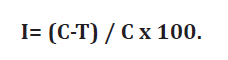Revista de la Facultad de Ciencias
Agrarias. Universidad Nacional de Cuyo. Tomo 56(2). ISSN (en línea) 1853-8665.
Año 2024.
Original article
Selection
of fungal isolates from Buenos Aires, Argentina, as biological control agents
of Botrytis cinerea and Sclerotinia sclerotiorum
Selección
de aislados fúngicos de Buenos Aires, Argentina, como agentes de control
biológico de Botrytis cinerea y Sclerotinia sclerotiorum
Ricardo Arturo
Varela Pardo1*,
Claudia Cristina
López Lastra2,
Romina Guadalupe
Manfrino2,
Darío Balcazar2,
Cecilia Mónaco3,
Eduardo Roberto
Wright1
1Universidad de Buenos Aires. Facultad de Agronomía. Cátedra de
Fitopatología. Av. San Martín 4453. Ciudad Autónoma de Buenos Aires. Argentina.
C1417DSE.
2Centro de Estudios Parasitológicos y de Vectores (CEPAVE)
CONICET-UNLP. Blvd. 120. La Plata. Provincia de Buenos Aires. Argentina. 1900.
3Universidad Nacional de La Plata. Facultad de Ciencias Agrarias
y Forestales. Curso de Fitopatología. Calle 60 y 119. La Plata. Argentina.
1900.
*varelapardo@gmail.com
Abstract
This work aimed to
select promising microorganisms as biological control agents (BCA). Forty-one
soil samples were obtained from florihorticultural farms located in Buenos
Aires, Argentina. Insect trap techniques and soil serial dilutions were used to
obtain isolates of entomopathogenic fungi and fungi of genera Trichoderma, respectively.
A total of 20 isolates included five Metarhizium and 15 Trichoderma.
The isolates were lyophilized and deposited as reference cultures in the
Mycological Collection of the Centro de Estudios Parasitológicos y de Vectores
(CEPAVE). We performed dual culture studies of the isolates collected against
the pathogens Botrytis cinerea Pers. (1797) and Sclerotinia
sclerotiorum (Lib.) de Bary (1884). Eleven isolates were selected for
growth promotion studies in tomato plants (Solanum lycopersicum L.). The
isolates of Metarhizium taii Liang & Liu (1991) CEP-722, CEP-723 Trichoderma
afroharzianum Chaverri, Rocha, Degenkolb & Druzhinina (2015) CEP-753
and CEP-754, molecularly identified by amplification of the ITS and TEF1α
zones, presented the best results in the dual culture and growth promotion
tests. Subsequent studies will evaluate virulence of fungal strains in insects.
Keywords: entomopathogenic
fungi, biological control agents, molecular identification, dual culture, plant
growth promotion
Resumen
El objetivo de este
trabajo fue seleccionar microorganismos promisorios como agentes de control
biológico (ACB). Se visitaron predios florihortícolas ubicados en Buenos Aires,
Argentina, de los cuales se obtuvo un total de 41 muestras de suelo. Se
utilizaron las técnicas de insecto trampa y diluciones seriadas de suelo para
la obtención de aislados de hongos entomopatógenos y hongos del género Trichoderma
respectivamente. Se obtuvieron un total de 20 aislados, cinco pertenecientes
al género Metarhizium y 15 aislados correspondientes al género Trichoderma.
Los aislados fueron liofilizados y depositados como cultivos de referencia en
la Colección Micológica del Centro de Estudios Parasitológicos y de Vectores
(CEPAVE). Se realizaron estudios de cultivos duales de los aislados
recolectados frente a los patógenos Botrytis cinerea Pers. (1797) y Sclerotinia
sclerotiorum (Lib.) de Bary (1884). Se seleccionaron 11 aislados para la
realización de estudios de promoción de crecimiento en plantas de tomate (Solanum
lycopersicum L.). Los aislados de Metarhizium taii Liang y Liu
(1991) CEP-722, CEP-723 y de Trichoderma afroharzianum Chaverri, Rocha,
Degenkolb y Druzhinina (2015) CEP-753 y CEP-754, identificados molecularmente
por medio de la amplificación de las zonas ITS y TEF1α, presentaron los mejores
resultados en las pruebas de cultivo dual y promoción de crecimiento. Se espera
avanzar en estudios posteriores que evalúen la virulencia de cepas de hongos en
insectos.
Palabras clave: hongos
entomopatógenos, agentes de control biológico, identificación molecular,
cultivo dual, promoción de crecimiento de las plantas
Originales: Recepción: 17/07/2023 - Aceptación: 13/06/2024
Introduction
Stem “wet rot”
caused by Sclerotinia sclerotiorum (Lib.) de Bary, (1884) and “grey rot”
caused by Botrytis cinerea Pers. (1797) stand among the economically
most important diseases in tomato (Solanum lycopersicum L.) Control of
these diseases has relied on benzimidazole and dicarboximide fungicides.
However, dicarboximide-resistant isolates are commonly detected (3). In this regard,
biological control programs sustained by isolation and subsequent selection of
antagonists (4) constitute a
valuable alternative. Among beneficial biota, nutrient-fixing and solubilizing
microorganisms produce plant growth-promoting substances, induce plant
resistance to diseases or behave as antagonists to phytopathogenic agents (32).
The genus Trichoderma
dominates the mycobiome of various ecosystems (10) with the ability
to colonize the rhizoplane, rhizosphere and roots, producing numerous
metabolites with antimicrobial and biostimulant activity. The plant growth
stimulating effect is probably generated by the interaction among growth
hormones synthesized by Trichoderma spp. and
plant defense hormones (8). Some
entomopathogenic fungi act as fungal growth inhibitors of phytopathogens (11,
12). The genus Metarhizium is composed of diverse common
soil fungi with multifunctional lifestyles and different nutrient acquisition
modes, either saprophytes, endophytes, and/or insect pathogens (37). Classically,
studies have focused on their entomopathogenic characteristics, but their
ability to inhibit phytopathogens was recently determined (13). Some studies have
evaluated the endophytic capacity and colonization methods of this genus (2).
Metarhizium is a genetically diverse taxon, and
colony color and conidial measurements of different species are not reliable
identification factors (22).
Alternatively, the molecular identification of Trichoderma is abundant,
with no standard process, except the recently proposed gene standardization
system for molecular identification (7).
This study intends to develop biological inputs based on native and/or
naturalized strains of Trichoderma and entomopathogenic fungi for
agricultural pest management.
Materials
and methods
Collection
of soil samples
Six agroecological
productions located in the province of Buenos Aires, Argentina were visited.
Agroecological production of Bernardo Castillo (Street 519, El Pato, Buenos
Aires. -34.905505, -58.200043); Organization 1610 (Street 1610, La Capilla,
Buenos Aires. -34.9046857, -58.2666433); Agroecological production Santa Elena
(Road Parque Pereyra Iraola, Pereyra, Buenos Aires. -34.83699, -58.093384); M.
