Revista de la Facultad de Ciencias
Agrarias. Universidad Nacional de Cuyo. Tomo 56(2). ISSN (en línea) 1853-8665.
Año 2024.
Original article
Gas
exchange in yellow melon (Cucumis melo) crop under controlled water
deficit (RDI) and application of a biostimulant
Intercambio
gaseoso en el cultivo de melón amarillo (Cucumis melo) bajo déficit
hídrico controlado (RDI) y aplicación de bioestimulantes
Alessandro Carlos
Mesquita1,
Welson Lima Simões2,
Luan David
Alcantara Campos1,
Marcos Brandão
Braga3,
Yuri Rafael Alves
Sobral4
1Universidade do Estado da Bahia. DTCS/III. s/n.
São Geraldo. CEP: 48.904-711. Juazeiro. Bahia.
2Embrapa Semiárido. Rodovia BR-428, Km 152. Zona Rural. Caixa
Postal 23. CEP: 56302- 970. Petrolina.
PE.
3Embrapa Hortaliças, Rodovia BR 060. Km 9 SN. Fazenda Tamandua.
CEP: 70351-970 Brasilia. Distrito Federal.
4Universidade do Estado da Bahia. DTCS/III. s/n.
São Geraldo. CEP: 48.904-711. Juazeiro. Bahia. Programa de Pós-Graduação em
Agronomia/Horticultura Irrigada. PPGHI.
* amesquita@uneb.br
Abstract
The São Francisco
River Valley region in Brazil is a major producer of irrigated melons, facing
stresses due to climate change. New strategies for crop management are
essential to maintain sustainable cultivation. This study aims to evaluate the
characteristics of melons under controlled irrigation deficit (RDI) and the use
of a biostimulant. The experiment followed a completely randomized design with
sub-subdivided plots. The main plots represented water levels: full irrigation
(100% soil water availability - SWA) and deficit levels (80%, 60%, and 40%
SWA). The subplots represented biostimulant application (with and without), and
the sub-subplots represented collection periods: time I (17 to 26 days after
planting - DAP), time II (27 to 36 DAP), and time III (37 to 46 DAP). The
variable analyzed was gas exchange. Water restriction affects melons; however,
some physiological characteristics show greater tolerance, demonstrating an
adaptive response to moderate water deficit (80% SWA), regardless of the
evaluation period. This allows for better water use efficiency. The
biostimulant applied was not effective in promoting adjustments in the
evaluated gas exchanges.
Keywords: Cucumis melo, physiology,
irrigation management
Resumen
En Brasil, el Valle
del Río San Francisco es reconocido como un importante productor de melón bajo
riego. Ante el estrés causado por el cambio climático, es crucial emplear
nuevas estrategias de gestión del cultivo para garantizar su sostenibilidad.
Por lo tanto, el objetivo de este estudio fue evaluar las características del
melón bajo déficit de riego controlado (RDI) y la aplicación de
bioestimulantes. El experimento se realizó en parcelas subsubdivididas. Las
parcelas representaron diferentes niveles de agua: riego completo (100% de
disponibilidad de agua del suelo - SWA) y niveles de déficit (80, 60 y 40%
SWA). La subparcela consideró el uso de bioestimulante (con y sin), mientras
que la subsubparcela abarcó los períodos de recolección: tiempo I (17 días
después de la siembra - DDS hasta 26 DDS), tiempo II (27 a 36 DDS) y tiempo III
(37 a 46 DDS). Las variables analizadas fueron el intercambio gaseoso. El melón
mostró ser afectado por la restricción hídrica; no obstante, ciertas características
fisiológicas mostraron ser más tolerantes, exhibiendo una respuesta adaptativa
atenuada. Específicamente, la aplicación de déficit hídrico moderado (80% SWA),
independientemente de la estación evaluada, permitió un mayor rendimiento en
la eficiencia del uso del agua (UEU). Sin embargo, el bioestimulante no
demostró ser eficiente para promover ajustes en el intercambio gaseoso.
Palabras clave: Cucumis melo, fisiología,
gestión del riego
Originales: Recepción: 28/07/2023 - Aceptación: 27/06/2024
Introduction
Melon (Cucumis
melo L.) is appreciated for its sweet flavor, functional and nutritional
traits, and significant economic value. It thrives in diverse environments and
management practices, particularly in the semi-arid Northeastern Brazil, where
it exhibits year-round growth due to its exceptional productivity (5, 24). The climatic conditions of the São
Francisco River Valley, characterized by high insolation and low rainfall, are
conducive to melon production, fostering high photosynthetic rates and minimal
disease incidence, thereby optimizing melon yields in the region (18).
The mid-region of
the São Francisco River Valley, notably the Juazeiro-Petrolina area, is a
prominent hub for melon production (14).
In response to historically limited water resources from the main reservoirs of
the São Francisco River, producers in these areas have increasingly adopted
agronomic techniques to enhance water use efficiency.
