Revista de la Facultad de Ciencias
Agrarias. Universidad Nacional de Cuyo. Tomo 56(1). ISSN (en línea) 1853-8665.
Año 2024.
Original article
Agrochemical
characterization of Vitis labrusca grape pomace and its effect on a
soil-plant system
Caracterización
agroquímica del orujo de uva de Vitis labrusca y su efecto sobre el
sistema suelo-planta
Hugo Merani
Victor2,
Alejandra
Bárcena3,
María Gabriela
Cano3,
María Florencia
Vianna1
1Universidad
Nacional de La Plata (UNLP). Facultad de Ciencias Naturales y Museo. Instituto
de Botánica Carlos Spegazzini. 122 y s/n. 1900 La Plata. Argentina.
2Universidad
Nacional de La Plata (UNLP). Facultad de Cs. Agrarias y Forestales. Centro de
Investigación de Suelos para la Sustentabilidad Agropecuaria y Forestal (CISSAF)
119 y 60. 1900 La Plata. Argentina.
3Universidad
Nacional de La Plata (UNLP). Instituto de Fisiología Vegetal (INFIVE). CCT-La
Plata-Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) Diag.
113 y 61. CC 327. 1900 La Plata. Argentina.
*mi.troncozo@conicet.gov.ar
Abstract
This study
characterized the agrochemical properties of V. labrusca grape pomace
(GP) and evaluated the effect on the rhizobacteria Azospirillum brasiliense and
horticultural crops, determining safety as fertilizer and/or mulching. Two
first bioassays were performed with the GP at different concentrations
evaluating toxicity on A. brasiliense, and on tomato and lettuce seeds.
A third bioassay evaluated GP mulching effects on tomato and lettuce plants
growing with amounts varying between 20 and 80 t ha-1. Agrochemical characterization
showed that GP is rich in potassium and phosphorus, with a low content of Na+
salts (SAR < 15). The GP at 2.5% (w v-1) significantly increased
survival rates of N2-fixing rhizobacteria. Results on seed
germination revealed lettuce was more susceptible to increasing GP
concentrations. The application of 20 t ha-1 of GP in greenhouse
experiments increased lettuce and tomato root biomass. Furthermore, the aerial
part of tomato showed no toxicity symptoms. These results open the possibility
of considering V. labrusca GP as mulching without prior treatment in
tomato crops.
Keywords: agrowaste,
revalorization, macronutrients, Azospirillum brasiliense, tomato,
lettuce
Resumen
Este estudio
tuvo como objetivo caracterizar las propiedades agroquímicas del orujo de uva
(GP) de V. labrusca y evaluar su efecto sobre Azospirillum
brasiliense y cultivos hortícolas para determinar su uso seguro como
fertilizante y/o mulching. Los bioensayos se realizaron con el GP a diferentes
concentraciones para evaluar su toxicidad sobre A. brasiliense y semillas
de tomate y lechuga. Para evaluar el efecto de GP sobre el crecimiento de
plantas de tomate y lechuga se aplicaron diferentes dosis como mulching en el
rango de 20-80 t ha-1. La caracterización agroquímica mostró que GP
es rico en potasio y fósforo; y tiene bajo contenido de sales de Na+
(SAR < 15). El GP al 2,5% (p v-1) fue responsable de un aumento
significativo en la tasa de supervivencia de las rizobacterias fijadoras de N2.
Los resultados de la germinación de las semillas revelaron que la lechuga fue
más susceptible al aumento de las concentraciones de GP. La aplicación de 20 t
ha-1 de GP en los experimentos de invernadero incrementó la biomasa
radicular de lechuga y tomate. Además, la parte aérea del tomate no presentó
síntomas de toxicidad. Estos resultados abren la posibilidad de considerar el
aprovechamiento de V. labrusca GP como mulching, sin utilizar ninguna tecnología
de tratamiento antes de su aplicación al suelo en cultivos de tomate.
Palabras claves:
residuo
agronómico, revalorización, macronutrientes, Azospirillum brasiliense, tomate,
lechuga
Originales:
Recepción: 19/08/2023 - Aceptación: 28/02/2024
Introduction
The wine
industry is one of the oldest and most important in the world. In 2019, wine
production was estimated at around 258 Mhl (27). Grapes for
winemaking belong to the Genus Vitis sp, being V. vinifera and V.
labrusca the two main producing species (20). V.
vinifera is cultivated in arid soils, poor in organic matter, while the
hybrid V. labrusca originated in eastern USA, grows in different
edaphoclimatic regions and is highly resistant to diseases (18,
24).
In South America, mainly in Argentina and Brazil, V. labrusca cultivated
areas have increased in the last decade (24, 40).
