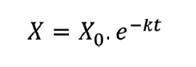Revista de la Facultad de Ciencias
Agrarias. Universidad Nacional de Cuyo. Tomo 57(1). ISSN (en línea) 1853-8665.
Año 2025.
Original article
Dry
mass production, nutrient accumulation and decomposition rate of cover crops
intercropped with a Theobroma cacao full-sun system
Producción
de materia seca, acumulación de nutrientes y tasa de descomposición de fitomasa
de cultivos de cobertura intercalados con Theobroma
cacao en un sistema a pleno sol
Claunita Novais
Alves1,
João Carlos
Medeiros1,
Maria Caroline
Aguiar Amaral1,
Ivan Pereira Santos
Silva1,
Paulo Henrique
Marques Monroe2,
Patrícia Anjos
Bittencourt Barreto-Garcia2,
George Andrade
Sodré3,
Paulo Cesar Lima
Marrocos4
1Universidade Federal do Sul da Bahia. Centro de Formação em
Ciências Agroflorestais. Rodovia Ilhéus/Itabuna. Km 30. 45600-970. Ilhéus. BA.
Brasil.
2Universidade Estadual do Sudoeste da Bahia. Estrada do Bem
Querer. 3293-3391. Candeias. 45083-900. Vitória da Conquista. BA. Brasil.
3Universidade Estadual de Santa Cruz. Rodovia Ilhéus-Itabuna. Km
16. Salobrinho. 45662-000. Ilhéus. BA. Brasil.
4Comissão Executiva do Plano da Lavoura Cacaueira-CEPLAC. Rodovia
Ilhéus/ Itabuna. Km 30. 45600-970. Ilhéus. BA. Brasil.
*jaqueline.rosa@ufsb.edu.br
Abstract
Cover crops play a
crucial role in promoting soil protection, enhancing organic matter content,
facilitating nutrient cycling, and improving overall soil quality. The
objective of this study was to evaluate the biomass production, nutrient
accumulation, and decomposition rate of cover crops intercropped with Theobroma
trees in a full-sun system. The research was conducted in Ilhéus, Bahia
state, Brazil. The experimental design employed randomized blocks with three
treatments, four decomposition times, and four replications. The treatments
consisted of three cover crops: 1) pigeon pea (Cajanus cajan); 2)
brachiaria (Urochloa decumbens); and 3) spontaneous vegetation.
Decomposition rates were evaluated using litter bags at specific intervals: 0,
47, 94, 116, and 136 days after field deposition. Dry biomass production and
nutrient accumulation by the cover crops were also measured. Spontaneous
vegetation and brachiaria treatments exhibited the highest potassium
accumulation, while no significant differences were observed among the
treatments for the other evaluated nutrients. Moreover, spontaneous vegetation
and brachiaria demonstrated higher decomposition rates, with 16.7% and 26.7% of
the deposited material remaining at the end of the 136-day study period,
respectively. In contrast, the decomposition rate of pigeon pea proved to be
slower, with a remaining dry mass of 38.3%, indicating longer persistence in
the soil, and consequently a greater half-life time. The cover crops
investigated in this study are regarded as promising options for intercropping
with cocoa, as they exhibit an average dry mass production of 10 Mg ha-1. This value falls
within the desired range for conservationist systems. When selecting species
for intercropping, it is crucial to consider the decomposition rates these
plants. This consideration ensures that the soil surface remains covered for an
extended duration, leading to enhanced conservation and improvement of the
soil’s physical, chemical, and biological properties. Soil conservation can be
effectively achieved by choosing cover crop species with slower decomposition
rates, thereby contributing to the overall health and quality of the soil.
