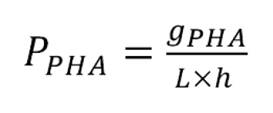Revista de la Facultad de Ciencias
Agrarias. Universidad Nacional de Cuyo. Tomo 57(2). ISSN (en línea) 1853-8665.
Año 2025.
Original article
Isolation
of Polyhydroxyalkanoate (PHA)-producing Azotobacter spp. from Crop Rhizospheres located in Lima, Peru
Selección
de Azotobacter spp. productores de
polihidroxialcanoatos (PHA) aislados de rizósfera de cultivos ubicados en la
región de Lima - Perú
Lisset Tupa-Andrade1,
Junior Caro-Castro1,
1Universidad Nacional Mayor de San Marcos. Facultad de Ciencias
Biológicas. Laboratorio de Ecología Microbiana. Lima-Perú.
*jleonq@unmsm.edu.pe
Abstract
PHAs
are polyesters found as internal granules in several microorganisms. Azotobacter
is known for its ability to produce PHA. This study aimed to isolate Azotobacter
from the rhizosphere of selected crops located in Lima and evaluate their
PHA-producing potential. Nile Red medium was used for PHA detection, and Sudan
Black B staining allowed microscopic observation. Biopolymer production and
quantification were carried out in Burk’s medium, PHA minimal medium (PHAMM),
and modified PHAMM. In Nile Red medium, 68.2% of strains produced PHA, with Azotobacter
AzoLur20 exhibiting the highest production, 2.1 g/L of PHA at 96 hours in
PHAMM. However, strain AzoLur19 showed higher productivity and stability,
achieving 0.06 g/L*h of PHA. Additionally, Sudan Black B staining in Burk’s
medium revealed larger Azotobacter cells with more defined granules.
AzoLur19 was classified as Azotobacter chroococcum. In conclusion, Azotobacter
species isolated from crops located in Lima can produce PHA with high
yields, with A. chroococcum as the predominant species.
Keywords:
Azotobacter, polyhydroxyalkanoate, PHA production,
bioplastics
Resumen
Los PHA son
poliésteres que se encuentran como gránulos internos en varios microorganismos.
Azotobacter se caracteriza por producir PHA. El objetivo de este estudio
fue aislar Azotobacter de la rizósfera de cultivos ubicados en la región
de Lima y evaluar su potencial productor de PHA. Se utilizó el medio Rojo de
Nilo para la detección de PHA y la tinción con Sudan Black B para la
observación microscópica. La producción y cuantificación del biopolímero se
realizó en medio Burk, medio mínimo PHA (PHAMM) y PHAMM modificado. El 68,2% de
las cepas produjeron PHA en el medio Rojo de Nilo, siendo la cepa de Azotobacter
AzoLur20 la más importante produciendo 2,1 g/L de PHA a las 96 horas en el
PHAMM; sin embargo, la cepa AzoLur19 fue la más productiva y estable con 0,06
g/L*h de PHA. En cuanto a la tinción con Sudan Black B en medio Burk, se
observaron células de Azotobacter de mayor tamaño y con gránulos más
definidos. Además, el análisis molecular de la cepa AzoLur19 la identificó como
Azotobacter chroococcum. En conclusión, Azotobacter sp. es capaz de producir PHA con un alto rendimiento,
destacándose la especie predominante A. chroococcum en la rizósfera de
varios cultivos.
Palabras clave: Azotobacter,
polihidroxialcanoato, producción de PHA, bioplásticos
Originales: Recepción: 07/09/2023
- Aceptación: 02/07/2025
Introduction
Petroleum-derived
plastics significantly contribute to global pollution. Their slow degradation
process can take several decades. As a result, the quest for eco-friendly
alternatives has become urgent, and polyhydroxyalkanoates (PHAs) are one
majorly investigated bioplastics. PHAs share similar properties with
petrochemical plastics and have various applications, including the production
of disposable everyday items, biomedical devices, pharmaceutical products,
agricultural materials, textiles, and nanotechnology products (11,
19, 25, 27).
PHAs
are polyesters produced and stored by various microorganisms as internal
granules, which serve as carbon and energy sources (26).
Although PHA-producing bacteria typically synthesize this compound under stress
conditions like an excess of carbon sources or a nutrient limitation, certain
exceptions, such as Azotobacter, can produce PHA without a stress
inducer (23).