Accurate water
management is crucial to meet the crop’s water requirements throughout its
growth stages, ensuring optimal productivity. Physiological growth analysis
serves as a valuable tool for understanding plant responses under varying
environmental conditions, enabling comparisons across different cultivation
systems (20).
Assessing drought
tolerance requires a comprehensive evaluation of multiple physiological
variables, such as water potential, stomatal conductance, temperature, and leaf
transpiration, which collectively indicate plant performance under water stress
(22). Parameters like transpiration,
stomatal conductance, and photosynthesis directly influence crop growth,
development, and yield, responding to soil water status and climatic variations
(9).
Water use
efficiency (WUE) metrics in agriculture facilitate the assessment of crop
responses to varying water availability conditions. WUE is defined as the ratio
of plant biomass production to the volume of irrigation water applied (11, 12).
Climate change
exacerbates environmental challenges, particularly in semi-arid regions (8), potentially escalating drought
vulnerabilities (7, 16) unless prompt
interventions are implemented. Water scarcity in arid and semi-arid regions,
such as the mid-region of the São Francisco River Valley, significantly impacts
regional development.
Regulated deficit irrigation (RDI) has emerged as a key strategy
in irrigation management, aiming to optimize water use efficiency by subjecting
fruit trees, including melons, to controlled water stress during specific
growth stages (1, 16). RDI entails
applying reduced irrigation water at critical plant growth phases, enhancing
WUE without compromising yield (3, 13, 14, 26, 27).
Various strategies
for applying water deficit alter soil water availability, influencing leaf
temperature variations and thereby affecting gas exchange and carbohydrate
accumulation (31), ultimately influencing
crop growth and productivity (28).
In addition to RDI,
another management approach to mitigate regional climatic impacts involves
biostimulant application. Biostimulants are formulations-comprising synthetic
or natural substances-that promote plant growth and development by enhancing
water and nutrient absorption. They influence vital plant processes, augmenting
growth attributes like chlorophyll content, leaf area (4), carbohydrate levels, and fruit quality (32).
Therefore, this
study aims to evaluate the physiological effects of RDI and biostimulant
application on melons throughout their cultivation cycle in a controlled
environment.
Material
and methods
The research was conducted during November and December in a
shaded environment (50% black mesh) at the experimental area of the Department
of Technology and Social Sciences (DTCS), Campus III, State University of Bahia
(UNEB), located in Juazeiro, BA, Brazil (9°25’09” S, 40°29’13” W, altitude
approximately 368 m). The local climate is classified as Bswh, semi-arid,
according to the Köppen classification, with an average annual rainfall of 540
mm. The experimental setup utilized 5-L containers filled with Fluvic Neosol
soil sampled from the 0-20 cm layer. Chemical analysis of the soil was
conducted at the UNEB Water, Soil and Limestone Laboratory (LASAC), with
results presented in table 1.
Table 1.
Results of the chemical analysis of the soil used in the research.
Tabla
1. Resultados del análisis químico
del suelo utilizado en la investigación.
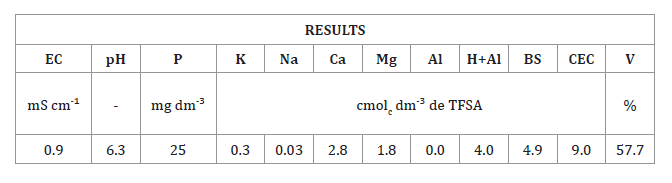
EC:
Electric conductivity; pH: soil pH; P: phosphorus; K: potassium; Ca: calcium;
Mg: magnesium; Al: aluminum; H+Al: potential acidity; BS: Bases sum; CEC:
cation exchange capacity; V: percentage of base saturation.
EC:
conductividad eléctrica; pH: pH del suelo; P: fósforo; K: potasio; Ca: calcio;
Mg: magnesio; Al: aluminio; H+Al: acidez potencial; BS: Suma de bases; CEC:
capacidad de intercambio catiónico; V: porcentaje de saturación de bases.
The experimental
design was completely randomized, consisting of four soil water availability
(SWA) levels (40%, 60%, 80%, and 100% SWA) and three water stress application
periods (time I: 17 to 26 days after planting (DAP); time II: 27 to 36 DAP; and
time III: 37 to 46 DAP), arranged in subdivided plots. Sixteen SWA combinations
were tested for each stress period: 100/100/100, 80/80/80, 60/60/60, 40/40/40,
100/80/80, 100/60/60, 100/40/40, 100/100/80, 100/100/60, 100/100/40, 100/80/100,
100/60/100, 100/40/100, 80/100/100, 60/100/100, and 40/100/100% SWA. During
non-stress periods, irrigation was adjusted to maintain soil water at field
capacity (100% SWA). The subplot factor included the absence or presence of a
biostimulant.
The biostimulant
(300 mL ha-1 concentration), obtained
through biological fermentation of organic compounds, was applied twice: six
days after transplant and pre-flowering. Each experimental unit provided data,
and irrigation depth was calculated for each treatment using graduated
cylinders, applied at two-day intervals.