Keywords: cocoa monoculture,
soil cover, Fabaceae, Poaceae
Resumen
Los cultivos de
cobertura desempeñan un papel crucial en la promoción de la protección del
suelo, el aumento del contenido de materia orgánica, la facilitación del ciclo
de nutrientes y la mejora de la calidad general del suelo. El objetivo del presente estudio fue evaluar la producción de biomasa, la
acumulación de nutrientes y las tasas de descomposición de los cultivos de
intercalados con árboles de cacao. La investigación se llevó a cabo en Ilhéus,
estado de Bahía, Brasil. El diseño experimental empleó un diseño de bloques
aleatorizados con tres tratamientos, cuatro tiempos de descomposición y cuatro
repeticiones. Los tratamientos consistieron en tres cultivos de cobertura: 1)
pigeon pea (Cajanus cajan), 2) braquiaria (Urochloa
decumbens) y 3) vegetación espontánea. Las tasas de descomposición se
evaluaron utilizando bolsas de descomposición a intervalos específicos: 0, 47,
94, 116 y 136 días después de la deposición en el campo. Se evaluó la
producción de biomasa seca y la acumulación de nutrientes por los cultivos de
cobertura. La producción promedio de biomasa seca fue de 10 Mg ha-1. Los tratamientos
de vegetación espontánea y braquiaria mostraron la mayor acumulación del
nutriente potasio. La vegetación espontánea y la braquiaria demostraron tasas
de descomposición más altas, con 16,7% y 26,7% de material remanente después de
136 días de estudio. Por el contrario, la descomposición del guandú resultó en
una persistencia más prolongada, con una materia seca restante de 38,3%, en
consecuencia, un mayor tiempo de vida media. Los cultivos de cobertura
investigados en este estudio se consideran opciones prometedoras para la
intercalación con cacao, ya que exhiben una producción de materia seca promedio
de 10 Mg ha-1.
Este valor se encuentra dentro del rango deseado para los sistemas conservacionistas.
Al seleccionar especies para la intercalación, es crucial considerar las tasas
de descomposición de estas plantas. Esta consideración asegura que la
superficie del suelo permanezca cubierta durante un período prolongado, lo que
conduce a una mejora en la conservación y las propiedades físicas, químicas y
biológicas del suelo. Al elegir especies de cultivos de cobertura con tasas de
descomposición más lentas, se puede lograr una conservación efectiva del suelo,
contribuyendo así a la salud y calidad general del mismo.
Palabras clave: monocultivo de
cacao, cobertura del suelo, Fabaceae, Poaceae
Originales: Recepción: 21/09/2023 - Aceptación: 12/06/2024
Introduction
The cocoa tree (Theobroma
cacao L.) is a plant species native to the Amazon and cultivated in
tropical countries of South America, West and Central Africa, India, and
Southeast Asia, holding significant economic importance in several countries (17). World cocoa
production is concentrated in a few key countries, such as Ivory Coast, Ghana,
Indonesia, Nigeria, Ecuador, Cameroon, and Brazil, which collectively account
for 88% of global production. Ivory Coast is the largest contributor, producing
approximately 39% of the total (14). Brazil stands as
the largest cocoa producer in South America and the seventh-largest producer
globally, having reported a production of 280 thousand tons in 2021 with a
planted area of 617 thousand hectares (14).
Cocoa cultivation in Brazil is predominantly concentrated in
four states: Bahia, Pará, Espírito Santo and Rondônia, with Bahia being the
leading producer, accounting for 100,864 tons in 2020 (4).
Moreover, cocoa farming represents the most important economic activity in the
southern region of Bahia. Cocoa is predominantly grown in an Agroforestry
System in southern Bahia, where the cocoa tree is cultivated in the understory
of the native Atlantic Forest, locally referred to as “cabruca” (33).
Another cultivation system that has been gaining prominence is monoculture,
also known as full-sun cultivation. In this case, the cocoa tree shading is
temporary, only occurring in the initial growth phase, and then the entire crop
cycle occurs in full sun. This system is used in countries considered as the
largest cocoa producers in the world (34),
and has been gaining ground in Brazil, including in non-traditional regions for
cocoa cultivation.
Cover crops can be
used to promote maintained soil quality and conservation in the full-sun cocoa
system. The association of perennial fruit trees with cover crops is already a
consolidated agricultural practice (12, 25, 27, 28, 30, 42), however, it has
not yet been studied in consortium with cocoa trees in a full-sun system,
warranting the need for studies to validate the production of phytomass,
nutrient accumulation and the decomposition rate of cover crops. Among the
various benefits, these plants can provide soil protection through litter
accumulation, promote nutrient cycling, increase biological activity, enhance
infiltration, and improve water storage in the soil (5,
25, 27, 29, 42), as well as increase the production of commercial crops, as
verified for citrus (19), and banana (20,
29).