Annually, the
wine industry produces 13 x 106 t of solid waste or by-products
worldwide, with grape pomace (GP) being the most abundant (10-13 Mt per year).
In terms of circular economy, GP could generate new products of different
values (7, 21), closing the
cycle as mulching or organic fertilizer due to its lignocellulosic nature (8). Mulching
improves soil structure, keeps moisture, moderates temperature, and increases
organic matter (OM), returning soil nutrient cycling environments to pristine
soil patterns (25). On the other
hand, when the agronomic purpose is to increase crop yield, organic fertilizers
of plant origin constitute an interesting agroecological alternative for
intensive crops like vegetables, flowers, and orchards (22). Nevertheless,
a proper agronomic management program incorporating lignocellulosic
by-products, such as GP, needs to characterize the product and establish an
appropriate use. Unlike the well-known physicochemical characteristics of V.
vinifera GP (6, 16, 34), the relevant
agrochemical properties of V. labrusca GP have not been studied yet.
Direct incorporation of V. vinifera GP into the soil could negatively
affect plant growth due to inhibitory compounds or given competition between
soil microorganisms for essential nutrients such as nitrogen (6). However, this
study only considers GP physicochemical properties, leaving aside other
sensitive parameters in the soil-plant systems.
Biological
indicators are living organisms, such as plants, animals, and/or microorganisms
exploited to detect toxic substances in terrestrial and aquatic ecosystems (45). The effect of
agrowastes on the soil-plant system can be evaluated by lettuce (Lactuca
sativa) and tomato (Solanum lycopersicum) as biological indicators,
as well as by the free-soil nitrogen-fixing bacterium Azospirillum
brasiliense (14, 36, 37, 41). The combination
of agrochemical characterization together with biological indicators enables
the development of appropriate agronomic management programs (22).
This study
characterized the agrochemical properties of V. labrusca var. Isabella
GP and evaluated toxicity effects on A. brasiliense, tomato and
lettuce determining GP safety as fertilizer or/and mulching.
Material
and methods
Study
area and GP sampling
The study
considered twenty-four ha of V. labrusca var Isabella at
“Cooperativa de la Costa’’ wine-cellar located in Berisso, Buenos Aires,
Argentina (34°53’22.79” S; 57°49’21.11” W (28). The soil is
Rendzic Leptosol (11) with an
A-horizon of 20-40 cm and a high content of well-humified organic matter, under
which there is an AC or C-horizon constituted by layers of small shells (1). In 2013, the
winery produced 350 hL of wine and a total of 12 t of GP, representing 25% of
grape total weight (29).
Samplings were
carried out in 2015, 2016 and 2017 seasons. Each year, 10 random GP samples
were collected immediately after grape pressing and blended to obtain a
compound sample. One part of the sample was separated to determine moisture
content. The rest was oven-dried at 60°C to constant weight and then divided
into sub-samples. These were stored in hermetically sealed in containers until
GP physicochemical characterization. Part of fresh material from the 2017
campaign was separated and stored at -20°C for subsequent bioassays.
Agrochemical
GP characterization
Ashes and OM
were determined by calcination (6). Percentage
contents of organic carbon (C) and total nitrogen (N) were determined after Walkley
and Black (1934)
and micro-Kjeldahl methods (5) respectively,
and the C/N ratio was calculated. Total phosphorus (P) was determined by the
yellow vanadate-molybdate method (19). Extractable
and exchange cations were obtained by the saturated paste extraction method and
the Ammonium acetate method 1N pH7, respectively (35). Calcium (Ca+2)
and Magnesium (Mg+2) were estimated by complexometry with 0.02 N
EDTA; Sodium (Na+) and Potassium (K+) by flame
photometry. Sodium adsorption ratio (SAR) was calculated using the following
equation (31) (eq.
1).
SAR
= (Na+ ext) / [(Ca+2 ext + Mg+2 ext /2]1/2
(1)
The Mg+2/K+,
Ca+2/Mg+2, and Ca+2/K+ ratios were
calculated using the obtained values for individual exchangeable cations (17). Cation
exchange capacity (CEC) was determined by steam distillation and percentage
base saturation (S) was calculated by eq. 2 (33).
S
(%) = (Σ exchangeable cations/CEC) *100 (2)
Electric
conductivity (EC) and pH values were measured in the aqueous soluble fraction
(1:5 ratio) by conductometry and potentiometry, respectively (19). Moisture
content (%) was determined by oven-drying samples at 60°C until constant weight
and calculating the difference between wet and dry weight. Water-soluble
phenols (WSP) were determined as described by Osono and Takeda
(2001).
Three technical replicates were used to determine each physicochemical
parameter. Values were averaged to obtain a unique value for each year. Mean,
range and t variance were estimated with three annual values. The
classification criterion proposed by Fernández
Linares et al. (2006) and Havlin et al.