Seedlings of the
‘Gold Mine’ cultivar, a yellow-type melon belonging to the inodorus group,
were grown in a greenhouse using commercial substrate in polystyrene trays with
128 cells for germination. At 12 DAP, seedlings were transplanted into 5-liter
pots filled with gravel for improved drainage, covered with a fine mesh to
prevent soil loss, and filled with soil.
Following a five-day acclimatization period, water deficit
treatments commenced at 17 DAP. Biostimulant application was individually
administered using a 20-mL syringe in the specified periods. Fertilization was
conducted via fertigation three times per week, tailored for the estimated
plant population based on a 0.3 x 2.0 m spacing.
During
each water stress period, gas exchange parameters including net photosynthesis,
leaf transpiration, stomatal conductance, and leaf temperature were analyzed
using a portable infrared CO2 analyzer (IRGA - LiCOR 6400XT) on
fully expanded leaves, between 10:00 am and 12:00 pm on sunny days. Water use
efficiency (WUE) was calculated as the ratio of photosynthetic rate to
transpiration.
Data
were subjected to analysis of variance (ANOVA) and significant differences were
determined using the Tukey test (p < 0.05). Statistical analyses were
performed using SISVAR 5.6 software (9).
Results and discussion
Despite
previous studies (4, 29) demonstrating
that biostimulants enhance plant metabolism and structure by improving water
and nutrient absorption and tolerance to water stress, the analysis of variance
in this study revealed no significant interaction between treatments or
exclusive differences in evaluated physiological variables compared to RDI. The
lack of biostimulant effects on physiological variables may be attributed to
the cultivation period, which coincided with the hottest time of the year,
likely impacting plant physiological and biochemical characteristics.
Nonetheless, there is limited literature discussing the biostimulant’s
influence on gas exchange parameters.
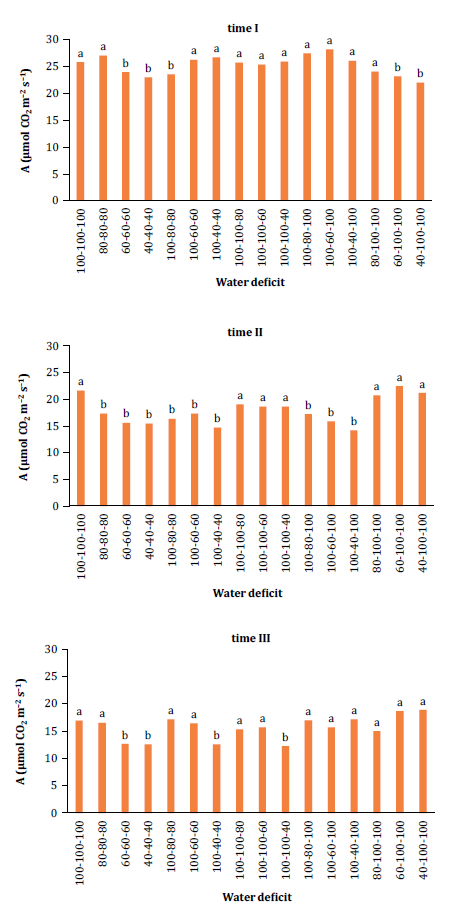
The analysis of photosynthesis data indicated statistical
significance (p < 0.01) and a significant interaction between time and water
deficit levels. Figure 1, illustrates the impact of water
deficit on photosynthesis levels, represented by A - net assimilation rate of
CO2 (μmol CO2 m-2 s-1).
Means
followed by the same letters do not differ at 5% probability level by Tukey’s
test.
Medias
seguidas de letras iguales no difieren al nivel de probabilidad del 5% por la
prueba de Tukey.
Figure
1. Changes in A - net assimilation rate CO2 (μmol CO2 m-2 s-1)
observed at different times (time I: 17 to 26 DAP; time II: 27 to 36 DAP; and
time III: 37 to 46 DAP) in relation to the interaction with water deficit
levels.
Figura
1. Cambios en A - tasa de
asimilación neta de CO2 (μmol CO2
m-2 s-1)
observados en diferentes momentos (Tiempo I: 17 a 26 días después de la siembra
- DAP; Tiempo II: 27 a 36 DAP y Tiempo III: 37 hasta 46 DAP) en relación con la
interacción con los niveles de agua.
Plants irrigated at 100% SWA during times I, II, and III
exhibited minimal reductions in photosynthetic activity. Moderate and severe
water deficits (60% and 40% SWA) at any time resulted in significantly lower
photosynthetic activity. As noted by Vendruscolo et al. (2017), adequate
irrigation enhances internal CO2 concentration in plants; however,
water availability directly limits photosynthesis, with high CO2
concentrations correlating with increased stomatal conductance (gs). Thus,
stomatal closure primarily restricts photosynthesis, as reduced stomatal
apertures hinder CO2 diffusion, a phenomenon observed in this study.