Azotobacter
species, primarily isolated from the rhizosphere, synthesize PHA
continuously during growth. They produce higher quantities when ammonium salts,
like ammonium acetate, are supplied to the culture medium (16).
Studies achieving high PHA production using Azotobacter mutant strains
include Burk medium with 0.12% NH4Cl (7)
and PHA minimal medium (PHAMM) with urea (20).
Both studies achieved high PHA production using Azotobacter mutant strains.
Although
further research is still needed, Azotobacter is recognized as a good
PHA producer (8),
easy-to-handle, non-pathogenic, and versatile microorganism. Among Azotobacter
species, A. chroococcum has recently received significant attention (1).
Currently, efforts enhancing PHA production and recovery, particularly the most
predominant type of PHA, polyhydroxybutyrate (PHB), focus on optimizing media
formulation for production, extraction methods, and quantification techniques (17,
22).
This
study evaluated the PHA-producing potential of Azotobacter isolated from
crop rhizospheres in Lima, Peru, determining physicochemical parameters for optimizing
PHA production.
Materials and Methods
Collection of Rhizosphere Samples
Rhizosphere samples
were collected from the districts of Lurin (latitude: -12.261063, longitude:
-76.888297) and Pachacamac (latitude: -12.166853, longitude: -76.857761) in
Lima, Peru. Three crops from Lurin (onion, corn, and sweet potato) and five
from Pachacamac (corn, strawberry, cucumber, chilli pepper, and sweet potato)
were selected. The samples were transported to the Laboratorio de Ecología
Microbiana at the Universidad Nacional Mayor de San Marcos.
Azotobacter Isolation and Selection
Microbial
isolation was performed according to Escobar et
al. (2011). Initially, 10 g of each sample were diluted in 90 mL of saline
(10-1). Then, a subsequent dilution (1:10) in saline obtained a
second dilution (10-2). The resulting dilution was seeded
on Ashby Mannitol Agar and Burk Agar solid media. Simultaneously, a selective
enrichment was performed in Ashby Sucrose Broth, later seeded in the mentioned
solid media. Isolated bacterial colonies were subcultured until pure strains
were obtained and preserved at -20°C in glycerol-containing media.
Biochemical Characterization of Azotobacter Strains
Strains
exhibiting presumptive morphological features of Azotobacter were
evaluated through biochemical tests, including sugar fermentation,
denitrification, urease testing, and cyst formation.
PHA Detection in Nile Red Medium
Azotobacter
strains were inoculated into Nile Red medium. PHA detection was
conducted using a 365 nm UV transilluminator (JUNYI brand, model JY02S),
reading every 24 hours for 4 days. Presence or absence of intense pink to
fuchsia fluorescence classified strains as PHA positive or negative,
respectively (5).
PHA Production in Liquid Culture Media
The
top five PHA-positive strains from Nile Red medium were subjected to
quantitative tests for PHA production in the following liquid media: A) Burk
medium with 2% glucose and 0.12% ammonium chloride (7); B) PHA minimal medium
(PHAMM), with 0.54 g/L urea and 2% sucrose (20); and C) a modified PHAMM
containing 2% glucose and 0.12% ammonium acetate. Aliquots were taken at
24-hour intervals, up to 96 hours, determining dry cell weight, Sudan Black B
cell staining, and medium absorbance. Retains were also processed for polymer
extraction using sodium hypochlorite and chloroform. The polymer was quantified
using sulfuric acid.
Analytical Methods for the Evaluation of PHA Production
Biopolymer
accumulation and productivity were evaluated according to Becerra
(2013):
A-
Percentage of PHA accumulation:
B-
PHA Productivity:
where:
gPHA = grams of PHA
gX = grams of biomass
L
= liters
h
= hours.
In
addition, ANOVA, Tukey’s test and correlation analysis (p < 0.05) were performed
using InfoStat v. 2020.
Molecular Characterization of Azotobacter
Bacterial DNA
extraction was performed using the GeneJET Genomic DNA Purification Kit (Thermo
Fisher, USA). Subsequently, the 16S rRNA gene was amplified by PCR with the
primers 27F (5’-AGAGTTTGATCCTGGCTCAG-3’) and 1492R (5’-GGTTACCTTGTTACGACTT-3’).
The PCR products were shipped to Macrogen Inc. (Seoul, Korea) for Sanger
sequencing. The obtained sequences were assembled and aligned with other 16S
rRNA gene sequences from different Azotobacter species recovered from
the GenBank database (2). Phylogenetic
inference was conducted using MEGA11 (2021), employing the
Neighbor-Joining method with 1000 bootstrap replications.