Crop residue
accumulation on the soil surface is influenced by the decomposition rate of
cover crops, which in turn is regulated by the physical and chemical conditions
of the soil, the material composition that is supplied, the presence of edaphic
fauna, microbial activity of the soil, and precipitation (47). In a study conducted
in the Cerrado biome of Goiânia, Brazil, the decomposition rates for pigeon pea
(Cajanus cajan L.) were
found to be 62% 60 days after the deposition of litter bags in the field (38). Also in the
Cerrado biome of Piauí state, Brazil, the decomposition rate at 314 days after
cutting was 83% for Urochloa eminii (Mez)
Davidse (sub. U. ruziziensis (R.Germ.
& C.M.Evrard) Crins) and 79% for pigeon pea (41). The dry matter
production and decomposition of Zea mays
and U. eminii (sub. U. ruziziensis)
straw were additionally evaluatedin an integrated crop-livestock system. The
obtained dry mass was 6.6 Mg ha-1 and the half-life time
was 115 days. At the end of the study, 36% of the crop residue was on the soil,
with a loss of 4.23 Mg ha-1 of dry matter (36).
Cover crops are widely used in intercrops with fruit trees,
especially species of brachiaria, and pigeon pea as an option for Poaceas and
Fabaceas, respectively. However, the use of cover crops in full-sun cocoa
systems has not yet been studied. Therefore, the present study was carried out
with the hypothesis that pigeon pea (Cajanus cajan) in consortium with
full-sun cocoa exhibit an accelerated decomposition rate compared to brachiaria
(Urochloa decumbens (Stapf) R.D.
Webste) and spontaneous vegetation. The objective of the present study was to
evaluate the phytomass production, nutrient accumulation, and decomposition
rate of cover crops intercropped with cocoa trees cultivated in a full-sun
system.
Materials
and methods
Characterization
of the study area
The experiment was
conducted at the cocoa research center (CEPEC-CEPLAC), in Ilhéus, Bahia state,
Brazil. The site was located at coordinates 14°47’55” S and 39°02’01” W.
According to the Köppen climate classification, the region has an Af-type hot
and humid tropical forest climate, without a distinct dry season. The average
annual precipitation exceeds 1,300 mm, distributed throughout the year, with an
average temperature of 23°C and relative humidity of 80%. Climatic data,
including temperature and precipitation, were recorded at the
CEPLAC/CEPEC/SERAM meteorological station during the experiment (figure 1).
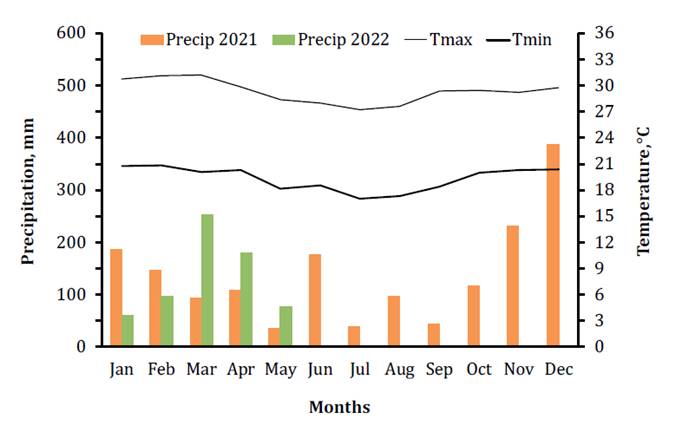
Source:
Meteorological station of CEPLAC/ CEPEC/SERAM.
Fuente:
Estación meteorológica de CEPLAC/CEPEC/ SERAM.
Figure
1. Meteorological data from the study period (December
2021 to May 2022).
Figura
1. Parámetros meteorológicos del
período de estudio (diciembre de 2021 a mayo de 2022).
The regional
topography is characterized as undulating, with an altitude of 60 m. The
experimental area soil is classified as a Typic Hapludalfs (34). The soil particle
size distribution was 320 g kg-1 sand, 338 g kg-1
silt and 342 g kg-1 clay. The chemical
properties of the soil before implementing the experiment are presented in table 1.
Table 1.
Soil chemical attributes before the experiment implemented in the 0-20 cm
layer.
Tabla
1. Indicadores químicos del suelo
antes de que se implementara el experimento en la capa de 0-20 cm.