At times I and III,
using 80% SWA showed statistical similarity (p < 0.05) to the control,
indicating no decrease in carbon assimilation or photosynthetic capacity under
moderate water deficit. This finding aligns with previous research (23), which evaluated gas exchange in melon plants
under different irrigation frequencies, and Ferrerira
(2011), which examined water stress in sesame plants, supporting our
results by reporting reduced photosynthetic activity with decreased irrigation
frequency.
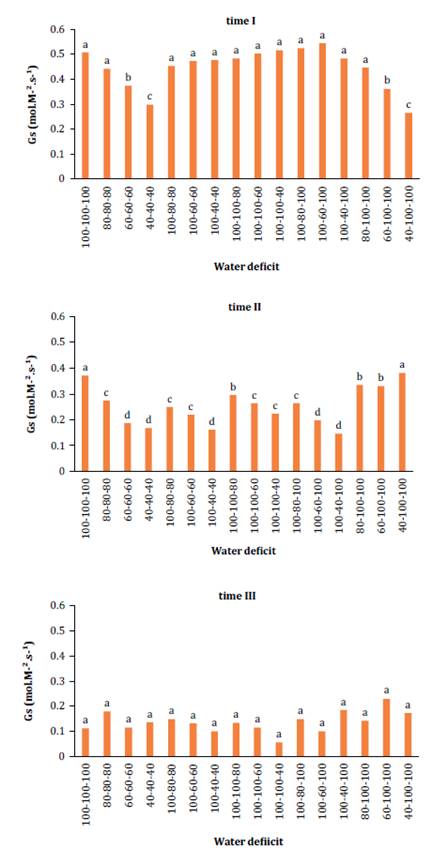
Stomatal
conductance (gs) data (figure 2), analyzed via ANOVA,
revealed a significant interaction between time and water deficit levels.
Examination across time periods indicated a decline in leaf surface water vapor
conductance over time (I, II, and III), correlating with the photosynthesis
data (figure 1), where photosynthetic levels decreased
correspondingly.
Means
followed by the same letters do not differ at 5% probability level by Tukey’s
test.
Medias
seguidas por letras iguales no difieren al nivel de probabilidad del 5% por la
prueba de Tukey.
Figure
2. Changes in stomatal conductance - (gs) (mol m-2 s-1)
at different times (time I: 17 to 26 DAP; time II: 27 to 36 DAP; and time III:
37 to 46 DAP) in relation to the interaction with water deficit levels.
Figura
2. Cambios en (gs) - conductancia estomática (mol m-2 s-1)
en diferentes tiempos (Tiempo I: 17 a 26 días después de la siembra - DAP;
Tiempo II: 27 a 36 DAP y Tiempo III: 37 a 46 DAP) en relación con la
interacción de los niveles de déficit hídrico.
During time I,
treatments maintaining 100% SWA exhibited the highest averages. The treatment
with 80% SWA statistically mirrored (p < 0.05) the control, allowing higher
water vapor conductance and photosynthesis levels (27.01 μmol.m2.s-1). Maximum and
minimum gs values per time period were as follows: 100% SWA (0.544 mol.m2.s-1)
and 40% SWA (0.264 mol.m2.s-1).
Time II showed a similar pattern to time I, with highest conductance
values in non-stressed plants and lowest in stressed plants: 100% SWA (0.381
mol.m2.s-1) and 40% SWA
(0.162 mol.m2.s-1), respectively.
This aligns with findings by Vendruscolo et al. (2017),
who reported increased gs with higher water availability in eggplants. However,
during time III, no significant differences in water deficit levels were
observed. As noted by Melo et al. (2014), studying gas exchanges aids in understanding melon responses
to soil water deficits and quantifying species’ acclimation to adverse
conditions. Reductions in gs throughout the phenological cycle reflect
photosynthetic adjustments, as melon development (15 DAP: vegetative, 30 DAP:
flowering, 45 DAP: fruiting) increases leaf area and stomatal density,
enhancing gas exchange efficiency. Without this efficiency, both melon types
risk excessive water loss, potentially leading to dehydration and hindered
growth.
Post the fourth
week under RDI, temporal variations significantly decreased compared to
controls (17), showing stress-induced
reductions in gs due to lowered leaf water potential. Figure 3,
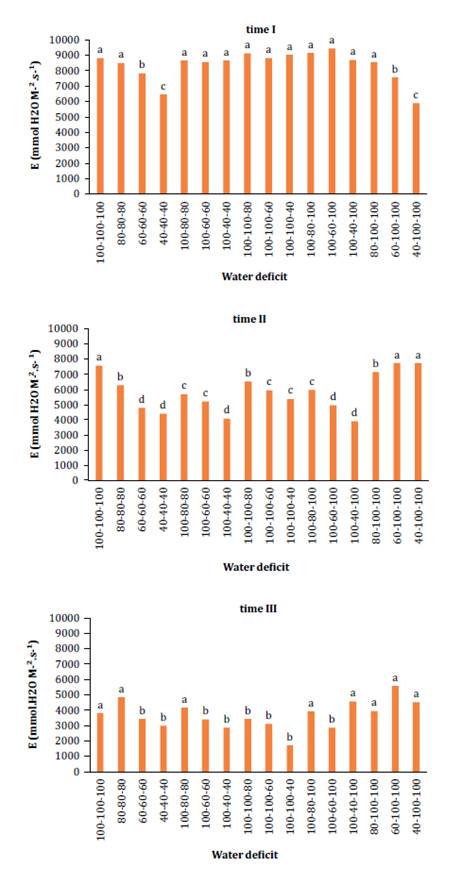
depicts leaf transpiration levels.