Results and discussion
Isolation and Characterization of Azotobacter sp.
Twenty-two Azotobacter strains were isolated. Eighteen
(82%) strains were recovered from Lurin (table 1). Although there
are no previous studies from this area, Lurin offers more suitable edaphic
conditions for Azotobacter than Pachacamac. Additionally, most isolates
were obtained from corn crops, as typically seen (3). Malynovska
et al. (2021) emphasized that the highest prevalence of Azotobacter occurs
in extensive crops, particularly in soils with fewer contaminants.
Table
1. Source and Origin of Azotobacter Strains.
Tabla
1. Fuentes y origen de las cepas de Azotobacter.
Qualitative Selection of PHA in Nile Red Medium
Fifteen of the 22 strains (68%) tested positive for PHA
detection using Nile Red medium. The highest fluorescence was observed between
72 and 96 hours of analysis (figure 1). The best results were obtained when acetone was used as a
solvent for the lipophilic dye Nile Red, as suggested by Giraldo
et al. (2020). When using agar medium as suggested by Carballo
(2003),
strong fluorescence was observed in the positive strains.
Figure 1. PHA
production by Azotobacter strains in Nile Red medium observed under a
365 nm transilluminator at A) 72 hours, and B) 96 hours.
Figura
1. Producción de PHA por cepas de Azotobacter
en medio Rojo de Nilo visto a través de un transiluminador (365nm) a A) 72
horas y B) 96 horas.
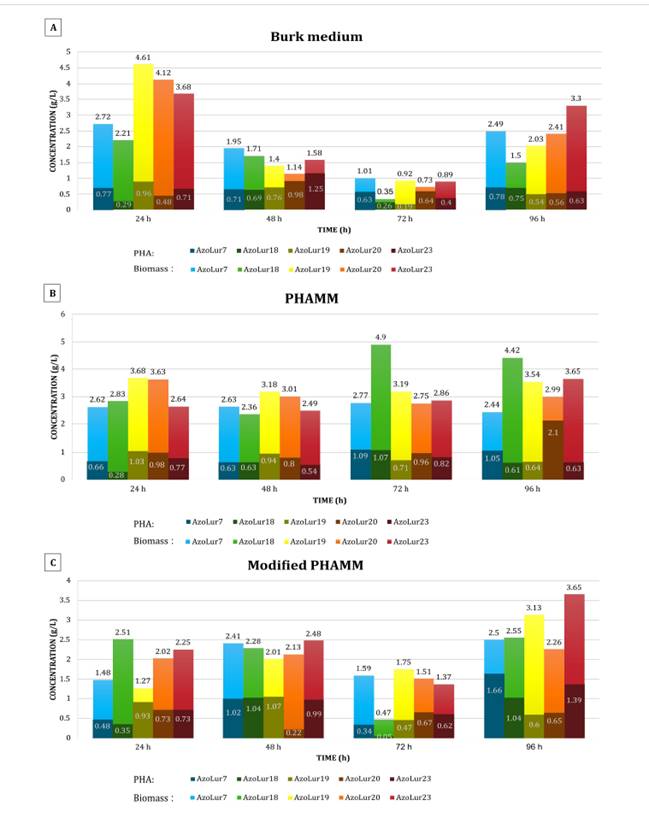
Quantitative Evaluation of PHA in Different Media
The
highest PHA producer strain in Burk medium was AzoLur23, generating 1.25 g
PHA/L at 48 hours from a biomass of 1.58 g/L (figure 2).
In contrast, Cerrone (2011)
reported 2.3 g PHA/L at 72 hours for a hyperproducing mutant strain using the
same medium. Our result is notably high.
A) Burk medium, B) PHAMM, and C) Modified PHAMM. /
A) Medio Burk, B) MMPHA y C) MMPHA modificado.
Figure
2. Determination of biomass and PHA production at 24,
48, 72, and 96 hours.
Figura 2. Evaluación
de la biomasa y producción de PHA a las 24, 48, 72 y 96 horas de evaluación.
The
highest PHA producer in PHAMM was AzoLur20, generating 2.1 g PHA/L at 96 hours
from a biomass of 2.99 g/L (figure 2).
Pei
et al. (2017) obtained 1.5 g PHA/L from one of their evaluated mutant
strains. Our higher result indicates that the AzoLur20 strain has potential for
future biopolymer biosynthesis.