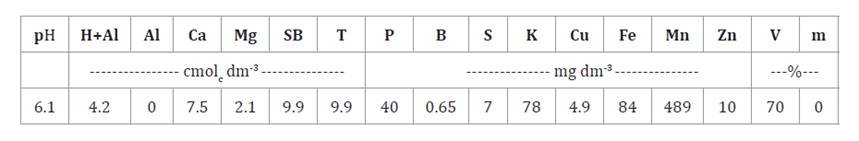
H+Al: potential acidity; Al:
aluminum; Ca: calcium; Mg: magnesium; P: phosphorus; SB: sum of bases; T: CEC a
pH 7; S: sulfur; K: potassium; Cu: copper; Fe: iron; Mn: manganese; Zn: zinc;
B: boron; V: base saturation; m: aluminum saturation.
H+Al: acidez potencial; Al:
aluminio; Ca: calcio; Mg: magnesio; P: fósforo; SB: suma de bases; T: CEC a pH
7; S: azufre; K: potasio; Cu: cobre; Fe: hierro; Mn: manganeso; Zn: zinc; B:
boro; V: saturación de bases; m: saturación de aluminio.
Experimental
area history
The experimental area (2000 m2)
was initially maintained until 2016 in an agroforestry system of cocoa with Erythrina
spp. This previous system was then subjected to clearcutting to implement a
monoculture cocoa. All plant residues were removed from the site, and
subsoiling was carried out to a depth of 0.50 m, followed by harrowing to
incorporate phosphate fertilization (144 kg ha-1 of P2O5) applied in the
form of single superphosphate. Liming was not used due to low active acidity
and adequate levels of Ca and Mg in the soil. The area remained fallow until
2019, during which time spontaneous vegetation growth occurred.
Then all vegetation
was cut (full mowing with brush cutter) in July 2019, and soil surface was
maintained with straw. Seedlings (6 months old) produced by cuttings from
plagiotropic branches of the cacao CEPEC 2002 clone were planted in holes with
dimensions 0.40 x 0.40 x 0.40 m, with spacing of 1.5m between plants and 4 m
inter-rows.
Experimental
design and treatments
The experiment was conducted in a randomized complete block
design with four replications. The experimental plots had an area of 9 m x 12
m, totaling 108 m2, with 18 cocoa trees by plot. One harrowing
operation was performed in all inter-rows of the cacao trees, followed by
manual sowing and incorporation of cover crop seeds. The cover crops
implemented in March 2020 were: 1) brachiaria (U. decumbens); (2) crotalaria (Crotalaria
breviflora DC); and (3) spontaneous vegetation. The following seed
quantities were used: brachiaria: 3.5 kg ha-1 and crotalaria: 15 kg
ha-1. The spontaneous vegetation consisted of germinating the existing
seed bank on the site, without the addition of external seeds.
In March of 2021,
the evaluation year of this study, the crotalaria treatment was replaced by
pigeon pea variety IAPAR 43, utilizing a seed rate of 45 kg ha-1.
There was no need to replant brachiaria and spontaneous vegetation treatments
in 2021, as the plants persisted in the plots. The spontaneous vegetation
treatment consisted of local native species, predominantly including Commelina benghalensis L. (10%); Bidens pilosa L. (3%); Cyperus odoratus L. (6%); Euphorbia heterophylla L. (5%); Rhynchospora nervosa (Vahl) Boeckeler (6%); Megathyrsus maximus (Jacq.) B.K. Simon & S.W.L. Jacobs (40%);
and Sorghum bicolor subsp. verticilliflorum (Steud.) de Wet ex
Wiersema & J.Dahlb. (30%), with the latter two species having the greatest
occurrence.
Determination
of dry mass and nutrient accumulation of cover crops
The cover crop
biomass was sampled in July 2021, four months after sowing the pigeon pea
treatment. A 0.5 m × 0.5 m metallic square was randomly thrown into each plot (10) and all plant
material within the square was cut close to the ground. The collected plant
material was dried in an oven at 65°C for 72 hours to determine the dry mass
production. Next, the nutritional composition of the dried plant samples was
analyzed to evaluate the nutrient accumulation in the cover crops. The nitrogen
(N); phosphorus (P); potassium (K); calcium (Ca); magnesium (Mg); sulfur (S);
iron (Fe); zinc (Zn); copper (Cu); manganese (Mn) and boron (B) concentrations
were determined (17). The nutrient
accumulation was calculated by multiplying the dry mass and the respective
nutrient concentrations in the cover crop biomass (21,
41).