Means
followed by the same letters do not differ at 5% probability level by Tukey’s
test.
Medias
seguidas de letras iguales no difieren al nivel de probabilidad del 5% según la
prueba de Tukey.
Figure
3. Changes in transpiration - (E) (mmol H2O
m-2 s-1)
at different times (time I: 17 to 26 DAP; time II: 27 to 36 DAP; and time III:
37 to 46 DAP) in relation to the interaction with water deficit levels.
Figura
3. Cambios en transpiración - (E)
(mmol H2O
m-2 s-1) en
diferentes tiempos (Tiempo I: 17 a 26 días después de la siembra - DAP; Tiempo
II: 27 a 36 DAP y Tiempo III: 37 a 46 DAP) al interactuar con los niveles de
déficit hídrico.
According to Lamaoui et al. (2018a), stomatal closure results
from reduced osmotic-water potential. Results also indicated melon’s limited
ability to maintain adequate leaf water potential, a trait some cultivars
manage due to biochemical characteristics that sustain photoassimilate
transport from shoots to roots, enabling enhanced water absorption (25).
For the variable
transpiration, there was a double-factor interaction for times and levels of
water deficit (figure 3).
At time I, plants
under 100% SWA exhibited higher transpiration rates, while those under moderate
deficit (80% SWA) were statistically similar (p < 0.05) to the control.
There was a slight reduction of 3.79% in maximum transpiration capacity
compared to the control treatment. By time II, plants irrigated at 100% SWA
continued to display higher transpiration rates, whereas those under water
stress exhibited significant reductions. Specifically, the moderate deficit
treatment (80% SWA) showed approximately a 20.89% decrease in transpiration
compared to its own performance at time I. Time III data indicated a decline in
transpiration rates as plants matured, though the pattern observed in previous
periods persisted. Water deficit consistently resulted in decreased
transpiration rates, with non-stressed treatments exhibiting the highest rates.
Notably, the moderate deficit treatment (80/80/80) did not significantly differ
at the 5% probability level from the control. Previous studies (unpublished
data) on melon physiology under varying irrigation levels suggest that applying
an 80% deficit does not reduce transpiration rates.
Research by Elmaghrabi et al. (2017), on the Sancho melon
cultivar suggested that this response might be attributed to melon metabolism,
which can maintain efficient photosynthesis with reduced stomatal opening and
lower intercellular CO2 levels without
compromising water use efficiency (WUE). Under optimal water conditions, melons
exhibit higher transpiration rates correlated with stomatal conductance (gs),
as stomata serve as the primary avenue for water loss, essential for water and
mineral absorption, CO2 uptake for
photosynthesis, growth, and plant cooling (23).
According to Lamaoui et al. (2018a), melon’s
high gs under normal conditions results from increased stomatal aperture,
leading to higher transpiration rates. Conversely, under stress conditions,
both transpiration rates and gs decrease. The authors noted that adequate
irrigation induces stomatal opening, enhancing melon photosynthetic rates and
mitigating stress effects.
Reducing irrigation
by 50% compared to conventional methods led to reduced transpiration rates
primarily due to stomatal closure, a phenomenon observed in various plant
species. According to Lamaoui et al. (2018b),
hormonal signaling in shoot long-distance signaling and hydraulic conductivity
control explain changes in stomatal activity.
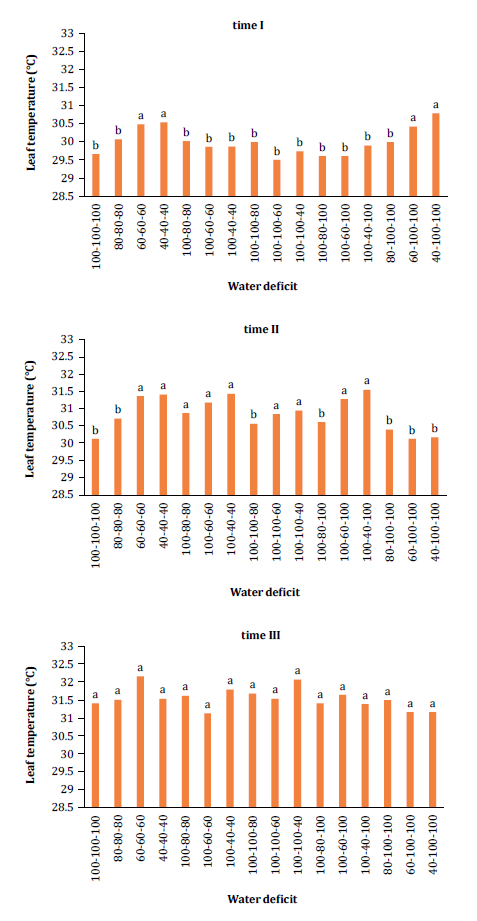
Significant
interaction effects were observed in leaf temperature results (figure
4).