Considering
the modified PHAMM, AzoLur7 was the highest PHA producer, generating 1.66 g
PHA/L at 96 hours from a biomass of 2.5 g/L (figure 2).
Although the result was lower than that obtained with the unmodified PHAMM, it
is still higher than the values reported by Pei et al.
(2017).
Biomass
production and PHA concentration followed a normal distribution across all
media (p > 0.05). Significant differences (p < 0.05) were observed when
comparing the biomass obtained in the Burk and PHAMM media with the biomass in
the modified PHAMM medium (figure 3A).
In contrast, no significant differences in PHA concentration were found among
the three media evaluated (figure 3B).
A)
biomass and B) PHA concentration across different production media. Lowercase
letters indicate significant differences (p < 0.05) among the production
media.
A) biomasa y B)
concentración de PHA en diferentes medios de producción. Las letras minúsculas
indican diferencias significativas (p < 0,05) entre los medios de
producción.
Figure
3. Statistical analysis of biomass and PHA
concentration across different production media.
Figura 3. Análisis
estadístico de la biomasa y concentración de PHA en diferentes medios de
producción.
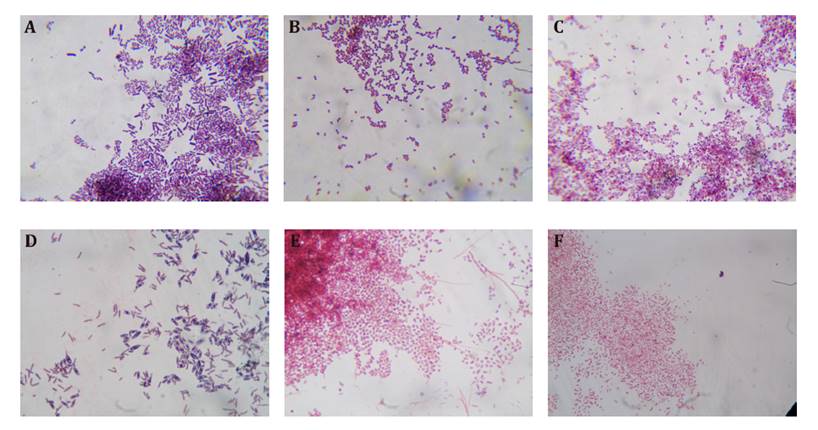
Staining with Sudan Black B
Cell staining with Sudan Black B dye was observed under a light
microscope, allowing the dark internal granules to be differentiated from the
pink-colored cytosol by the counterstain safranin, as described by Mohammed
et al. (2019). Cells from Burk medium were larger, and the granules were more
prominent compared to the cells recovered from PHAMM and modified PHAMM media (figure 4). Metals present
in the composition of the latter two media may have affected cell growth and
development, as previously noted by Lara et al. (2010).
A) AzoLur19 in Burk medium, B) AzoLur23 in PHAMM, C)
AzoLur19 in modified PHAMM, D) AzoLur19 in Burk medium, E) AzoLur19 in PHAMM,
F) AzoLur23 in modified PHAMM.
A)
AzoLur19 en medio Burk, B) AzoLur23 en MMPHA, C) AzoLur19 en MMPHA modificado,
D) AzoLur19 en medio Burk, E) AzoLur19 en MMPHA, F) AzoLur23 en MMPHA
modificado.
Figure 4. Cell
staining with Sudan Black B dye, observed under an optical microscope at 24
hours (A, B, and C) and 96 hours (D, E, and F).
Figura
4. Tinción de células con Negro de
Sudán B vistas al microscopio óptico a las 24 horas (A, B y C) y a las 96 horas
(D, E y F).
PHA Organic Extraction
As
previously mentioned (9),
sodium hypochlorite and chloroform effectively extracted the PHA biopolymer.