(1999)
allowed interpretation.
Bioassays
with GP application
Three bioassays
determined GP safety as fertilizer or/and mulching: a) GP toxicity evaluation
on a N fixing microorganism (A. brasiliense), b) GP toxicity evaluation
on germination of tomato and lettuce seeds; and c) GP effect on growth
parameters of tomato and lettuce plants.
a)- GP toxicity
evaluation on a N fixing microorganisms (A. brasiliense)
GP effects on
survival of A. brasiliense CECT 590 T were evaluated according to Saparrat
et al. (2010).
A bacterial suspension (100 μl) was inoculated in 900 μl of the GP sterilized
by filtration (0.22 μm millipore membrane) at different concentrations: 2.5,
3.5, 5, and 10% (w v-1). Controls were made using sterile distilled
water and 4 replicates per treatment were utilized. Cultures were grown at 28°C
and 150 rpm for 24 h. Colony-forming units (CFU mL-1) were estimated
by the dilution and plating method using the selective medium Congo red. The
data were transformed to logarithms and analyzed by ANOVA and a Tukey test (p
< 0.05).
b)- GP toxicity
evaluation on germination of tomato and lettuce seeds
GP phytotoxicity
was evaluated after the effects observed on germination and root growth of
lettuce and tomato according to Tiquia et al.
(1996)
with modifications. Seeds of each species were placed on filter paper inside
Petri dishes (9 mm diameter) in contact with 3 ml of the GP at different
concentrations: 2.5, 5, 10, and 20% w v-1. Negative and positive
controls consisted of paper soaked with sterile distilled water and 1 M of a
CuSO4 solution, respectively. Four replicates per treatment were
incubated at 25°C for 7 days in the dark. Number of germinated seeds and root
length (mm) were determined. Relative germination percentage (G) (eq.
3),
relative root length (RL) (eq. 4), and germination index (GI) (eq.
5)
were calculated using the following equations:
RL
(%) = (mean root length at X GP concentration/ mean root length in negative
control) *100 (4)
A 2 mm primary
root defined seed germination. The data obtained were analyzed by ANOVA and
Tukey test (p < 0.05). Percentual data were transformed using arc sen
√p, before statistical analysis.
c) GP effect on
growth of tomato and lettuce plants
The effect of GP
on lettuce and tomato plants was determined using ten plants per treatment and
according to Sampedro et al. (2004) with modifications.
Seedlings with three expanded leaves were placed in 5 L pots filled with a
mixture of soil and sand in a 1:1 ratio (v v-1). GP mulching was
added to the pots at different doses: 20, 40, and 80 t h-1 (wet
weight equivalent to 60, 120 and 240 g of GP per pot). The plants were grown
for 1 month in a greenhouse and periodically watered. Pots without GP were set
up as control. The number of expanded leaves per plant was estimated by direct
counting. Leaf greenness (GrI) was measured using a portable chlorophyll meter
(SPAD-502, Minolta Corp. Japan), randomly selecting three expanded leaves per
plant. Total leaf area per plant was captured by photography and analyzed using
J image software (38). Plants were
harvested and soil adhered to roots was washed with running water. The aerial
part of the plants and roots were oven-dried at 60°C to constant weight
determining aerial and root dry biomass. The data were analyzed for each plant
species comparing dose effects. A Kruskal-Wallis test and a non-parametric
multiple contrast as post hoc test (p < 0.05) analyzed the
number of expanded leaves. The remaining data were analyzed by ANOVA and Tukey
test (p < 0.05).
Results
Agrochemical
GP characterization
The C/N ratio
< 25 suggested an equilibrate decomposition of OM. Among extractable
cations, GP showed high levels of K+ and low Na+, high
CEC (> 40), and a varied macronutrient supply (exchangeable cations, high
content of K+, followed by Ca+2, and low Mg+2).
The GP exhibited a high base saturation index (S > 40), high EC (> 4 dS m-1),
extremely acidic pH, and high hygroscopicityy (> 70% humidity, table
1).
Table
1. Physicochemical characteristics of V.
labrusca var. isabella GP.
Tabla 1. Características
fisicoquímicas del OU de V. labrusca var. isabella.
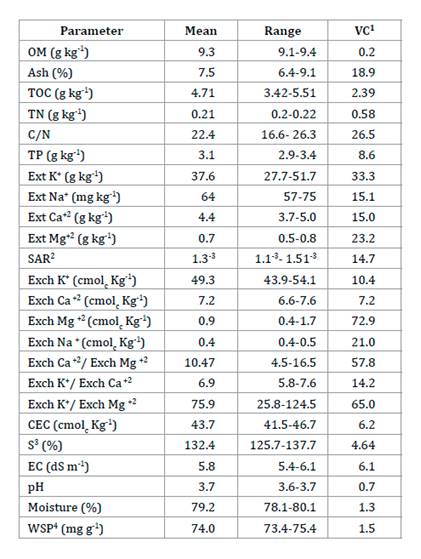
1, Variance coefficient. 2, Sodium adsorption ratio.