Means
followed by the same letters do not differ at 5% probability level by Tukey’s
test.
Medias
seguidas de letras iguales no difieren al nivel de probabilidad del 5% según la
prueba de Tukey.
Figure
4. Changes in leaf temperature (oC) at different times (time I: 17 to 26 DAP;
time II: 27 to 36 DAP; and time III: 37 to 46 DAP) in relation to the
interaction with water deficit levels.
Figura
4. Cambios en la temperatura foliar (oC) en
diferentes momentos (Tiempo I: 17 a 26 días después de la siembra - DAP; Tiempo
II: 27 a 36 DAP y Tiempo III: 37 a 46 DAP) al interactuar con los niveles de
déficit hídrico.
Analysis across
time periods (I, II, and III) indicated a gradual increase in leaf temperature
over time, driven by rising ambient temperatures typical of November and
December, the study period. During times I and II, most treatments receiving
100% SWA showed lower leaf temperatures compared to water deficit treatments
(60% and 40% SWA). Notably, plants under 80% SWA did not exhibit increased leaf
temperatures, contrasting with other stressed treatments and statistically
equating to the control (p < 0.05). Similar findings were reported by Vieira et al. (2019), in studies on melon plants
subjected to various water stress levels, where conductance and transpiration
closely influenced leaf cooling. Water deficit indirectly raises leaf
temperature by limiting cooling mechanisms through chemical signaling that
prompts stomatal closure.
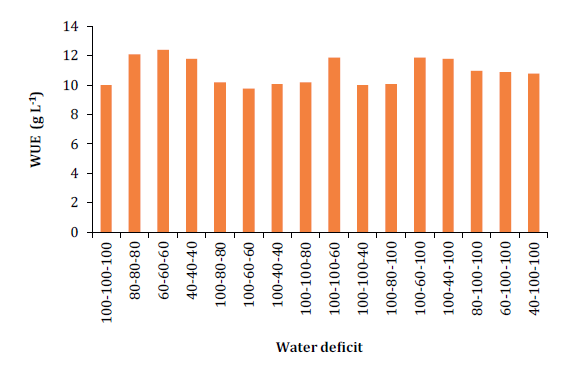
The WUE graph (figure 5) illustrates how WUE is directly influenced by water
deficit.
Figure 5. Variation in
water use efficiency (WUE) of melon plants in relation to different water
deficits.
Figura 5. Variación en la
eficiencia del uso del agua (WUE) de las plantas de melón en interacción con
diferentes déficits hídricos.
Full irrigation (100/100/100) resulted in lower WUE compared to
treatments subjected to higher water stress levels. Comparing the WUE of the
40/40/40 treatment with the control, there was a 14.7% increase, while the
60/60/60 treatment showed a 25.2% superiority in WUE over the control. Similar
results were reported by Al-Mefleh et al. (2012)
and Nascimento et al. (2011), in studies on
melon subjected to varying irrigation depths (50%, 75%, 100%, and 125% ETc),
highlighting that lower irrigation levels generally lead to higher WUE values.
Conclusion
Water restriction
impacts melon plants, but certain physiological traits exhibit greater
tolerance and adaptive responses, notably under moderate water deficit (80%
SWA), irrespective of the season. Consequently, this approach enhances water
use efficiency (WUE).
However, the biostimulant applied during the cultivation period
does not effectively induce adjustments in gas exchange.
1.
Ahmadi-Mirabad, A.; Lotfi, M.; Roozban, M. R. 2014. Growth, yield, yield
component sand water-use efficiency in irrigated cantaloupe sunder fulland
deficit irrigation. Electronic Journal of Biology. 10(3): 79-84.
2.
Al-Mefleh, N. K.; Samarah, N.; Zaitoun, S.; Al-Ghzawi, A. A. 2012. Effect of
irrigation levels on fruit characteristics, total fruit yield and water use
efficiency of melon under drip irrigation system. Journal of Food, Agriculture
& Environment. 10(2): 540-545. https://www. researchgate.net/publication/233859372
3.
Bassoi, L. H.; Gonçalves, S. O.; Santos, A. R. L.; Silva, J. A.; Lima, A. C. M.
2011. Influência de manejos de irrigação sobre aspectos de ecofisiologia e de
produção da videira cv. Syrah/Paulsen 1103. Irriga. 16(4): 395-402. DOI:
10.15809/irriga.2011v16n4p395
4.