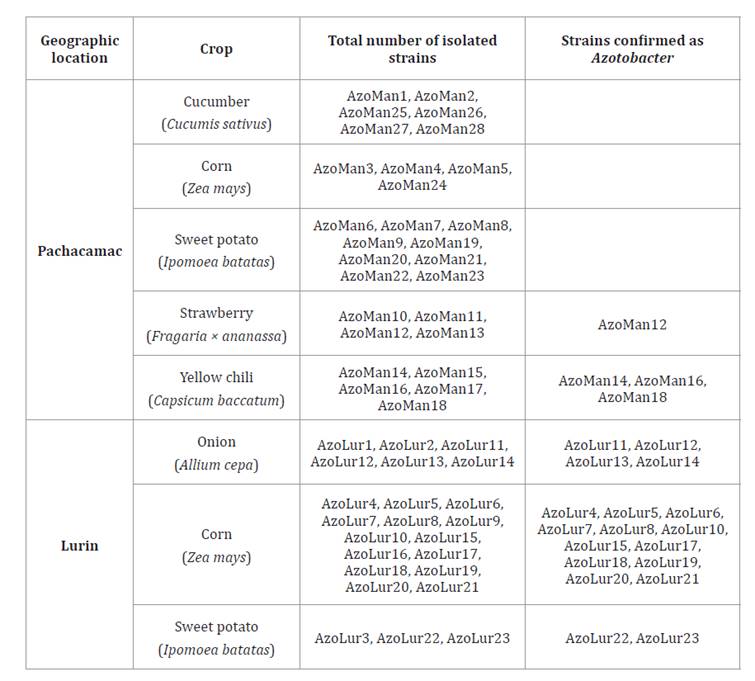
PHA Accumulation and Productivity
According to strain evaluation, the highest biopolymer
accumulation in PHAMM was 70.23% at 96 hours for the AzoLur20 strain (table 2).
Table
2. Percentage of PHA accumulation in
selected strains in Burk medium.
Tabla
2. Porcentaje de acumulación de PHA de
las cepas seleccionadas en medio Burk.
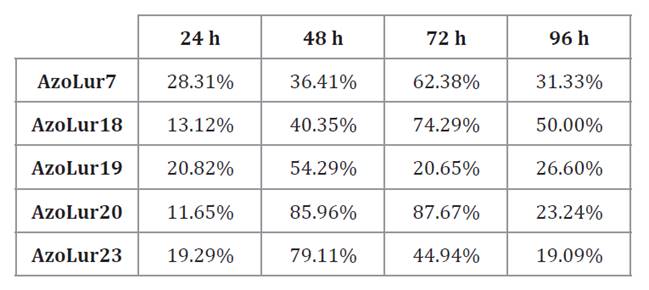
In contrast, the highest accumulation in the modified PHAMM
medium was 74.33% at 96 hours for the AzoLur23 strain (table 3).
Table
3. Percentage of PHA accumulation in
selected strains in PHAMM.
Tabla
3. Porcentaje de acumulación de PHA de
las cepas seleccionadas en MMPHA.
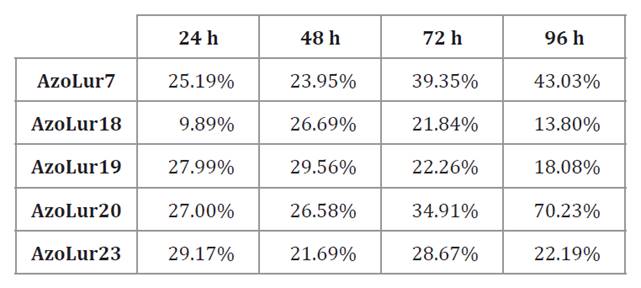
Additionally, the highest PHA accumulation across all strains
was observed in Burk medium with the AzoLur20 strain, reaching 87.67% at 72
hours (table
4).
El-Nahrawy
et al. (2018) reported similar results, using ammonium sulfate (NH4)2SO4
with a final concentration of 0.2%. In contrast, Castillo et al. (2017) utilized the
same culture medium but achieved 80% PHA accumulation, lower than that observed
in our study.
Table
4. Percentage of PHA accumulation in
selected strains in the modified PHAMM.
Tabla
4. Porcentaje de acumulación de PHA de
las cepas seleccionadas en PHAMM modificado.
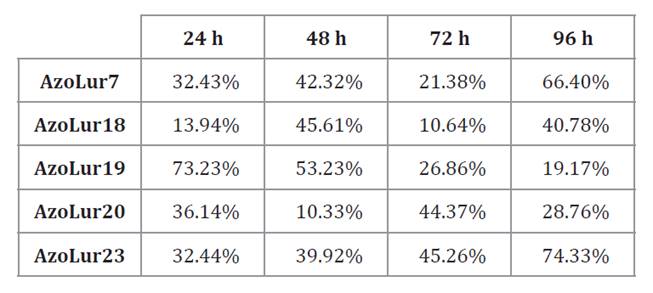
Considering
productivity, AzoLur19 strain achieved the highest value of 0.04 g/L*h across
all three tested media. Upon repeating the evaluation in triplicate,
productivity values reached up to 0.06 g/L·h in both PHAMM and modified PHAMM.