G. Agroecológica (Esteban Echeverría, Buenos Aires. -34.8672710, -58.4608800);
Cooperative UTT Jaúregui. (Luján, Buenos Aires. -34.6204249, -59.1764168); and
the experimental plot of Cátedra de Horticultura, Facultad de Agronomía de la
Universidad de Buenos Aires (Av. San Martín 4453, C.A.B.A. -34.594101,
-58.484467). Fourty-one soil samples were obtained from cultures of cabbage (Brassica
oleracea var. capitata); basil (Ocimum basilicum); corn (Zea
mays); lettuce (Lactuca sativa); tomato (Solanum lycopersicum);
zucchini (Cucurbita pepo); bell pepper (Capsicum annuum); cherry
tomato (Solanum lycopersicum var. cerasiforme); chives (Allium
fistulosum); leek (Allium ampeloprasum var. porrum); fennel (Foeniculum
vulgare); beets (Beta vulgaris); broccoli (Brassica oleracea var.
italica); brussels sprouts (Brassica oleracea var. gemmifera);
chard (Beta vulgaris var. cicla); carrot (Daucus carota);
artichoke (Cynara cardunculus var. scolymus); broad bean (Vicia
faba); turnip (Brassica rapa subsp. rapa); kale (Brassica
oleracea var. sabellica); peas (Pisum sativum); and arugula (Eruca
vesicaria). Sample number and species varied by establishment. As sampling
criterion, soil from more vigorous plants within the same plot was also
sampled, for later obtention of growth-promoting microorganisms (20,
39, 42). Five random subsamples within a crop row were collected for
each sample from the first 20 cm below ridge surface with crop roots. Then,
they were mixed into a single homogeneous sample with approximately 500 g from
each crop, soil and rhizosphere, holding the greatest biodiversity (24). Fungal greatest
abundance is found in the superficial layers or soil horizons (21). Samples were
arranged in plastic bags indicating date, culture and origin, later transported
to the laboratory in a closed expanded-polystyrene container and processed
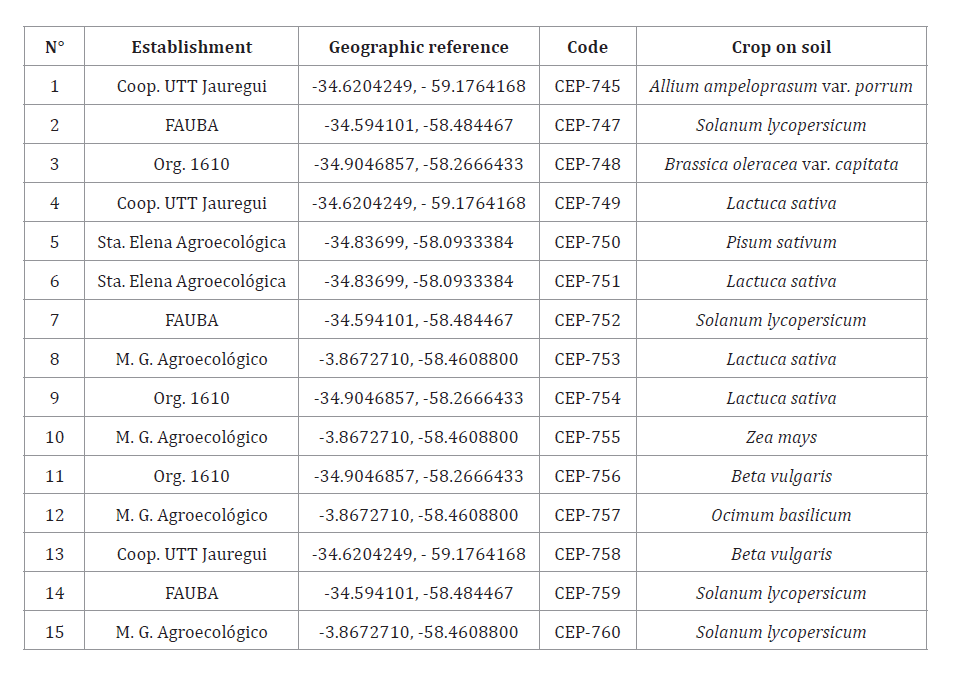
within 24 hours (tables 4 and 5).
Table 4. Metarhizium isolates
obtained from soil samples.
Tabla
4. Aislados del género Metarhizium obtenidos
de las muestras de suelo.
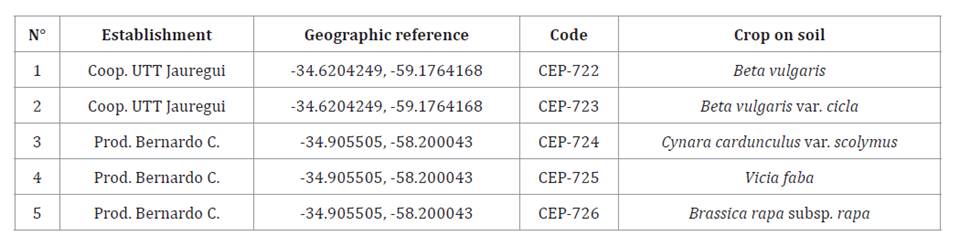
Table 5. Trichoderma isolates
obtained from soil samples.
Tabla
5. Aislados del género Trichoderma obtenidos
de las muestras de suelo.
Isolation
of Metarhizium, Trichoderma, Botryris cinerea and Sclerotinia
sclerotiorum fungi
From the collected
soil samples, the insect trap technique was used with larvae of Tenebrio
molitor L. from stage L3 to L4 as bait insects (1). Samples were
sieved and 300 g were placed in 500 ml plastic containers with five larvae
each, moistened with 20 ml of sterile distilled water and incubated at 18°C 65%
relative humidity and 14:10 h light-darkness photoperiod. T. molitor carcasses
prospected after seven days. Dead larvae with external mycosis were washed with
sterile distilled water and placed in a humid chamber to increase sporulation.
External mycelium present in T. molitor corpses (figure 5A) was obtained via
direct isolation from the sporulated corpses, using a previously sterilized
loop and subsequent sowing in Sabouraud Dextrose Agar culture medium, SDYA
(Merck, Germany) with the addition of 5% chloramphenicol inside a 90 mm
diameter Petri dish.
Figure
5. A) Detail of T. molitor larva corpse with sporulation
of isolate CEP-722; B) Conidiophores, phialides and conidia of isolate CEP-753;
C) Conidiophores, phialides and conidia of isolate CEP-754; D) Conidiophores,
phialides and conidia of isolate CEP-722, E) Conidiophores, phialides and
conidia of isolate CEP- 723; and F) Mycelium, conidia and chlamydospores of the
isolate CEP-722.
Figura
5. A) Detalle de cadáver de larva de T. molitor con
esporulación del aislado CEP-722; B)Conidióforos, fiálides y conidios del
aislado CEP-753; C) Conidióforos, fiálides y conidios del aislado CEP-754; D)
Conidióforos, fiálides y conidios del aislado CEP-722; E) Conidióforos,
fiálides y conidios del aislado CEP-723; y F) Micelio, conidio y clamidosporas
del aislado CEP-722.
Trichoderma, fungi were isolated via
serial dilution. Five grams of each soil sample were suspended in 100 ml of
sterile distilled water in an Erlenmeyer and vortexed for one hour. Serial
dilutions were made until reaching x106 spores/ml.