Batista, G. S.; Silva, J. L.; Rocha, D. N. S.; Souza, A. R. E.; Araújo, J. F.;
Mesquita, A. C. 2019. Crescimento inicial do meloeiro em função da aplicação de
biofertilizantes no cultivo orgânico. Revista Brasileira de Agropecuária
Sustentável. 9(2): 24-32. DOI: 10.21206/rbas.v9i2.3072
5.
Bigaran Aliotte, J. T.; Ramos de Oliveira, A. L. 2022. Multicriteria decision
analysis for fruits and vegetables routes based on the food miles concept.
Revista de la Facultad de Ciencias Agrarias. Universidad Nacional de Cuyo.
Mendoza. Argentina. 54(1): 97-108. DOI: https:// doi.org/10.48162/rev.39.069
6.
Elmaghrabi, A. M.; Rogers, H. J.; Francis, D.; Ochatt, S. J. 2017. PEG induces
high expression of the cell cycle checkpoint gene WEE1 in embryogenic callus of
Medicago truncatula: Potential link between cell cycle checkpoint
regulation and osmotic stress. Front. Plant Sci. 8: 1479.
7.
FAO - A agricultura irrigada pode contribuir no aumento da produção de
alimentos no Brasil. 2018. http://www.fao.org/brasil/noticias/detail-events/pt/c/1110333/.
(Access: 10 may. 2021).
8.
Faria, L. N.; Soares, A. A.; Donato, S. L. R. 2016. Irrigação com déficit
hídrico controlado na cultura da mangueira no semiárido baiano. Engenharia
Agrícola, Jaboticabal. 36(3): 387-398.
9.
Feitosa, S. S.: Albuquerque, M. B.; Oliveira, A. P. 2016. Fisiologia do Sesamum
indicum L. sob estresse hídrico e aplicação de ácido salicílico. Irriga,
Botucatu. 21(4): 711-723. DOI: 10.15809/ irriga.2016v21n4p711-723
10.
Ferreira, D. F. 2011. Sisvar: um sistema computacional de análise estatística.
Ciência e Agrotecnologia. 35(6), 1039-1042. DOI:
10.1590/S1413-70542011000600001
11.
Frizzone, J. A.; Souza, F.; Lima, S. C. R. V. 2015. Manejo de Irrigação:
quando, quanto e como irrigar. Curso de capacitação. Brasília: Agencia Nacional
das Águas-ANA. 62 p.
12.
Ghrab, M.; Ayadi, M.; Gargouri, K.; Chartzoulakis, K.; Gharsallaoui, M.;
Bentaher, H.; Psarras, G.; Mimoun, M. B.; Masmoudi, M. M.; Mechlia, N. B. 2014.
Long-term effects of partial rootzone drying (PRD) on yield, oil composition
and quality of olive tree (cv. Chemlali) irrigated with saline water in arid
land. Journal of Food Composition and Analysis. 36(1): 90-97. DOI:
10.1016/j.jfca.2014.05.005
13.
Hortifruit-Anuário Brasileiro de Horti & Fruti. 2020. In: Melão, p. 88.
http:// www.editoragazeta.
com.br/sitewp/wpcontent/uploads/2020/05/HORTIFRUTI_2020. (Acces: 10 may. 2021).
14.
Lacerda, F. H. D.; Pereira, F. H. F.; da Silva Neves, D.; da Costa Borges, F.
Q.; Júnior, J. E. C. 2012. Aplicação exógena de prolina na redução do estresse
salino em meloeiro. Revista Verde de Agroecologia e Desenvolvimento
Sustentável. 7(3): 218-227.
15.
Lamaoui, M.; Chakhchar, A.; Kharrassi, E. L.; Wahbi, Y. S.; Modafar, C. E.
2018a. Morphological, physiological, and biochemical responses to water stress
in melon (Cucumis melo) subjected to regulated deficit irrigation (RDI)
and partial rootzone drying (PRD). J. Crop Sci. Biotechnol. 21: 407-416. DOI:
10.1007/s12892-018-0122-0
16.
Lamaoui, M.; Jemo, M.; Datla, R.; Bekkaoui, F. 2018b. Heat and drought stresses
in crops and approaches for their mitigation. Frontiers and Chemistry. 6(26):
1-14. DOI: 10.3389/ fchem.2018.00026
17.
Lima, D. S.; Simões, W. L.; Silva, J. A. B.; Amorim, M. N.; Salviano, A. M.;
Costa, N. D. 2020. Pele de sapo’ melon grown under different irrigation depths
and bioestimulant rates in the Semi-arid region of Brazil. Comunicata
Scientiae. 11: e3303. DOI: 10.14295/CS.v11i0.330
18.
Melo, A. S.; Dias, V. G.; Dutra, W. F.; Dutra, A. F.; Sá, F. V. S.; Brito, M.
E. B.; Viégas, P. R. A. 2020. Physiology and yield of piel de sapo melon (Cucumis
Melo L.) under water deficit in semi-arid region, Brazil. Bioscience
Journal. 36(4): 1251-1260. DOI: 10.14393/BJ-v36n4a2020-48168
19. Melo, D. M.; Charlo, H. C. O.;
Castoldi, R.; Braz, L. T. 2014. Dinâmica do crescimento do meloeiro rendilhado
‘Fantasy’ cultivado em substrato sob ambiente protegido. Revista Biotemas.