These results were comparable to those reported by Ramirez et al.
(2011), who reported a productivity of 0.04 g/L·h in Azotobacter OPN
mutant strain using Burk medium with ammonium acetate.
No significant differences were observed in AzoLur19
productivity between 24 and 48 hours. However, at 96 hours, significant
differences in productivity were noticed in Burk medium compared to PHAMM and
modified PHAMM. This disparity arose because, at 96 h, the culture in Burk
medium was in exponential phase, whereas cultures growing in both PHAMM and
modified PHAMM were in stationary phase (figure 5). Although values
obtained by AzoLur19 strain did not exceed the ones reported by Cerrone
(2011)
and Pei
et al. (2017), the analysis provided insight about strain behavior in
different culture media and PHA production over time. Additionally, it supports
the proposal of modified PHAMM for potential PHA production.
A) Burk medium, B) PHAMM and C) modified PHAMM. Abs.
1: Absorbance 1. Abs. 2: Absorbance 2. Abs. 3: Absorbance 3. Conc. 1:
Concentration 1. Conc. 2: Concentration 2. Conc. 3: Concentration 3.
A) medio Burk, B) MMPHA y C) MMPHA modificado. Abs. 1:
Absorbancia 1. Abs. 2: Absorbancia 2. Abs. 3: Absorbancia 3. Conc. 1:
Concentración 1. Conc. 2: Concentración 2. Conc. 3: Concentración 3.
Figure 5. Comparison
between cell growth and PHA production of AzoLur19 strain.
Figura
5. Comparación entre el crecimiento
celular y la producción de PHA de la cepa AzoLur19.
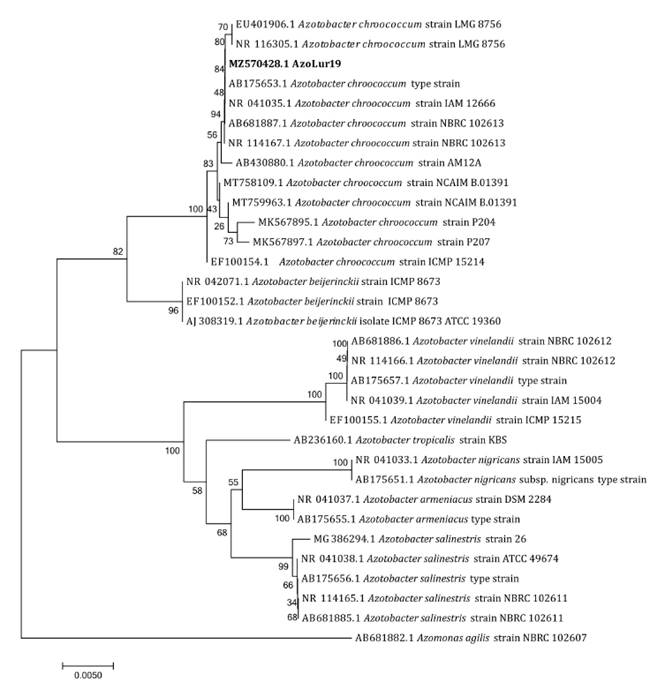
Molecular Analysis
Based on BLASTN and phylogenetic analysis, the AzoLur19 strain
was identified as Azotobacter chroococcum (GenBank accession number:
MZ570428.1) (figure
6).
Previous biochemical tests are consistent with this analysis. Aasfar et
al. (2021) noted that A. chroococcum is predominant in the
rhizosphere of several crops. Before this, Kim and Chan (1998) had demonstrated
its high capacity to produce PHA.
The 16S rRNA sequence from Azomonas agilis was
selected as outgroup.
La
secuencia de ARNr 16S de Azomonas agilis se eligió como grupo externo.
Figure 6. Phylogenetic
tree of the 16S rRNA gene of the AzoLur19 strain and other sequences of Azotobacter
sp., inferred using the Neighbor-Joining method with 1000 bootstrap
replicates.
Figura
6. Árbol filogenético del gen 16S rRNA
de la cepa AzoLur19 y otras secuencias de Azotobacter sp. inferido utilizando el método de unión de vecinos y 1000 de bootstrap.