Concentrations were determined with a Neubauer chamber, with each dilution
inoculated in 90 mm diameter Petri dishes with Potato Glucose Agar, APG
(Merck, Germany), and 2% streptomycin. The plates were incubated at 20-22°C for
72 hours. When fungal colonies developed, they were replicated in Petri dishes
with APG (Merck, Germany) until purification.
Phytopathogenic fungi isolates from B. cinerea and S.
sclerotiorum were obtained from the mycological bank of phytopathology
(Facultad de Agronomía de la Universidad de Buenos Aires), with identification
code BC18 and SS18 and pathogenicity tested on tomato (S. lycopersicum).
After isolation and before bioassays, visual prospection of the isolates was
carried out under a microscope (OLYMPUS BX51, Japan), identifying fungal types.
Monosporic isolates
were preserved on sterile filter paper and were lyophilized, deposited and
preserved (14) as reference
cultures in the mycological collection of the “Centro de Estudios
Parasitológicos y de Vectores” (CEPAVE) (CONICET-UNLP), La Plata, Argentina.
Laboratory
tests in dual cultures of Metarhizium and Trichoderma isolates
against B. cinerea and S. sclerotiorum
Ninety mm diameter Petri dishes were filled with 12 ml of
APG (Merck, Germany) or 12 ml of SDYA (Merck, Germany) for Trichoderma and
Metarhizium trials, respectively. Once culture medium solidified, two 10
mm diameter discs with seven-days mycelial growth were placed on the medium, 70
mm apart, one containing Trichoderma spp. or Metarhizium
spp. and the other containing B. cinerea or
S. sclerotiorum according to each treatment. A disk of each isolate
(pathogens and antagonists) was inoculated as control against a disk of APG
and/or SDYA without microorganisms. Petri dishes were incubated at 22°C
and maintained under fluorescent lights with a 14:10 h light-darkness
photoperiod in a completely randomized design, with eight replicates per
treatment. Growth radius of colonies considering Trichoderma isolates
against phytopathogenic fungi were measured with a millimeter ruler, at 1, 2,
3, 4, 5 and 6 days of trial with B. cinerea and at 1, 2, 3, 4 and 5 days
with S. sclerotiorum. Considering Metarhizium isolates against
phytopathogenic fungi, colony radius was measured at 4, 5, 6 and 7 days in both
cases. The number of measurement days per trial differs according to growth
rate in phytopathogen control treatments. Pathogen percentage inhibition (I)
was calculated using the following equation:
where:
(I) = Percentage of
mycelium growth inhibition
C = Pathogen growth
on control plates
T = Pathogen growth
in dual culture plates (19).
The data were
analyzed by ANOVA and Tukey test (p> 0.05) with InfoStat software (version
2016e) (9).
Growth
promotion assays in tomato plants var. platense (S.
lycopersicum) inoculated with a spore suspension of isolates of the genera Metarhizium
and Trichoderma
Tomato plants (S. lycopersicum) var. platense
were inoculated with a spore suspension of Metarhizium sp. CEP-722,
CEP-723, CEP-724, CEP-725 and CEP-726 or the Trichoderma sp. CEP-745,
CEP-749, CEP-751, CEP-752, CEP-753 and CEP-754, selected after in vitro growth
inhibition tests of B. cinerea and S. sclerotiorum. The test was
conducted in a biotherium chamber under controlled conditions at an average
temperature of 24°C, average relative humidity of 70%, and a 18-6 h
light-darkness photoperiod, using high-pressure sodium vapor lamps (Philips Son
T Agro 250 W, China). Seeds of tomato (S. lycopersicum) var. platense were sown in commercial substrate (Grow Mix
Multipro, Argentina) in seedling trays with 50 x 50 mm holes and irrigated with
sterile distilled water. Spore suspensions of Trichoderma spp. and Metarhizium spp. isolations
were obtained in sterile distilled water standardized to a concentration of 1 x
107 spores/ml. Tomato
plants (S. lycopersicum) were inoculated at 7, 21 and 35 days after
being sown (31 days in the case of the genus Metarhizium) with 2 ml of
conidial suspension on the substrate. Measurements were made 49 days after
sowing in Trichoderma spp. and 37 days in Metarhizium
spp. Plants were watered to field capacity with sterile distilled water
throughout the study. The completely randomized block design had seven
repetitions (seven plants) for each treatment (spore suspension of the Metarhizium
sp. and Trichoderma sp. isolates selected). The variables analyzed
were stem length (mm), stem diameter at cotyledon height (mm) and aerial fresh
weight (g), using a millimeter rule, graduated metal caliper and precision
balance, respectively. An ANOVA and means comparison with Tukey test (p >
0.05) were performed with InfoStat (2016e version) (9).
Morphological
characterization, molecular identification and phylogenetic analysis of the
isolates CEP-722, CEP-723, CEP-753 and CEP-754
Four isolates, two of the genus Metarhizium and two of
the genus Trichoderma, were selected for best results on dual culture
and promotion growth assays. Isolates were identified at genus level based on
microscopic traits contrasted with taxonomic keys (5,
18). Once the material was mounted in lactophenol/cotton blue
(0.01% w/v), shape and size of conidia, conidiogenous cells (phialide),
mycelium and other traits were observed under an optical microscope (OLYMPUS
BX51, Japan) and photographed with a digital camera (Sony DSCP73, Japan).
Measurements were based on 25 observations per microstructure (conidia,
phialide and chlamydospore) and average calculations. Molecular analysis and
identification of the isolates included mycelium production in three 90 mm
diameter Petri dishes with APG (Merck, Germany) as culture medium for Trichoderma
and SDYA (Merck, Germany) for Metarhizium, kept at 23° ± 1°C for seven
and 14 days, respectively. Then, mycelium was placed in 1.5 ml Eppendorf tubes.
Tubes containing fungal material were placed in a container with liquid
Nitrogen for eight minutes. DNA extraction was performed using the DNeasy
extraction kit from Qiagen (Germany) according to manufacturer instructions.
Extracted DNA was quantified using a micro-volume spectrophotometer (Nanodrop,
Thermo Fisher Scientific, United States) and stored in a freezer. PCR was
performed to amplify 2 DNA regions: 1) The ribosomal DNA region comprising the
3’ end of the 18S gene (small ribosomal subunit, SSU). The ITS1 internal spacer
sequence (internal transcribed spacer 1), the 5.8S gene, the internal
transcribed spacer 2 (ITS2) sequence and the 5’ end of the 28S gene (long ribosomal
subunit), using the universal primers ITS4 (5’ -TCCTCCGCTTATTGATATGC-3’) and
ITS5 (5’-GGAAGTAAAAGTCGTAACAAGG-3’) (30).
2) The 5’ region of the Elongation Factor 1-Alpha (TEF1α) gene with the primers
EF1 983F (5’-ATGGGTAAGGARGACAAGAC-3’) and EF” 2218R
(5’-ATGGGTAAGGARGACAAGAC-3’) (23).
Amplification reactions were carried out in a final volume of 50 μl, containing
25 μl Mastermix Promega 2x (GoTaq, USA), 17 μl nuclease-free water, 2 μl PRIMER-F,
2 μl PRIMER- R and 4 μl of DNA for each isolate. Table 1
shows the thermocycling processes for the ITS1 and TEF1α regions.