The cover crop
shoot management was performed with a brush cutter, and the residues were
maintained on the soil surface. The mowings were repeated in the following
months from July 2020: September 2020, November 2020, January 2021, March 2021,
July 2021, September 2021, November 2021, January 2022 and March 2022. Two
annual fertilizations were performed on the cocoa tree after cutting the cover
crops at a dose of 50 kg ha-1 of N per application in
the form of urea in July and January of each year (2020 and 2021). No other
fertilizations were carried out, neither for the cocoa tree nor the cover
crops.
Decomposition
rate of cover crops
The plant material collected in July 2021 was separated
according to each cover crop treatment and then dried in an oven. Afterwards,
it was fragmented into pieces of approximately five centimeters. Portions of 11
g of the fragmented plant material were weighed and packed in litter bags. The
litter bags were made with nylon fabric, with a mesh size of 2 mm and
dimensions of 0.20 m x 0.20 m. Four litter bags for each cover crop treatment
were distributed in the rows of cocoa trees in each experimental plot in direct
contact with the soil surface. The decomposition rate of the cover crop
residues was evaluated in the field during December 2021 to April 2022. The
following litter bag collection times were considered: 0, 47, 94, 116 and 136
days after deposition in the field. A litter bag was collected from each
treatment after each period. The material was removed from the litter bag, and
washed in distilled water under a screen through a 0.053 mm mesh to remove soil
particles. The material was then dried in a forced-air circulation oven at 65°C
until reaching constant weight (45).
Finally, the material was weighed to obtain the remaining dry mass. The
remaining mass percentage (R%) was calculated using the relationship between
the final dry weight (Wf) and the initial dry weight (Wi), according to the
expression: R%= (Wf/Wi) x 100. The exponential model proposed in equation
(1) was used to describe the decomposition rate of the residues (44).
were:
X = amount of dry
phytomass remaining after a period of time t, in days
X0 = initial amount of dry
phytomass
k = residue decomposition constant
The half-life time was calculated using the value of k, which
represents the time required for the decomposition of half of the initial plant
residues. This was obtained through the simple exponential linearization model (18),
calculated by equation (2).
Chemical
characterization of residues after decomposition
The material
remaining in the litter bag collected after the final decomposition period was
washed and dried in an oven at 65℃ for chemical characterization. Three
subsamples of each treatment were separated, ground and the total nitrogen
content was determined by the Kjeldahl method (13). Additionally, the
lignin and cellulose concentrations were determined using the acid detergent
fiber (ADF) method (48).
Statistical
analysis
The data analysis
was conducted considering the following factors: treatment (cover crops); the
decomposition period and characterization of the residues after decomposition;
the collection time of the litter bags; and the interaction of these factors.
The data were analyzed for homogeneity of error variances by the Cochran test
and normality by the Lilliefors test, followed by analysis of variance (ANOVA);
mean comparison in significant cases was applied by the Tukey’s test
(p<0.05), using the RStudio program, version 3.5.0 (R-Development
Core Team 2019).
Results
and discussion
The precipitation
in the period which included the growth of cover crops (March to June 2021) was
412mm and the temperature ranged from 31°C to 18°C (figure 1).
Dry
mass production and nutrient accumulation
Higher dry mass production was observed in the brachiaria
treatment (11.9 Mg ha-1),
followed by spontaneous vegetation (10.3 Mg ha-1)
and pigeon pea (7.9 Mg ha-1)
(figure
2).
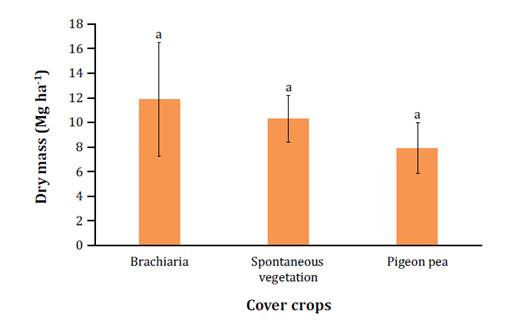
Letras iguales
indican que no hay diferencias entre tratamientos según la prueba de Tukey
(p<0,05). Las barras indican los valores del error estándar de las medias.
Figure
2.
Dry mass production (March - July 2021) of cover crops intercropped with cocoa
trees.