27(2): 19-29. DOI: 10.5007/2175-7925.2014v27n2p19
20.
Melo, J. M. M.; Marinho, L. B.; Vargens, F. N.; Sousa Filho, J. R.; Deon, M.
D.; Melo, A. M. Y. 2017. Crescimento de meloeiro submetido ao estresse hídrico
com e sem micorrização no Vale do Submédio São Francisco. Revista Brasileira de
Agricultura Irrigada-RBAI. 11(2): 1261 -1270. DOI: 10.7127/rbai.v11n200595
21.
Nascimento, S. P.; Bastos, E. A.; Araújo, E. C. E.; Filho, F. R. F.; Silva, E.
M. 2011. Tolerância ao déficit hídrico em genótipos de feijão-caupi. Revista
Brasileira de Engenharia Agrícola e Ambiental. 15(8): 853-860. DOI:
10.1590/S1415-43662011000800013
22.
Pereira filho, J. V.; Bezerra, F. M. L.; Chagas, K. L.; Silva, T. C.; Pereira,
C. C. M. S. 2015. Trocas gasosas e fitomassa seca da cultura do meloeiro
irrigado por gotejamento nas condições semiáridas do nordeste. Revista
Brasileira de Agricultura Irrigada. 9(3): 171-182. DOI: 10.7127/rbai. v9n300286
23.
Pires, M. M. M. L.; Santos, H. A.; Santos, D. F.; Vasconcelos, A. S.; Aragão,
C. A. 2013. Produção do meloeiro submetido a diferentes manejos de água com o
uso de manta de tecido não tecido. Horticultura Brasileira. 31: 304-10. DOI:
10.1590/S0102-05362013000200021
24.
Rewald, B.; Raveh, E.; Gendler, T.; Ephrath, J. E.; Rachmilevitch, S. 2012.
Phenotypic plasticity and water flux rates of Citrus root orders under
salinity. Journal of Experimental Botany. 63(7): 2717-2727.
25.
Romero-Conde, A.; Kusakabe, A.; Melgar, J. C. 2014. Physiological responses of
citrus to partial rootzone drying irrigation. Scientia Horticulturae. 169(1):
234-38. DOI: 10.1016/j. scienta.2014.02.022
26.
Sampaio, A. H. R.; Filho, M. A. C.; Coelho, E. F.; Daniel, R. 2014. Indicadores
fisiológicos da lima ácida tahiti submetida à irrigação deficitária com
secamento parcial de raiz. Irriga, Botucatu. 19(2): 292-301. DOI: 10.15809/irriga.2014v19n2p292
27.
Santos, A. R.; Donato, S. R. L.; Coelho, E. F.; Junior, P. R. F. C.; Castro, I.
N. 2016. Irrigation deficit strategies on physiological and productive
parameters of ‘Tommy Atkins’ mango. Revista Caatinga. 29(1): 173-182. DOI:
10.1590/1983-21252016v29n120rc
28.
Silva, F. G.; Dutra, A. F.; Oliveira, I. M.; Filgueira, L. M. B.; Melo, A. S.
2015. Trocas gasosas e fluorescência da clorofila em plantas de berinjela sob
lâminas de irrigação. Revista Brasileira de Engenharia Agrícola e Ambiental.
19(10): 946-952. DOI: 10.1590/1807- 1929/agriambi.v19n10p946-952
29.
Vendruscolo, E. P.; Martins, A. P. B.; Seleguini, A. 2016. Promoção no
desenvolvimento de mudas olerícolas com uso de bioestimulante. Journal of
Agronomic Sciences, Umuarama. 5(2): 73-82.
30.
Vendruscolo, E. P.; Rabelo, R. S.; Campos, L. F. C.; Martins, A. P. B.;
Semensato, L. R.; Seleguini A. 2017. Alterações físico-químicas em frutos de
melão rendilhado sob aplicação de bioestimulante. Revista colombiana de
ciencias hortícolas. 11(2): 459-463. DOI: 10.17584/ rcch.2017v11i2.7413
31.
Vieira, D. A.; Mesquita, A. C.; Marinho, L. B.; Souza, V.; Aidar, S. T.;
Carvalho, M. M. P. 2019. Gas exchanges of melon under water stress in the
Submedium region of the São Francisco River Valley. Acta Scientarum Agronomy.
41(13): e42686. DOI: 10.4025/actasciagron. v41i1.42686
32. Vieira, D. A.; Carvalho, M. M. P.;
Rodrigues, B. A.; Marinho, L. B.; Mesquita, A. C. 2020. Metabolic behavior in
the allocation of biomass of melon cultivars under water deficit conditions.
Research, Society and Development. 9(7): 1-19. DOI: 10.33448/rsd-v9i8.5128