3, Base saturation index. 4, Water-soluble phenols. Extractable (Ext) and
interchange (Exch) cations. n= 3 annual samples.
1, Coeficiente de variación. 2, Radio de adsorción
de sodio. 3, Índice de saturación de bases. 4, Fenoles hidrosolubles. Cationes
extractables (Ext) e intercambiables (Exch). n= 3 muestras anuales.
GP
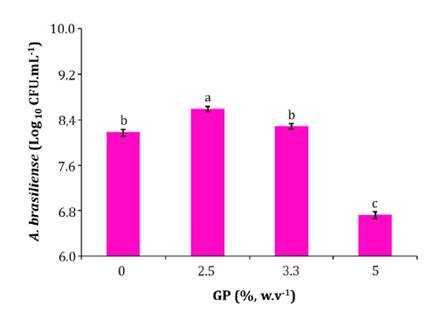
toxicity evaluation on a N fixing microorganism (A. brasiliense)
GP
concentrations showed significant differences in A. brasiliense growth
(F= 684.50; p <0.001). No CFU of the diazotrophic bacteria was
recovered at 10% GP concentration (figure 1).
Means followed by the same letter are not
significantly different (Tukey test, p < 0.05).
Los datos son medias de cuatro réplicas ± D.E.
Medias seguidas por la misma letra no presentan diferencias significativas
(Test de Tukey, p < 0,05).
Figure 1. A.
brasiliense growth after 24
h of incubation in several concentrations of GP (%, w v-1). The data
are means of four replicates ± S.D.
Figura 1.
Crecimiento de A. brasiliense después de 24 h de incubación en distintas
concentraciones de OU (%, p v-1).
GP
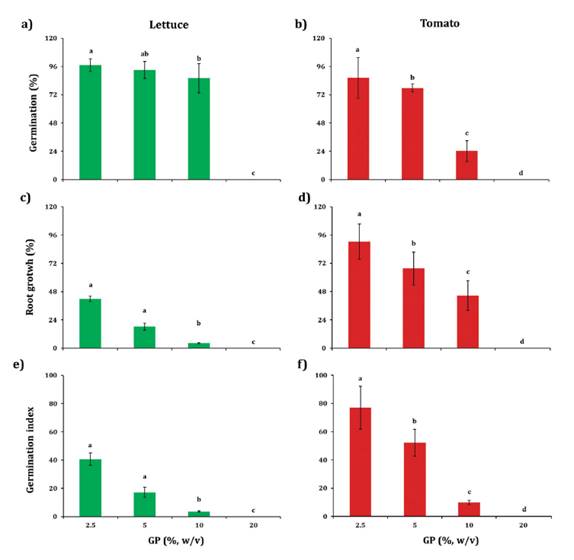
toxicity evaluation on germination of tomato and lettuce seeds
Significant
differences were found in relative germination (G) (F= 86.04; p <0.001),
relative root length (RL) (F= 583.56; p <0.001) and germination index
(GI) (F= 348.69; p <0.001) when lettuce seeds were incubated at
different GP concentrations. The G values at 2.5, 5 and 10% GP concentration
equalled or exceeded 90%. The RL and GI, registered values below 50% indicating
a toxic effect of GP at all concentrations evaluated (figure 2a, c, e).
Mean ± S.D. of four replicates followed by the same
letter are not significantly different (Tukey test, p < 0.05).
Los datos son medias de cuatro réplicas ± D.E.
Medias seguidas por la misma letra no presentan diferencias significativas
(Test de Tukey, p < 0,05).
Figure 2.
Effect of GP addition on germination, root length and germination index of
lettuce and tomato plants.
Figura 2. Efecto
de la adición del OU sobre la germinación, longitud radicular e índice de
germinación de plantas de lechuga y tomate.
For tomato,
significant differences were found in G (F= 368.32; p <0.001), RL (F=
145.96; p <0.001) and GI (F= 278.02; p <0.001). The toxic
effect in G, RL and GI increased with GP concentration (figure 2b, d, f).
GP
effect on growth of tomato and lettuce plants.
Significant
differences were found in expanded leaf number (EL) (H= 8.54; p=
0.0259), greenness index (GrI) (F= 14.21; p < 0.0001), total leaf
area (TLA) (F= 95.71; p < 0.0001), aerial dry weight (ADW) (F= 77.05;
p < 0.0001) and root dry weight (RDW) (F= 40.54; p <
0.0001) of lettuce. The TLA, ADW and RDW significantly decreased with GP doses
over 40%. The addition of 20 t ha-1 of GP as mulch showed an
increase in RDW.