Figura 2. Producción
de materia seca (Marzo - Julio 2021) de cultivos de cobertura intercalados con
cacao.
The dry matter
production in the three treatments is considered adequate for conservationist
agricultural systems, as the values were between 6 Mg ha-1 and 12 Mg ha-1, which is the
average amount necessary to provide sufficient soil coverage and ensure
beneficial effects on the physical, chemical, and biological attributes of the
soil, especially in tropical climate regions (1,
43).
It is important to
note that the dry mass production is influenced by the location and time of
cultivation, climatic conditions, soil fertility, species used, and cutting age
(24,
39). These factors (table 1 and figure
2)
were also responsible for the dry mass production obtained in the present
study. A study (23) in the
municipality of Diamantina, Minas Gerais state, Brazil, implementing a
no-tilling system using cover crops observed a dry mass of 4.54 Mg ha-1
for spontaneous vegetation and 4 Mg ha-1 for pigeon pea,
constituting lower values than those observed in the current study (10.3 and
7.9 Mg ha-1 for spontaneous
vegetation and pigeon pea, respectively). These same authors reported a
production of 11.2 Mg ha-1 for Urochloa decumbens,
which is a similar result to that found in the present investigation. Regarding
the spontaneous vegetation treatment, the high dry mass production observed in
this study can also be attributed to the occurrence of two grasses with great
biomass production capacity, Megathyrsus
maximus (sub. Panicum maximum L.)
and Sorghum
bicolor subsp. verticilliflorum
(sub. S. arundinaceum), present in
the experimental area. Furthermore, the adequate precipitation conditions
during the growth of cover crops (figure 1) were a key factor
in the dry mass achieved (24, 28, 39).
When evaluating
different cover crop species in an orange orchard in Cruz das Almas, Bahia
state, Brazil, Carvalho
et al. (2021) reported the highest dry matter production of 11 Mg ha-1
for Urochloa decumbens and U. eminii. In this same
study, spontaneous vegetation had the lowest dry matter production, which
differs from the results of the current investigation. The discrepancy in the
performance of spontaneous vegetation between the two studies could be
attributed to differences in factors such as soil fertility, climatic
conditions, and the specific species composition of the spontaneous vegetation (23,
28, 39).
Significant differences regarding nutrient accumulation (table
2) were only observed for potassium (K+).
Table 2.
Nutrient accumulation (March-July 2021) of cover crops intercropped with cocoa
trees.
Tabla 2. Acumulación
de nutrientes (Marzo-Julio 2021) de plantas de cobertura intercaladas con
cacao.
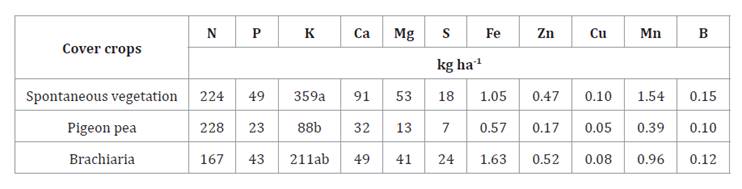
Averages followed by the same
letter in the columns do not differ from each other, ns: not significant by
Tukey’s test (p<0.05). N: nitrogen; P: phosphorus; K: potassium; Ca:
calcium; Mg: magnesium; S: sulfur; Fe: iron; Zn: zinc; Cu: copper; Mn:
manganese; B: boron.
Promedios seguidos por la misma letra en las
columnas no difieren entre sí, ns: no significativo por la prueba de Tukey
(p<0,05). N: nitrógeno; P: fósforo; K: potasio; Ca: calcio; Mg: magnesio; S:
azufre; Fe: hierro; Zn: zinc; Cu: cobre; Mn: manganeso; B: boro.
The spontaneous vegetation treatment exhibited the highest K+
accumulation, reaching 359 kg ha-1,
which was significantly greater than the pigeon pea treatment, presenting the
lowest K+ accumulation at 88 kg ha-1. A substantial K+
accumulation was also observed in the brachiaria treatment although
it did not differ significantly from the other treatments. The results indicate
a direct relationship between potassium accumulation by the treatment and dry
matter production (figure 2
and table
2). The K+ accumulation in the
spontaneous vegetation was approximately five times greater than that of the
pigeon pea treatment. The high dry matter production and considerable K+
accumulation in the spontaneous vegetation suggest that this
treatment may provide similar nutrient cycling benefits as those observed with
purposefully cultivated cover crops species (39).