Table 1. Thermocycling
processes for the ITS1 and TEF1α regions.
Tabla 1. Procesos
de termociclados para las regiones ITS1 y TEF1α.
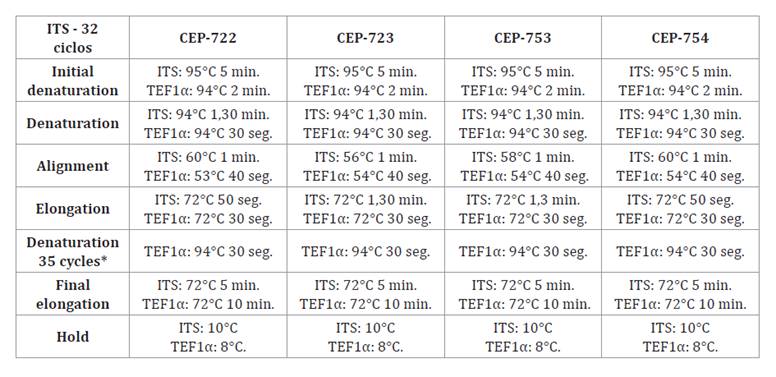
*
TEF1α region only. * Solo región TEF1α.
Electrophoresis was performed in 1% agarose gels (UNQ Biological
Products, Argentina) stained with Ethidium bromide in 0.5x Buffer TBE
(Roti-Gelstain, Germany), applying a voltage of 90 V for 50 min. Five μl of
each reaction was mixed with 1 μl of loading buffer (Productos Biológicos UNQ,
Argentina). A molecular weight marker was added (Ladder 100 bp, PB-L), to
determine PCR product size. Gels were visualized with a UV transilluminator
(Analytik-Jena, Alemania). The ribosomal region was expected at 530 bp, while
the TEF1α gene was 620 bp. Positive reactions were stored at -20°C. Samples
were then sent to Macrogen (South Korea) for purification and sequencing. The
free software “Chromas” (38) cleaned the
obtained sequencing then aligned using the online software “Clustal-Omega” (35). Agreement between
the base pairs replicated by the forward and reverse primers of the four isolates
analyzed was verified using the free software “Genedoc” (25). The sequences
obtained were compared with those available in Genbank for each molecular
marker (41). Sequences were aligned with 16
homologues and contrast of reference strains of Metarhizium with the
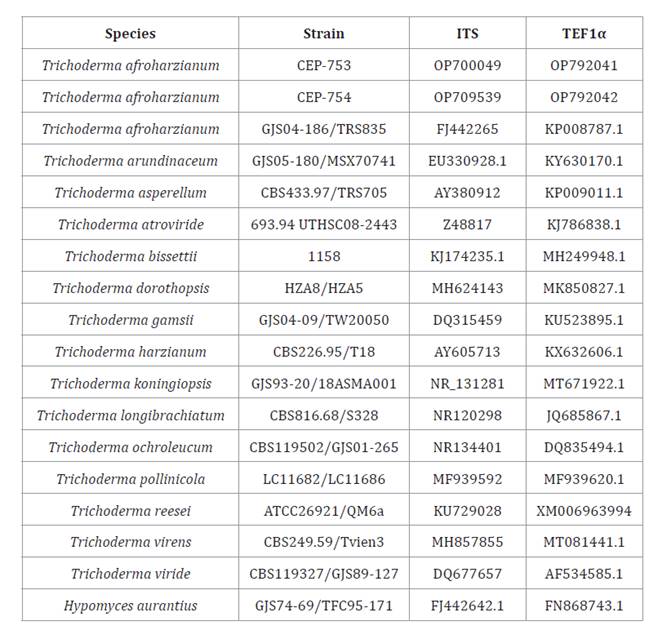
sequencing of the ITS and TEF1α areas of Gutierrez et al. (2019) (table 2) and with 15
homologous species and a contrast obtained from the “Trichokey” data software (7), for Trichoderma
(table
3).
The phylogenetic tree was constructed with “Mr. Bayes” (version 3.2.7) (17), “Tracer” (version
1.7.2) (30) and “FigTree” (version 1.4.4)
softwares (29).
Table 2. ITS and TEF1α gene sequences used for molecular
identification of isolates CEP-722 and CEP-723.
Tabla
2. Secuencias de genes ITS y TEF1α
utilizadas para la identificación molecular de los aislados CEP-722 y CEP-723.
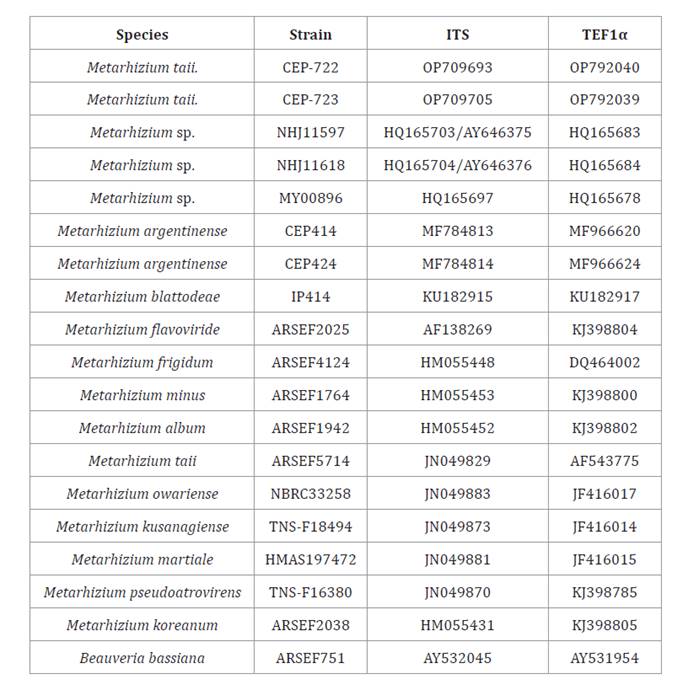
Table 3. ITS and TEF1α gene sequences used for molecular
identification of isolates CEP-753 and CEP-754.
Tabla
3. Secuencias de genes ITS y TEF1α
utilizadas para la identificación molecular de los aislados CEP-753 y CEP-754.
Results
Isolates with access numbers CEP-722, CEP-723, CEP-724, CEP-725
and CEP-726 for Metarhizium spp. (table 4) and CEP-745,
CEP-747, CEP-748, CEP-749, CEP-750, CEP-751, CEP-752, CEP-753, CEP-754,
CEP-755, CEP-756, CEP-757, CEP -758, CEP-759 and CEP-760 for Trichoderma spp.
(table 5) were obtained and
admitted to the mycological bank of the “Centro de Estudios Parasitológico y de
Vectores” (CEPAVE) (CONICET-UNLP), La Plata, Argentina.