For tomato
plants, significant differences were found in GrI (F= 6.72; p= 0.0038),
TLA (F= 46.47; p < 0.0001), ADW (F= 21.00; p < 0.0001) and
RDW (F= 30.54; p < 0.0001). The GP presented the highest toxic effect
on ADW and TLA at doses of 40-80 t ha-1. In contrast, GrI
significantly increased at the maximum dose. Also, a non-toxic effect of GP was
observed on RDW at 20 t ha-1 (table 2).
Table
2. Expanded leaf number (EL), greenness
index (GrI), total leaf area (TLA), aerial dry weight (ADW) and root dry weight
(RDW) of lettuce and tomato plants grown with increasing GP doses.
Tabla 2.
Número de hojas expandidas (EL), Índice de verdor (GrI), Área foliar total
(TLA), Biomasa seca área (ADW) y biomasa seca radicular (RDW) de plantas de
lechuga y tomate en respuesta a diferentes dosis crecientes de OU.
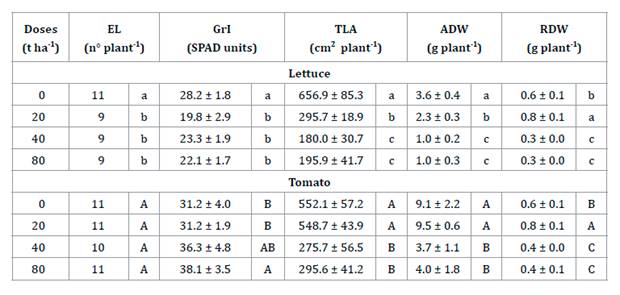
Data means ± S.D. of 10 replicates. For each row and
vegetal species, different letters indicate significant differences (p <
0.05; Tukey test or Kruskal-Wallis test).
Los datos son medias de 10 réplicas ± D.E. Para cada
fila y especie vegetal, diferentes letras indican diferencias significativas (p
< 0,05; Test de Tukey o Kruskal-Wallis).
Discussion
This study found
high OM contents in V. labrusca GP, as reported for V. vinifera and
other agroindustrial by-products like solid olive mill “alperujo” (Olea
europaea L.) and coffee pulp (2, 3, 4, 6). Unlike oil
and coffee by-products, grape pomaces have high NT content and a C/N ratio
nearly under the recommended limits for organic fertilizing (< 25) (6,
22).
Depending on the
origin, commercial organic fertilizers have diverse amounts of essential
nutrients (23). Bustamante
et al. (2008)
found 1.15 g kg-1 total P for V. vinifera, while in this
study V. labrusca showed a higher total P content, resulting in a more
attractive by-product for the agronomic industry.
While most
organic fertilizers usually require EC values under 4 dS m-1, the
value obtained in this study was higher and considered detrimental to plant
growth (22). However, the
main salts found in V. labrusca GP are Ca+2 and/or Mg+2
chlorides and sulfates (SAR < 15). In this sense, the pomace could be
considered a good alternative to animal manure causing soil disintegration due
to concentrated Na+ salts (9, 10). Furthermore, V.
labrusca pomace exhibited a CEC value similar to that of highly productive
soils (>45), probably given by the available functional groups negatively
charged (phenols, carboxylic acids, etc.) found in OM (15). Hence, soil
addition of GP might reduce leaching, thus increasing essential cations
availability (39).
Soils with a
saturation base close to 100% exhibit alkaline pH (14). In this
study, the pomace showed an elevated base saturation while being an acidic
by-product, probably given to the presence of organic acids like malic and tartaric,
common in grapes, that together with K+ and its effect over the diminished free
tartaric acid, define by-product acidity (3). On the other
hand, the high moisture content in this by-product (79.2%) is within the range reported
for V. vinifera pomace and other agroindustrial wastes such as alperujo
and coffee pulp ranging between 64-80% (3, 4).
Introducing OM
into the system could differentially affect soil microorganisms and plants,
depending on application dose and tolerance ranges (26,
41).
A. brasiliense is a soil-free nitrogen-fixing bacteria producing several
plant signalling molecules like phytohormones (auxins and gibberellins) (13,
32).
The positive effect of GP on this diazotrophic bacteria has not been previously
reported using other by-products (37).
When seeds were
exposed to 10% GP concentration, primary roots showed a brownish colour in both
plant species, probably given by cell necrosis caused by toxic compounds in GP
or polymerization of compounds (chromophores) in root exudates as a defence
response. A similar symptom was reported in tomato roots as an allelochemical
effect of Sicyos deppei (Cucurbitaceae) (33). Some phenols
act as allelochemical compounds related to polar narcosis (structural and
functional alteration of cell membrane), oxidation uncoupling, alterations in
electrophilicity, hydrophobicity, and dissociation and union of H in
biomolecules (30, 44). Since V.
labrusca pomace is rich in soluble phenolic compounds, these substances
could be related to the mentioned root symptoms.
