosemary essential oil fractions obtained by molecular
distillation and their effect on oxidative stability of sunflower oil. Food
Chem. (242): 9-15.
36. Mlalila, N.;
Hilonga, A.; Swai, H.; Devlieghere, F.; Ragaert, P. 2018. Antimicrobial
packaging based on starch, poly (3-hydroxybutyrate) and poly
(lactic-co-glycolide) materials and application challenges. Trends Food Sci
Technol. 74: 1-11.
37. Mojtahed, R.;
Niakousari, M.; Hashemi, H.; Saharkhiz, M.; Mousavi, A. 2018. Study of
two-stage ohmic hydro-extraction of essential oil from Artemisia aucheri Boiss:
Antioxidant and antimicrobial characteristics. Food Research International.
107(9): 462.
38. Monot, M.;
Eckert, C.; Hoys, S.; Collignon, A.; Janoir, C.; Candela, T. 2018. Anaerobe
Clostridium difficile forms variable bio films on abiotic surface. 4-7.
39. Navia, D.;
Gordillo, M.; Hernández, J.; Poveda L. 2019. Optimization of physical, optical
and barrier properties of films made from cassava starch and rosemary oil. J
Polym Environ. 27(1): 127-140.
40. Park, B.;
Chelliah, R.; Wei, S.; Park, J.; Forghani, F.; Park, Y.; Cho, M.; Park, D.; Oh,
D. 2018. Unique biomarkers as a potential predictive tool for differentiation
of Bacillus cereus group based on real-time PCR. Microb Pathog. 115:
131-137.
41. Pelissari, F.;
Andrade-mahecha, M.; José, P.; Cecilia, F. 2013. Optimization of process
conditions for the production of films based on the flour from plantain bananas
(Musa paradisiaca). LWT-Food Science and Technology. 52(1): 1-11.
42. Pérez-Recalde,
M.; Ruiz, I.; Hermida, É. 2018. Could essential oils enhance biopolymers
performance for wound healing? A systematic review. Phytomedicine. 38: 57-65.
43. Priya, B.;
Gupta, V.; Pathania, D.; Singha, A. 2014. Synthesis, characterization and
antibacterial activity of biodegradable starch/PVA composite films reinforced
with cellulosic fibre. Carbohydr Polym. (9): 109-171.
44. Rodríguez, M.;
Osés, J.; Ziani, K.; Maté, J. 2006. Combined effect of plasticizers and
surfactants on the physical properties of starch based edible films. 39:
840-846.
45. Romani, V.;
Prentice-Hernández, C.; Martins, V. 2017. Active and sustainable materials from
rice starch, fish protein and oregano essential oil for food packaging. Ind
Crops Prod. 97: 268-274.
46. Sani, I.;
Masoudpour-Behabadi, M.; Sani, M.; Motalebinejad, H.; Juma, A.; Asdagh, A.;
Eghbaljoo, H.; Khodaei, S.; Rhim, J.; Mohammadi, F. 2023. Value-added
utilization of fruit and vegetable processing by-products for the manufacture
of biodegradable food packaging films. Food Chemistry. Elsevier. 405.
47. Seligra, P.;
Medina, C.; Famá, L.; Goyanes, S. 2016. Biodegradable and non-retrogradable
eco-films based on starch-glycerol with citric acid as crosslinking agent.
Carbohydr Polym. 74: 138-166.
48. Shariatinia,
Z.; Fazli, M. 2015. Mechanical properties and antibacterial activities of novel
nanobiocomposite films of chitosan and starch. Food Hydrocoll. (46): 112-124.
49. Shen, X.; Wu,
J.; Chen, Y.; Zhao, G. 2010. Antimicrobial and physical properties of sweet
potato starch films incorporated with potassium sorbate or chitosan. Food
Hydrocoll. 24(4): 285-290.
50. Souza, A.;
Benze, R.; Ferrão, E.; Ditchfield, C.; Coelho, A.; Tadini, C. 2012. Cassava
starch biodegradable films: Influence of glycerol and clay nanoparticles
content on tensile and barrier properties and glass transition temperature. LWT
- Food Science and Technology. 46(1): 110-117.
51. Souza, A.;
Goto, G.; Mainardi, J.; Coelho, A.; Tadini, C. 2013. Cassava starch composite
films incorporated with cinnamon essential oil: Antimicrobial activity,
microstructure, mechanical and barrier properties. LWT - Food Science and
Technology. 54(2): 346-352.
52. Souza, V. G.
L.; Pires, J.; Rodrigues, P.; Lopes, A.; Fernandes, F.; Duarte, M.; Coelhoso,
I.; Fernando, A. 2018. Bionanocomposites of chitosan/montmorillonite
incorporated with Rosmarinus officinalis essential oil: Development and
physical characterization. Food Package Shelf Life. 16: 148-156.
53. Torkar, K.; Bedenić, B. 2018. Antimicrobial susceptibility
and characterization of metallo-β- lactamases, extended-spectrum β-lactamases
and carbapenemases of Bacillus cereus isolates. Microb Pathog. 118: 140-145.
54. Vimala, K.;
Yallapu, M.; Varaprasad, K.; Reddy, N.; Ravindra, S.; Naidu, N.; Raju, M. 2011.
Fabrication of curcumin encapsulated chitosan-PVA silver nanocomposite films
for improved antimicrobial activity. J Biomater Nanobiotechnol. 01: 55-64.
55. Weighing, S.
2002. Separation C rate of grip. Standard test method for tensile properties of
thin plastic sheeting 1. 1: 1-10.
56. Yahia, R.;
Owda, M.; Abou-Zeid, R.; Abdelhai, F.; El-Gamil, H.; Abdo, A.; Ali, A. 2023.
Biodegradable, UV absorber and thermal stable bioplastic films from waxy corn
starch/polyvinyl alcohol blends. Biomass Convers Biorefin.
https://doi.org/10.1007/s13399-022-03683-8
57. Yahyaoui, M.;
Gordobil, O.; Herrera, R.; Abderrabba, M.; Labidi J. 2016. Development of novel
antimicrobial films based on poly (lactic acid) and essential oils. REACT. 109:
1-8.
58. Yang, J.;
Ching, Y. C.; Ching, K. Y.; Ran, X.; Al-Hada, N. M.; Sui, X. 2023. Preparation
and characterization of starch-based bioplastic films modified by citric
acid-epoxidized soybean oil oligomers. J Polym Environ. 31(3): 954-964.
59. Zhou, H.; Ren,
J.; Li, Z. 2017. Antibacterial activity and mechanism of pinoresinol from Cinnamomum
camphora leaves against food-related bacteria. Food Control. (9): 79-192.