Percentage
inhibition of growth of B. cinerea and S. sclerotiorum caused by
fungi of the genera Trichoderma and Metarhizium
Considering growth speed and physical conditions of the Petri
dishes, results on dual culture trials are presented for day 4 for Trichoderma
and day 7 for Metarhizium. Percentage growth inhibition of the
pathogens stabilized after mycelial contact with the treatments or when an
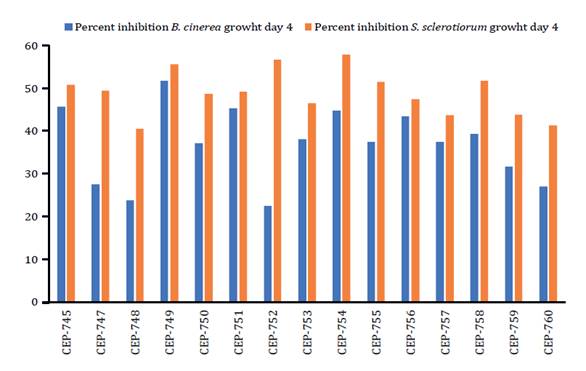
inhibition halo was generated. Trichoderma isolates with the highest
inhibition percentages at day four of measurement in the dual culture tests
against the pathogen B. cinerea were CEP-745, CEP-749, CEP-751, CEP-754
and CEP-756, these being 46.04; 51.75; 45.40; 45.40; and 43.18%, respectively.
Inhibition percentage of S. sclerotiorum in Petri dishes on day 4
of measurement reached 55.88; 56.75; 58.25; 52.13; and 51.75% for isolates
CEP-749, CEP-752, CEP-754, CEP-755 and CEP-758 respectively, presenting the
highest mean values (figure
1).
Figure
1. Growth inhibition (%) of B.
cinerea and S. sclerotiorum by isolates of the genus Trichoderma at
day 4 of measurement.
Figura
1. Medias del porcentaje de inhibición
del crecimiento de B. cinerea y S. sclerotiorum generado
por aislados del género Trichoderma al día 4 de medición.
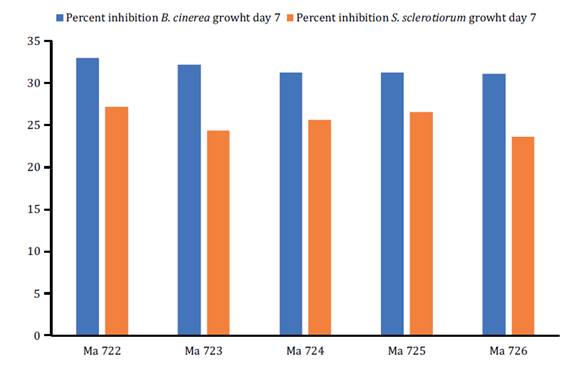
No significant differences were observed in growth percentage of
B. cinerea at day seven among the different treatments of spores
suspension Metarhizium isolates, but the highest mean values were
recorded for CEP-722 and CEP-723, being 32.97 and 32.19%, respectively. The
CEP-722 isolate presented the highest values in inhibition percentage of S.
sclerotiorum at day seven, reaching a mean value of 27.34%. This was the
only treatment with statistically significant differences concerning treatment
CEP-726, which presented the lowest inhibition percentages (23.59%) against S.
sclerotiorum (figure
2).
Figure
2. Growth inhibition (%) of B. cinerea and S.
sclerotiorum by isolates of the genus Metarhizium at day 7 of
measurement.
Figura
2. Medias del porcentaje de inhibición
del crecimiento de B. cinerea y S. sclerotiorum generado
por aislados del género Metarhizium al día 7 de medición.
Growth
promotion of tomato plants (S. lycopersicum) var. platense
inoculated with six isolates of Trichoderma and five isolates of Metarhizium
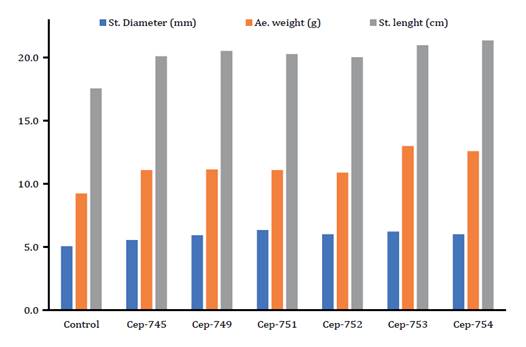
Considering all variables studied in the Trichoderma assays,
control treatment presented the lowest mean values after application. Regarding
stem diameter, the plant inoculated with spore suspension of the isolates
CEP-751, CEP-752, CEP-753 and CEP-754 presented higher mean values than the
plant inoculated with CEP-745, CEP-749 and the control. Stem length reached the
highest mean value (21.37 cm) for the plant inoculated with spore suspension of
the isolates CEP-754. Aerial weight mean was highest for the plants inoculated
with spore suspension of CEP-753 and CEP-754, reaching 13.02 and 12.59 g,
respectively (figure
3).
Figure
3. Mean values of stem diameter,
aerial weight and stem length of tomato plants (S. lycopersicum)
inoculated with spore suspensions of Trichoderma isolates in growth
promotion assays.
Figura
3. Medias registradas en el diámetro
de tallo, peso aéreo y longitud de tallo de plantas de tomate (S.
lycopersicum) inoculadas con suspensiones de esporas de aislados del género
Trichoderma en los ensayos de promoción del crecimiento.
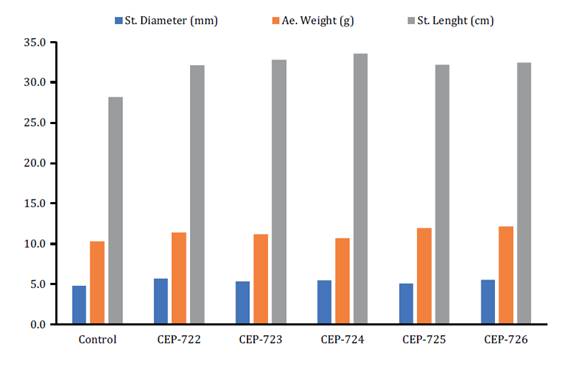
Stem diameter, stem length and aerial weight of all treatments
with Metarhizium fungi differed from the control. Regarding stem
diameter, treatments inoculated with a spore suspension of the isolates
CEP-722, CEP-724 and CEP-726 presented differences from the control, with mean
values of 5.71, 5.50 and 5.57 mm respectively. Stem length of tomato plants
inoculated with CEP-724 stood out with a mean of 33.54 cm followed by
treatments inoculated with CEP-723 and CEP-726, with mean values of 32.84 and
32.44 cm respectively. Regarding aerial weight, the treatment with CEP-726
presented 12.15 g, the highest mean value (figure 4).
Figure
4. Mean values of stem diameter,
aerial weight and stem length of tomato plants (S. lycopersicum)
inoculated with spore suspensions of Metarhizium isolates in growth
promotion assays.
Figura
4. Medias registradas en el diámetro
de tallo, peso aéreo y longitud de tallo de plantas de tomate (S.
lycopersicum) inoculadas con suspensiones de esporas de aislados del género
Metarhizium en los ensayos de promoción del crecimiento.