In the plant
experiments, the stimulant effect of GP on root systems at the lowest dose
could be due to greater nutrient availability (9). In intensive
crops, such as lettuce and tomato, the recommended dose of an organic
fertilizer aimed at maintaining soil productive capacity is 40 t ha-1
(42). The results obtained in this study
would limit GP utilization as an organic fertilizer, but applications at the
lowest dose could be used as mulching in tomatoes and possibly in other crops
with similar tolerance ranges.
Conclusions
V. labrusca GP presents
physicochemical characteristics associated with soil health. Its high content
of phosphorus and potassium, as well as low sodium and low SAR values
differentiate this winery by-product from the one derived from V. vinifera.
Low concentrations of V. labrusca GP promotes A. brasiliensis and
tomato root system without altering aerial biomass, an effect known as
hormesis. Toxicity symptoms of V. labrusca GP on plant growth at highest
doses restricts its usage as an organic fertilizer. However, further ongoing
field experiments will evaluate the effect of GP as mulching on tomato growth
and other microorganisms indicating soil biological quality.
Acknowledgements
Special thanks
to Dr. Mario C.N. Saparrat for his support during the development of the experiments
and critical reading of the manuscript. The authors are grateful for the
financial support provided by Agencia Nacional de Promoción Científica y
tecnológica (PICT 2019-00207 to Mario C.N. Saparrat; PICT 2021-00056 to María
Inés Troncozo)
1. Abbona, E.
A.; Sarandón, S. J.; Marasas, M. E.; Astier, M 2007. Ecological sustainability
evaluation of traditional management in different vineyard systems in Berisso,
Argentina. Agriculture, ecosystems & environment. 119(3-4): 335-345.
https://doi.org/10.1016/j.agee.2006.08.001
2. Alburquerque,
J. A.; Gonzálvez, J.; García, D.; Cegarra, J. 2004. Agrochemical
characterisation of “alperujo”, a solid by-product of the two-phase
centrifugation method for olive oil extraction. Bioresour Technol. 91: 195-200.
https://doi.org/10.1016/S0960-8524(03)00177-9
3. Boulton, R.
1980. The relationships between total acidity, titratable acidity and pH in
wine. Am J Enol Vitic 31.
4. Braham, J.
E.; Bressani, R. 1979. Coffee pulp: composition, technology and utilization.
IDRC. Ottawa. ON. CA.
5. Bremner, J.
M.; Mulvaney, S. C. 1982. Nitrogen Total, in: Page, A. L.; Miller, R. H.;
Keeney, D. R. (Eds.), Methods of Soil Analysis: Chemical and Microbiological
Properties. Soil Science Society of America, Madison (WI). p. 595-624.
6. Bustamante,
M. A.; Moral, R.; Paredes, C.; Pérez-Espinosa, A.; Moreno-Caselles, J.;
Pérez-Murcia, M. D. 2008. Agrochemical characterisation of the solid
by-products and residues from the winery and distillery industry. Waste Manag.
28: 372-380.
7. Corbin, K.
R.; Hsieh, Y. S. Y.; Betts, N. S.; Byrt, C. S.; Henderson, M.; Stork, J.;
DeBolt, S.; Fincher, G. B.; Burton, R. A. 2015. Grape marc as a source of
carbohydrates for bioethanol: Chemical composition, pre-treatment and
saccharification. Bioresour Technol. 193: 76-83.
8. Devesa-Rey,
R.; Vecino, X.; Varela-Alende, J. L.; Barral, M. T.; Cruz, J. M.; Moldes, A. B.
2011. Valorization of winery waste vs. the
costs of not recycling. Waste Manage. 31: 2327-2335.
9. Diacono, M.;
Montemurro, F. 2011. Long-term effects of organic amendments on soil fertility.
Sustain Agr. 2: 761-786.
10. Diacono, M.;
Montemurro, F. 2015. Effectiveness of Organic Wastes as Fertilizers and
Amendments in Salt-Affected Soils. Agriculture. 5: 221-230.
11. FAO. 1998.
ISSS-ISRIC-FAO. World reference base for soil resources. Acco Press, Leuven,
Belgium.
12. Fernández
Linares, L. C.; Rojas Avelizapa, N.G.; Roldán Carillo, T. G.; Ramírez Islas, M.