Conclusions
Azotobacter strains are predominant in the rhizospheres of several crops. A.
chroococcum is easily isolated and characterized, while being an effective
PHA producer, especially after 24 and 48 hours of incubation. In this study,
PHA detection was effectively achieved using the Nile Red medium, facilitating
the selection of top-producing strains and allowing for production and
quantitative evaluation. Additionally, staining with Sudan Black B enabled the
observation of PHA intracellular granules. Finally, the AzoLur19 strain
exhibited high productivity in both PHAMM and modified PHAMM, highlighting its
potential for PHA synthesis.
1.
Aasfar, A.; Bargaz, A.; Yaakoubi, K.; Hilali, A.; Bennis, I.; Zeroual, Y.;
Kadmiri, I. 2021. Nitrogen Fixing Azotobacter Species as Potential Soil
Biological Enhancers for Crop Nutrition and Yield Stability. Frontiers in
Microbiology. 12: 1-19.
2.
Altschul, S.; Madden, T.; Schaffer, A.; Zhang, J.; Zhang, Z.; Miller, W.;
Lipman, D. 1997. Gapped BLAST and PSI- BLAST: a new generation of protein
database search programs. Nucleic Acids Research. 2(17): 3389-3402.
3.
Arsita, R.; Karim, H.; Hala, Y.; Iriany, N.; Jumadi, O. 2020. Isolation and
identification of nitrogen-fixing bacteria in the corn rhizosphere (Zea mays
L.) originating from Jeneponto Regency, South Sulawesi. IOP Conf. Series:
Earth and Environmental Science. 484: 012051.
4.
Becerra, M. 2013. Producción de un polímero tipo polihidroxialcanoato (PHA)
empleando residuos de la producción de biodiesel. Tesis de Magister en
Ciencias, Microbiología. Facultad de Ciencias. Universidad Nacional de
Colombia. Bogotá. Colombia. 129 p.
5.
Carballo, M.; Iglesias, Y.; Martínez, J.; Solano, R.; Fernández, A.;
Villaverde, M. 2003. Evaluación de la producción de polihidroxialcanoatos por
cepas bacterianas marinas. Revista Biología. 17(1): 52-57.
6.
Castillo, C.; Flores, C.; Segura, D.; Espín, G.; Sanguino, J.; Cabrera, E.;
Barreto, J.; Díaz, A.; Peña, C. 2017. Production of polyhydroxybutyrate (PHB)
of high and ultra-high molecular weight by Azotobacter vinelandii in
batch and fed-batch cultures. Journal of Chemical Technology &
Biotechnology. 92: 1809-1816.
7.
Cerrone, F. 2011. Producción de poliésteres biopoliméricos (PHAs) desde
alpeorujo por medio de bacterias fijadoras de nitrógeno. Tesis doctoral,
Doctorado europeo. Instituto del agua. Universidad de Granada. Granada. España.
152 p.
8.
Chávez, M. 2021. Metabolitos de Azotobacter spp. con
posible aplicación biotecnológica. Tesis de grado en Biología. Facultad de
Ciencias Biológicas. Benemérita Universidad Autónoma de Puebla. Puebla. México.
108 p.
9.
El-Nahrawy, S.; El-Kodoos, R.; El-Sayed, B.; El-Shouny, W. 2018. Production of
Poly-β-hydroxybutyrate (PHB) by Azotobacter sp. Isolated from Different
Sources. Environmental Biodiversity and Soil Security. 2: 183-192.
10.
Escobar, C.; Horna, Y.; Carreño, C.; Mendoza, G. 2011. Caracterización de cepas
nativas de Azotobacter spp. y su efecto en el
desarrollo de Lycopersicon esculentum Mill. “tomate” en Lambayeque.
Scientia Agropecuaria. 2(1): 39-49.
11.
Gatea, I.; Sabr, A.; Abdul, E.; Abbas, A.; Halob, A.; Mahmood, M. 2019.
Isolation and characterization of local Azotobacter isolate producing
bio-plastics and consuming waste vegetable oils. IOP Conf. Series: Earth and
Environmental Science. 388: 012082.
12.
Giraldo, J.; Castaño, G.; Rivera, F. 2020. Bacteria from industrial waste:
potencial producers of polyhydroxyalkanoates (PHA’s) in Manizales, Colombia.
Environmental Monitoring and Assessment. 192(7): 480.
13.
Kim, B.; Chang, H. 1998. Production of poly(3-hydroxybutyrate)
from starch by Azotobacter chroococcum. Biotechnology Letters. 20(2):
109-112.