Morphological
characterization, molecular identification and phylogenetic Analysis of the
isolates CEP-722, CEP-723, CEP-753 and CEP-754
Figures 5D, 5E and 5F show microscopic
traits of the isolates CEP-722 and CEP-723. Conidiogenesis occurs in a dense
hymenium; conidiophores branch repeatedly at wide angles resembling candelabra;
conidiogenous cells are clavate or cylindrical, with a rounded to conical apex,
no obvious neck; the apical wall thickens progressively as conidia are produced
in long chains, adhering laterally to form prismatic (palisade) columns. The
CEP-722 isolate was the only one presenting chlamydospores (figure 5F). Microscopic
measurements and morphological traits of CEP-722 and CEP-723 coincide with Metarhizium
taxonomic keys (18). Microscopic
traits of isolates CEP-753 and CEP-754 are hyaline conidiophores,
smooth-walled, up to 5 μm wide near the base, gradually tapering to about 2 μm
wide near the apex, with relatively conspicuous septa distant; side branches
borne at right angles, singly or in whorls of 2-3, gradually increasing in
length. Phialides occur in whorls of 2-5, solitary and alternate, or more
irregularly arranged, particularly towards the apex of the conidiophore.
Terminals are more elongated and generally not constricted at the base. Conidia
are unicellular, diluted green in color, smooth-walled, short cylindrical and
almost oblong, with obtusely rounded apex (figures 5B and 5C). Microscopic
measurements and morphological traits of CEP-753 and CEP-754 coincide with Trichoderma
taxonomic keys (5).
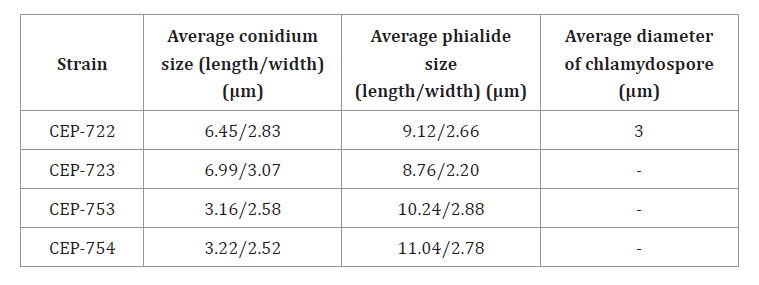
Table 6, shows average
measurements of each microstructure.
Table 6. Average measurements of reproductive structures of isolates
CEP-722, CEP-723, CEP-753 and CEP-754.
Tabla
6. Promedios de mediciones de
estructuras reproductivas de los aislados CEP-722, CEP-723, CEP-753 y CEP-754.
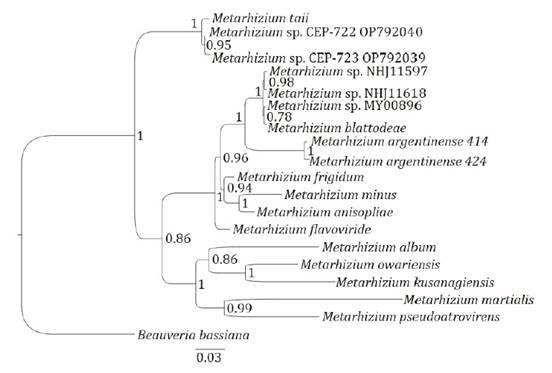
Isolates CEP-722 and CEP-723 had 100% homology to each other,
considering the sequences used for ITS and TEF1α markers of Metarhizium spp.
Therefore, CEP-722 and CEP-723 correspond to the genus Metarhizium and
present 0% genetic variability with the species Metarhizium taii (Genbank
access code ARSEF5714). The taxonomic classification for CEP-722 and
CEP-723 with GenBank reference codes ITS: OP709693/TEF1α: OP792040 and ITS:
OP709705/TEF1α: OP792039, is Fungi; Ascomycota; Pezizomycotina;
Sordariomycetes; Hypocreales; Clavicipitaceae; Metarhizium (Sorokin,
1883):
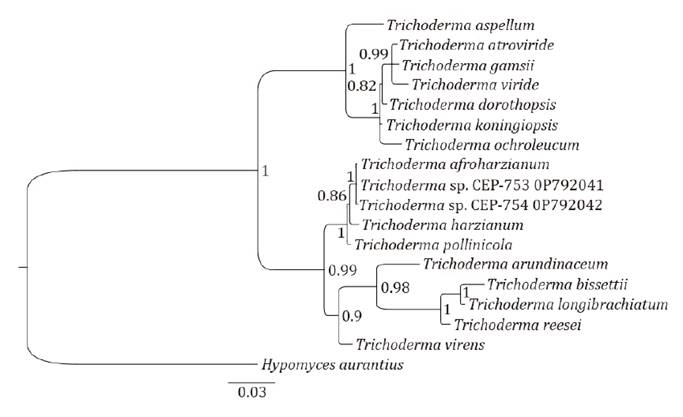
Metarhizium taii. Isolates CEP-753 and CEP-754 presented 100% homology
to each other considering reference sequences used for ITS and TEF1α markers of
Trichoderma spp. Phylogenetic analysis shows CEP-753 and CEP-754
correspond to Trichoderma and present 0% genetic variability with the
species Trichoderma afroharzianum, Genbank access code GJS04-186/TRS835.
The taxonomic classification for CEP-753 and CEP-754 with reference codes ITS:
OP700049/TEF1α: OP792041 and ITS: OP709539/TEF1α: OP792042, respectively, is
Fungi; Ascomycota; Euascomycetes; Hypocreales; Hypocraceae; Trichoderma and
Hypocrea (Rifai,
1969):
Trichoderma afroharzianum. Analytical “runs” of the sequences of
selected microorganisms were carried out with MrBayes software (version 3.2.7).
Databases were prepared according to published references. Figure 6 and figure 7 show the
phylogenetic trees for CEP-722 and CEP-723 isolates of Metarhizium and
for CEP-753 and CEP-754 isolates of Trichoderma, respectively.
Node
values represent maximum likelihood probabilities.
Los
valores entre las diagonales representan el valor de probabilidad de máxima
verosimilitud.
Figure
6. Phylogenetic tree of ITS and TEF1α regions of
CEP-722 and CEP-723 isolates.
Figura
6. Árbol filogenético de las regiones
ITS y TEF1α de los aislados CEP-722 y CEP-723.
Tree
node values represent maximum likelihood probability values.
Los
valores entre las diagonales representan el valor de probabilidad de máxima
verosimilitud.
Figure
7. Phylogenetic tree of ITS and TEF1α
regions of CEP-753 and CEP-754 isolates.
Figura
7. Árbol filogenético de las regiones
ITS y TEF1α de los aislados CEP-753 y CEP-754.
Discussion
Trichoderma spp. and Metarhizium spp. isolates
evaluated against the phytopathogens Botrytis cinerea and Sclerotinia
sclerotiorum in this study showed varying effects according to strain and
isolate, as previously found (16, 34). In our in
vitro studies, the Metarhizium taii strains CEP-722 and CEP-723, and
the Trichoderma afroharzianum strains CEP-753 and CEP-754 presented
different inhibition levels against different phytopathogens. This, because
biological control agents of Trichoderma use varied mechanisms, like
antifungal compounds, competition for nutrients, parasitism or pathogen
inhibition, antibiosis, lytic enzymes (23) and systemic
resistance (26, 28). Growth promotion
of tomato plants (S. lycopersicum) inoculated with spore suspensions of Metahrhizium
and Trichoderma fungi constitutes an important background when
designing fertilization strategies in the cultivation of tomatoes (S.
lycopersicum). The selected strains CEP-753 and CEP-754 of T.
afroharzianum could be considered for nutritional management of crops. Our
results agree with previous studies reporting a growth promotion in tomato
plants (S. lycopersicum) var. platense
inoculated with entomopathogenic fungi (33). Strains CEP-722
and CEP-723 of M. taii inhibit B. cinerea and S. sclerotiorum,
in agreement with studies on the interaction of entomopathogenic and
phytopathogenic microorganisms (11). Employing
indigenous microorganisms could be a promising alternative to external
inoculants, potentially reducing production costs and without introducing
foreign microorganisms into the environment (6).