E.; Zegarra Martinez, H. G.; Uribe Hernández, R.; Reyes ävila R. J.; Flores
Hernández, D.; Arce Ortega, J. M. 2006. Manual de técnicas de análisis de
suelos aplicadas a la remediación de sitios contaminados. Instituto Mexicano
del Petróleo, México.
13. Fukami, J.;
Cerezini, P.; Hungria, M. 2018. Azospirillum: benefits that go far beyond biological
nitrogen fixation. AMB Express 2018(8): 1-12.
https://doi.org/10.1186/S13568-018-0608-1
14.
Funes-Pinter, I.; Salomón, M. V.; Martín, J. N.; Uliarte, E. M.; Hidalgo, A.
2022. Effect of bioslurries on tomato Solanum lycopersicum L and lettuce
Lactuca sativa development. Revista de la Facultad de Ciencias Agrarias.
Universidad Nacional de Cuyo. Mendoza. Argentina. 54(2): 48-60. DOI:
https://doi.org/10.48162/rev.39.082
15. Garrido
Valero, S. 1994. Interpretación de análisis de suelos, N° 5/93. Instituto
Nacional de Reforma y Desarrollo Agrario. Madrid.
16. González
Fernández, J. L.; Eraso Luca de Tena, F.; Pérez Lanzac, J.; Medina Blanco, M.
1991. Influencia del procesado y fecha de recogida sobre la composición química
de los orujos de uva. Arch Zootec. 40: 379-389.
17. Havlin, J.
L.; Beaton, J. D.; Tisdale, S. L.; Nelson, W. L. 1999. Soil fertility and
fertilizers, 6th ed. Prentice Hall, Upper Saddle River. New York.
18. Hernández,
J. D.; Trujillo, Y. Y.; Osorio, D. S. D. 2011. Phenolic potential determination
and yeasts identification with significant leavens in Isabella grape (Vitis
labrusca) from Villa del Rosario (Norte de Santander) for wine making.
Vitae. 18: 17-25.
19. Jackson, M.
L.; Barak, P 2005. Soil chemical analysis: advanced course: a manual of methods
useful for instruction and research in soil chemistry, physical chemistry of
soils, soil fertility, and soil genesis. Parallel Press. University of
Wisconsin-Madison Libraries.
20. Kurt-Celebi,
A.; Colak, N.; Hayirlioglu-Ayaz, S.; Veličkovska, S. K.; Ilieva F.;
Esatbeyoglu, T.; Ayaz, F. A. 2020. Accumulation of phenolic compounds and
antioxidant capacity during Berry development in Black ‘Isabel’ Grape (Vitis
vinifera L. x Vitis labrusca L.). Molecules. 25: 3845-3845.
21. Mateo, J.
J.; Maicas, S. 2015. Valorization of winery and oil mill wastes by microbial
technologies. Int Food Res J. 73: 13-25.
https://doi.org/10.1016/J.FOODRES.2015.03.007
22. Mazzarino,
M. J.; Satti, P. 2012. Compostaje en la Argentina: experiencias de producción,
calidad y uso. Editorial UNRN.
23. Möller, K.;
Schulthei, U. 2015. Chemical characterization of commercial organic
fertilizers. Arch Agron Soil Sci. 61: 989-1012.
24.
Nascimento-Gavioli, M. C. A.; Rockenbach, M. F.; Welter, L.; Guerra, M. P.
2019. Histopathological study of resistant (Vitis labrusca L.) and
susceptible (Vitis vinifera L.) cultivars of grapevine to the infection
by downy mildew. J Hortic Sci Biotechnol. 95: 521-531.
https://doi.org/10.1080/14620316.2019.1685411
25. Ngosong, C.;
Okolle, J. N.; Tening, A. S. 2019. Mulching: A sustainable option to improve
soil health. Soil Fertility Management for Sustainable Development. p. 231-249.
26. Obiakor, M.
O.; Wilson, S. C.; Tighe, M.; Pereg, L. 2019. Antimony Causes Mortality and
Induces Mutagenesis in the Soil Functional Bacterium Azospirillum brasilense
Sp7. Water Air Soil Pollut 230 1-14.
https://doi.org/10.1007/S11270-019-4232-8
27. OIV
International Organization of Vine and Wine 2019 n.d. Statistical report on
world vitiviniculture (WWW Document).
https://www.oiv.int/es/technical-standards-and-documents/statistical-analysis/annual-assessment
(accessed 10.6.22).
28. Osono, T.;
Takeda, H. 2001. Organic chemical and nutrient dynamics in decomposing beech
leaf litter in relation to fungal ingrowth and succession during 3-year
decomposition processes in a cool temperate deciduous forest in Japan. Ecol
Res. 16: 649-670.
29. Otero, J.
2013. Factores de la reactivación de un producto agroalimentario típico: el
vino de la costa de Berisso, Argentina. Cuadernos de Desarrollo Rural. 10(71):
37-58.