14.
Lara, C.; García, L.; Oviedo, L. 2010. Medio de cultivo utilizando
residuos-sólidos para el crecimiento de una bacteria nativa con potencial
biofertilizante. Revista Colombiana de Biotecnología, 12(1): 103-112.
15.
Malynovska, I.; Yula, V.; Asanishvili, N.; Ptashnik, М.; Lyubchich, A. 2021.
Influence of crop species on quantity and physiological activity of rhizosphere
microorganisms. Ukrainian Journal of Ecology, 11(1): 286-290.
16.
Martinez, M.; Gonzalez, J.; Rodelas, B.; Pozo, C.; Salmeron, V. 1995.
Production of poly- β-hydroxybutyrate by Azotobacter chroococcum H23 in
chemically defined medium and alpechin médium. Journal of Applied Bacteriology.
78: 413-418.
17.
McAdam, B.; Brennan, M.; Mcdonal, P.; Mojicevic, M. 2020. Producción de
polihidroxibutirato (PHB) y factores que afectan sus características químicas y
mecánicas. Polímeros. 12(12): 2908.
18. Mohammed, S.; Panda, A.; Ray, L. 2019. An investigation for
recovery of polyhydroxyalkanoates (PHA) from Bacillus sp. BPPI-14 and Bacillus
sp. BPPI-19 isolated from plastic waste landfill. International Journal of
Biological Macromolecules. 134: 1085-1096.
19.
Navia Porras, D. P.; Poveda Perdomo, L. G.; Cuervo Mulet, R. A.; Esparza
Estrada, J.; Hernández Umaña, J. 2024. Antibacterial activity and
physicochemical characterization of bioplastic films based on cassava (Manihot
esculenta Crantz) starch and rosemary (Salvia rosmarinus) essential
oil. Revista de la Facultad de Ciencias Agrarias. Universidad Nacional de Cuyo.
Mendoza. Argentina. 56(2): 126-136. DOI: https://doi.org/10.48162/rev.39.136
20.
Pei, M.; Kumar, S.; Woan, L.; Bor, J.; Najimudin, N. 2017. Characterization of
polyhydroxyalkanoate production by mutant Azotobacter vinelandii.
Malaysian Applied Biology. 46(1): 1-8.
21.
Ramírez, M. E.; Herrera, S. L.; Domínguez, M.; Romo, Á.; Segura, D.; Peña, C.
2011. Evaluación de la producción y peso molecular del polihidroxibutirato
(PHB) sintetizado por diversas cepas mutantes de A. vinelandii. Sociedad
Mexicana de Bioquímica, Congreso Nacional 2011. Querétaro. Cartel CV-68.
22.
Ramos, A. 2019. Extracción, purificación y modificación de un biopolímero del
tipo poli (3-hidroxibutirato) obtenido de la fermentación de ácidos grasos con B.
cepacia. Tesis de Maestría en Ingeniería Química. Facultad de Ingeniería.
Universidad Nacional de Colombia. Bogotá. Colombia. 192 p.
23.
Sharma, V.; Sehgal, R.; Gupta, R. 2021. Polyhydroxyalkanoate (PHA): Properties
and Modifications. Polymer, 212: 123161.
24.
Tamura, K.; Stecher, G.; Kumar, S. 2021. MEGA11: Molecular Evolutionary
Genetics Analysis Version 11, Molecular Biology and Evolution. 38(7):
3022-3027.
25.
Vega, O. 2016. Extracción y caracterización estructural de un PHA, obtenido de
residuos de cáscaras de yuca y piña mediante procesos de fermentación; y su
aplicación en la fabricación de fibras por electrospinning. Tesis de doctor en
Ingeniería. Facultad de Ingeniería. Universidad de Antioquia. Medellín.
Colombia. 163 p.
26.
Villota-Calvachi, G.; González, K.; Marulanda, S.; Galeano, N.; Velasco, D.;
Ocampo, L.; Castañeda, L.; Giraldo, C.; Rodríguez, N. 2022. Aislamiento y
caracterización de bacterias productoras de biopolímeros a partir de efluentes
industriales. Revista Colombiana de Biotecnología. 24(1): 27-45.
27. Zambrano, H.; Riera, M. 2021. Desafío de los polihidroxialcanoatos
como solución al problema de los plásticos de un solo uso. Publicaciones en
Ciencia y Tecnología. 15(1): 15-26.