Conclusion
Metarhizium taii strains CEP-722 and CEP-723 and Trichoderma afroharzianum CEP-753
and CEP-754 were best candidates as biological control agents against Botrytis
cinerea and Sclerotinia sclerotiorum. These strains constitute
valuable tools for disease management and interesting ingredients for
nutritional management of tomato (S. lycopersicum).
Acknowledgment
To Dr. Sebastián Arturo Pardo Seguel and Nicole Tomasic for the
translation. This work was supported by CONICET (doctoral scholarship) and
Universidad de Buenos Aires, Argentina. (UBACYT 20020160100066BA).
1.
Aguilera-Sammaritano, J. A.; Lopez-Lastra, C. C.; Leclerque, A.; Vazquez, F.;
Toro, M. E.; D’ Alessandro, C. P.; Cuthbertson, A. G. S.; Lechner, B. E. 2016.
Control of Bemisia tabaci by entomopathogenic fungi isolated from arid
soils in Argentina. In Biocontrol Science and Technology. 26(12): 1668-1682.
DOI: 10.1080/09583157.2016.1231776
2.
Allegrucci, N.; Velazquez, M. S.; Russo, M. L.; Pérez, M. E.; Scorsetti, A. C.
2017. Endophytic colonisation of tomato by the entomopathogenic fungus Beauveria
bassiana: the use of different inoculation techniques and their effects on
the tomato leafminer Tuta absoluta (Lepidoptera: Gelechiidae). DOI:
10.1515/jppr-2017-0045
3.
Arias, L. A.; Tautiva, L. A.; Piedrahíta, W.; Chaves, B. 2007. Evaluación de
tres métodos de control del Moho blanco (Sclerotinia sclerotiorum (Lib.)
de Bary) en lechuga (Lactuca sativa L.). In Agronomía Colombiana. 25(1):
131-141.
4.
Bettiol, W.; Morandi, M. A. V. 2009. Biocontrole de doenças de plantas: Uso e
perspectivas. In EMBRAPA, Jaguariúna. 341 p.
5.
Bissett, J. 1984. A revision of the genus Trichoderma. I. Section
Longibrachiatum sect. nov. In Canadian Journal of Botany. 62(5): 924-931. DOI:
10.1139/b84-131
6. Boenel, M.;
Fontenla, S.; Solans, M.; Mestre, M. C. 2023. Effect of yeast and mycorrhizae
inoculation on tomato (Solanum lycopersicum) production under normal and
water stress conditions. Revista de la Facultad de Ciencias Agrarias.
Universidad Nacional de Cuyo. Mendoza. Argentina. 55(2): 141-151. DOI:
https://doi.org/10.48162/rev.39.116
7. Cai, F.;
Druzhinina, I. 2021. In honor of John Bissett: authoritative guidelines on
molecular identification of Trichoderma. In Fungal Diversity. (107):
1-69. DOI: 10.1007/s13225-020- 00464-4. https://www.trichokey.com/
8.
Carná, M.; Repka, V.; Skupa, P.; Sturdík, E. 2014. Auxins in defense
strategies. In Biología. 69(10): 1255-1263. DOI: 10.2478/s11756-014-0431-3
9. Di Rienzo, J.;
Balzarini, M.; Gonzalez, L.; Casanoves, F.; Tablada, M.; Walter Robledo, C.
2010. Infostat: software para análisis estadístico.
https://www.infostat.com.ar/index.php
10.
Ghorbanpour, M.; Omidvari, M.; Abbaszadeh-Dahaji, P.; Omidvar, R.; Kariman, K.
2018. Mechanisms underlying the protective effects of beneficial fungi against
plant diseases. In Biological Control. 117: 147-157. DOI:
10.1016/j.biocontrol.2017.11.006
11.
Gómez-De La Cruz, I.; Pérez-Portilla, E.; Escamilla-Prado, E.; Martínez-Bolaños,
M.; Carrión-Villarnovo, G. L.; Hernández-Leal, T. I. 2017. Selection in
vitro of mycoparasites with potential for biological control on coffee leaf
rust (Hemileia vastatrix). In Revista Mexicana de Fitopatología. 36(1):
172-183. DOI: 10.18781/r.mex.fit.1708-1
12.
Gothandapani, S.; Boopalakrishnan, G.; Prabhakaran, N.; Chethana, B. S.;
Aravindhan, M.; Saravanakumar. M.; Ganeshan, G. 2014. Evaluation of
entomopathogenic fungus against Alternaria porri (Ellis) causing purple
blotch disease of onion. In Archives of Phytopathology and Plant Protection.
48(2): 135-144. DOI: 10.1080/03235408.2014.884532
13.
Guigón-López, C.; Holguín-Ibarra, P. D.; Torres-Zapien, J. H.; García-Cruz, I.;
Villapando, I.; Salas-Salazar, N. A. 2021. Metarhizium anisopliae reduces
conidial germination and mycelium growth of the apple gray mold Botrytis
cinerea. In Biological Control. 160: 1-9. DOI:
10.1016/j.biocontrol.2021.104660
14.
Gutierrez, A. C.; Tornesello-Galván, J.; Manfrino, R. G.; Hipperdinger, M.;
Falvo, M.; D’ Alessandro, C.; López-Lastra, C. C. 2017. Organización y
conservación de la colección de hongos patógenos y simbiontes de insectos y
otros artrópodos del CEPAVE (CONICET-UNLP), La Plata, Argentina. In Revista
argentina de Microbiología. 49(2): 183-188. DOI: 10.1016/j. ram.2016.09.007
15.
Gutierrez, A. C.; Leclerque, A.; Manfrino, R. G.; Luz, C.; Ferrari, W. A. O.;
Barneche, J.; García, J. J.; Lopez Lastra, C. C. 2019. Natural occurrence in
Argentina of a new fungal pathogen of cockroaches, Metarhizium argentinense sp.
nov. In Fungal Biology. 123(5): 364-372. DOI: 10.1016/j.funbio.2019.02.005
16.
Hidayah, B. N.; Khangura, R.; Dell, B. 2022. Biological control potential of
Trichoderma species and bacterial antagonists against Sclerotinia
sclerotiorum on canola in western Australia. In International Journal
Agriculture and Biology. 27(3): 215-227. DOI: 10.17957/ IJAB/15.1919
17. Huelsenbeck, J.
P.; Ronquist, F. 2001. MRBAYES: Inferencia bayesiana de filogenia. (versión 3.2.7, 2019). Bioinformática. 17: 754-755.
https://github.com/NBISweden/MrBayes/tree/ v3.2.7a
18.