30. Ren, S.
2003. Phenol mechanism of toxic action classification and prediction: a
decision tree approach. Toxicol Lett. 144: 313-323.
https://doi.org/10.1016/S0378-4274(03)00236-4
31. Richards, L.
A. 1954. Diagnosis and improvement of saline and alkali soils. Agriculture
Handbook N° 60. Washington DC.
32. Rodríguez
Larramendi, L. A.; Salas-Marina, M. Á.; Hernández García, V.; Campos Saldaña,
R. A.; Cruz Macías, W.; López Sánchez, R. 2023. Seed treatments with salicylic
acid and Azospirillum brasilense enhance growth and yield of maize plants (Zea
mays L.) under field conditions. Revista de la Facultad de Ciencias Agrarias.
Universidad Nacional de Cuyo. Mendoza. Argentina. 55(1): 17-26. DOI:
https://doi.org/10.48162/rev.39.092
33.
Romero-Romero, T.; Sánchez-Nieto, S.; San Juan-Badillo, A.; Anaya, A. L.;
Cruz-Ortega, R 2005. Comparative effects of allelochemical and water stress in
roots of Lycopersicon esculentum Mill. (Solanaceae). Plant Sci. 168:
1059-1066.
34. Rondeau, P.;
Gambier, F.; Jolibert, F.; Brosse, N. 2013. Compositions and chemical
variability of grape pomaces from French vineyard. Ind Crops Prod. 43: 251-254.
35. SAGPyA 2004.
Sistema de apoyo metodológico a los laboratorios de suelos, agua, vegetales y enmiendas
orgánicas (SAMLA)
36. Sampedro,
I.; Aranda, E.; Martín, J.; García-Garrido, J. M.; García-Romera, I.; Ocampo,
J. A. 2004. Saprobic fungi decrease plant toxicity caused by olive mill
residues. Appl Soil Ecol. 26: 149-156. https://doi.org/10.1016/j.apsoil.2003.10.011
37. Saparrat, M.
C. N.; Jurado, M.; Díaz, R.; Romera, I. G.; Martínez, M. J. 2010.
Transformation of the water soluble fraction from “alpeorujo” by Coriolopsis
rigida: The role of laccase in the process and its impact on Azospirillum
brasiliense survival. Chemosphere. 78: 72-76.
https://doi.org/10.1016/j.chemosphere.2009.09.050
38. Schneider,
C. A.; Rasband, W. S.; Eliceiri, K. W. 2012. NIH Image to ImageJ: 25 years of
image analysis. Nat Methods. 9: 671-675. https://doi.org/10.1038/nmeth.2089
39. Scotti, R.;
Bonanomi, G.; Scelza, R.; Zoina, A.; Rao, M. A. 2015. Organic amendments as
sustainable tool to recovery fertility in intensive agricultural systems. J
Soil Sci Plant Nutr. 15: 333-352.
40. Sisterna,
M.; Ronco, L.; Voget, C.; Marasas, M.; Abbona, E.; Romero, M.; Daniele, J.;
Artaza, S.; Otero, J.; Sepúlveda, C.; Avila, G.; Loviso, C.; Orosco, E.;
Bonicatto, M.; Condes, C.; Velarde, I. 2010. American Grapevine Culture and
Research in Berisso, Argentina. Am J Plant Sci. 3: 38-53.
41. Tiquia, S.
M.; Tam, N. F. Y.; Hodgkiss, I. J. 1996. Effects of composting on phytotoxicity
of spent pigmanure sawdust litter. Environ Pollut. 93: 249-256.
42. Vazquez, M.
E.; Terminiello, A. 2012. Recuperación de suelos degradados de pequeños
productores del cinturón hortícola del Gran La Plata. Valoración del problema y
estrategias correctivas. FCAyF-UNLP.
43. Walkley, A.;
Black, I. A. 1934. An examination of the Degtjareff method for determining soil
organic matter, and a proposed modification of the chromic acid titration
method. Soil Sci. 37: 29-38. https://doi.org/10.1097/00010694-193401000-00003
44. Wang, X.;
Sun, C.; Wang, Y.; Wang, L. 2002. Quantitative structure-activity relationships
for the inhibition toxicity to root elongation of Cucumis sativus of
selected phenols and interspecies correlation with Tetrahymena pyriformis.
Chemosphere. 46: 153-161. https://doi.org/10.1016/S0045-6535(01)00133-3
45. Zaghloul,
A.; Saber, M.; Gadow, S.; Awad, F. 2020. Biological indicators for pollution
detection in terrestrial and aquatic ecosystems. Bulletin of the National
Research Centre. 44(1): 1-11. https://doi.org/10.1186/s42269-020-00385-x