Humber, R. A.; Lacey, L. A. 2012. Manual of techniques in invertebrate
pathology. In Lacey, L. A. (Ed.). Academic Press, London. 151-187.
19.
Joshi, B. B.; Bhatt, R. P.; Bahukhandi, D. 2010. Antagonistic and plant growth
activity of Trichoderma isolates of Western Himalayas. In Journal of
Environmental Biology. 31(6): 921-928.
20.
Kovács, C.; Csótó, A.; Pál, K.; Nagy, A.; Fekete, E.; Karaffa, L. Kubicek, C.
P.; Sándor, E. 2021. The biocontrol potential of endophytic Trichoderma
fungi isolated from Hungarian grapevines. Part I. Isolation, identification
and in vitro studies. Pathogens. 10(12): 1612. DOI: 10.3390/
pathogens10121612
21.
Lavelle, P.; Spain, A. V. 2001. Soil ecology. In Dordrecht, NL. Kluwer Academic
Publishers.
22.
Lomer, C. J.; Bateman, R. P.; Johnson, D. L.; Langewald, J.; Thomas, M. 2001.
Biological control of locusts and grasshoppers. In Annual Review of Entomology.
46(1): 667-702. DOI: 10.1146/ annurev.ento.46.1.667
23.
Marra, R.; Ambrosino, P.; Carbone, V.; Vinale, F.; Woo, S. L.; Ruocco, M.;
Ciliento, R.; Lanzuise, S.; Ferraioli, S.; Soriente, I.; Gigante, S.; Turrà,
D.; Fogliano, V.; Scala, F.; Lorito, M. 2006. Study of the three-way
interaction between Trichoderma atroviride, plant and fungal pathogens
by using a proteomic approach. Current Genetics. 50: 307-321. DOI:
10.1007/s00294-006- 0091-0
24.
Nannipieri, P.; Ascher, J.; Ceccherini, M.; Landi, L.; Pietramellara, G.;
Renella, G. 2003. Microbial diversity and soil functions. In European journal
of soil science. 54(4): 655-670. DOI: 10.1046/j.1351-0754.2003.0556.x
25. Nicholas, K.
B.; Nicholas Jr, H. B.; Deerfield II, D. W. 1997. embnet.
news. GeneDoc: Analysis and Visualization of Genetic
Variation, 4, 14. (https://genedoc.software.informer.com/2.7/)
26.
O’Brien, P. A. 2017. Biological control of plant diseases. Australasian Plant
Pathology. 46: 293-304. DOI:10.1007/s13313-017-0481-4
27.
O’ Donnell, K.; Ward, T. J.; Robert, V. A. R. G.; Crous, P. W.; Geiser, D. M.;
Kang, S. 2015. DNA sequence-based identifcation of Fusarium: current status and
future directions. In Phytoparasitica. 43(5): 583-595. DOI:
10.1007/s12600-015-0484-z
28.
Pieterse, C. M.; Zamioudis, C.; Berendsen, R. L.; Weller, D. M.; Van Wees, S.
C.; Bakker, P. A. 2014. Induced systemic resistance by beneficial microbes.
Annual Review of Phytopathology. 52: 1-5. DOI:10.1146/annurev-phyto-082712-102340
29. Rambaut, A.
2009. FigTree. Tree figure drawing tool. http://tree. bio.
ed. ac. uk/software/figtree/. http://tree.bio.ed.ac.uk/software/figtree/
30. Rambaut, A.;
Drummond, A. J.; Xie, D.; Baele, G.; Suchard M. A. 2018. Posterior
summarisation in Bayesian phylogenetics using Tracer 1.7. Systematic Biology.
syy032. DOI: 10.1093/ sysbio/syy032. https://beast.community/tracer
31. Rifai, M. A.
1969. Sarawakus Lloyd, a genus of the pyrenomycete family Hypocreaceae.
Reinwardtia. 7(5): 561-578.
32.
Rivera, M. C.; Wright, E. R. 2013. Interacciones entre fitopatógenos y
microorganismos benéficos en la rizósfera. In García-de Salamone, I. E.;
Vázquez, S.; Penna, C.; Cassán, F. (Eds.). Rizósfera, biodiversidad y
agricultura sutentable. Asociación Argentina de Microbiología. 33-46.
33.
Russo, M. L.; Scorsetti, A. C.; Vianna, M. F.; Cabello, M.; Ferreri, N.;
Pelizza, S. 2019. Endophytic effects of Beauveria bassiana on corn (Zea
mays) and its herbivore, Rachiplusia nu (Lepidoptera: Noctuidae).
Insects. 10(4): 110. DOI: 10.3390/insects10040110
34.
Sarven, S.; Hao, Q.; Deng, J.; Yang, F.; Wang, G.; Xiao, Y.; Xiao, X. 2020.
Biological control of tomato gray mold caused by Botrytis cinerea with
the entomopathogenic fungus Metarhizium anisopliae. Pathogens. 9(3):
213. DOI: 10.3390/pathogens9030213
35.
Sievers, F.; Higgins, D. G. 2014. Clustal Omega, accurate alignment of very
large numbers of sequences. Multiple sequence alignment methods, 105-116.
(https://www.ebi.ac.uk/ Tools/msa/clustalo/)
36. Sorokin, N.
1883. Plant parasites causing infectious diseases of man and animals. Vol. 2.
Edition of the Chief Military Medical Directorat, St Petersburg. 168-169.
37. Stone, L. B.
L.; Bidochka, M. J. 2020. The multifunctional lifestyles of Metarhizium:
evolution and applications. In Applied Microbiology and Biotechnology. 104:
9935-9945. DOI: 10.1007/ s00253-020-10968-3
38.
Technelysium, P. 2012. Chromas Lite version 2.1. South Brisbane, Queensland,
Australia, 817. https://technelysium.com.au/wp/chromas/
39.
Wang, X.; Wang, C.; Li, Q.; Zhang, J.; Ji, C.; Sui, J.; Liu, Z.; Song, X.; Liu,
X. 2018. Isolation and characterization of antagonistic bacteria with the
potential for biocontrol of soil‐borne wheat diseases. Journal of Applied
Microbiology. 125(6): 1868-1880. DOI: 10.1111/ jam.14099
40.
White, T. J.; Bruns, T. D.; Lee, S. B.; Taylor, J. W. 1990. Amplification and
direct sequencing of fungal ribosomal RNA genes for phylogenetics, In Innis M.
A.; Gelfand, D. H.; Sninsky, J. J.; White, T. J. (Eds.). PCR protocols: a guide
to methods and applications. Academic Press, San Diego. 18(1): 315-322.
41.
Zhang, Z.; Schwartz, S.; Wagner, L.; Miller, W. 2000. A greedy algorithm for
aligning DNA sequences. In Journal Computational Biology; Journal Computation
Molecular Cell Biology. 7(1-2): 203-214. DOI: 10.1089/10665270050081478
42. Zheng, X.; Wang, J.; Chen, Z.; Zhang, H.; Wang, Z.; Zhu, Y.;
Liu, B. 2019. A Streptomyces sp. strain: Isolation,
identification, and potential as a biocontrol agent against soilborne diseases
of tomato plants. Biological control. 136: 104004. DOI:
10.1016/j.biocontrol.2019.104004