Therefore, when spontaneous plant communities possess the characteristics of
high biomass production and nutrient accumulation, they can be considered
viable alternatives for cover cropping, as they can deliver the benefits of
implanted species without the establishment cost.
Forage
grasses are reported to exhibit great potential for K+ absorption
and subsequent accumulation, which is then returned to the soil following the
decomposition of plant residues (6).
Grasses in no-tillage systems can leverage their deep root system, remove
nutrients from depth and subsequently return them to the soil surface through
decomposition of their plant residues (36).
Studies indicate that brachiaria grasses are highly efficient in K+
cycling, corroborating the observations made in the present study (22,
40, 45). It is noteworthy that high values regarding nitrogen (N)
accumulation were observed across the treatments, ranging from 167 to 228 kg ha-1,
although no statistically significant differences were detected (table
2). The ability of cover crops to accumulate N is primarily
dependent on the specific species used (32).
In a study conducted in the state of Piauí, Brazil, the N accumulation observed
for pigeon pea was similar to the values reported in the current study (260 kg
ha-1); however, the accumulated N value for Urochloa eminii (sub. U.
ruziziensis) was higher compared to the present findings (253 kg ha-1)
(41).
Decomposition rate and half-life (T1/2)
The decomposition of cover crop residues decreased exponentially
over time. During the 136-day evaluation period, the pigeon pea treatment
exhibited the lowest loss of dry mass in its residues. In contrast, the brachiaria
and spontaneous vegetation treatments showed greater degradation of their
residues, with similar patterns observed between these two treatments (figure
3).
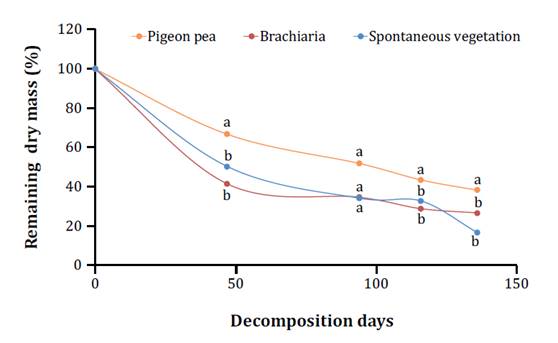
Diferentes
letras minúsculas indican diferencias entre tratamientos para materia seca
remanente, el mismo día de descomposición, utilizando la prueba de Tukey
(p<0,05).
Figure
3.
Remaining dry mass (%) of cover crops, intercropped with cocoa trees.
Figura 3. Materia
seca restante (%) de cultivos de cobertura, intercalados con árboles de cacao.
The highest
decomposition rates were observed for brachiaria and spontaneous vegetation
treatments, with only 41.5% and 50.4% of residues remaining, respectively, in
the first 47 days after deposition in the field (figure 3). These treatments
differed significantly from the pigeon pea treatment for this evaluation
period, which exhibited a higher residue remaining percentage of 66.8% (figure 3). By the end of
the evaluation period (136 days), the spontaneous vegetation and brachiaria
treatments continued to display the highest decomposition rates, with only
16.7% and 26.7% of the deposited material remaining, respectively. These values
differed from the pigeon pea treatment, which had the highest remaining dry
mass on the soil of 38.3% (figure
3).
In evaluating the
decomposition rate of cover crops in the Cerrado biome, it was observed that
the pigeon pea treatment exhibited a higher remaining dry mass (37%) after 154
days of deposition compared to spontaneous vegetation (23%) and brachiaria
(21%) (46), corroborating what was observed in the
present study. However, a contradictory result was observed in a study
conducted in the Cerrado, Góias state, Brazil. In this aforementioned study,
high decomposition rates were reported for brachiaria (48%) and pigeon pea
(65%) 150 days after cutting (16), constituting
contrasting results to what was observed in our study, especially for pigeon
pea. The authors suggest that the elevated decomposition rate in this case may
have been driven by the soil being maintained in a moist condition through
irrigation during the decomposition period.
The spontaneous vegetation and brachiaria treatments displayed
the highest decomposition coefficient (k) of 0.011496 g g-1 d-1 and 0.009154 g g-1
d-1,
respectively. Consequently, these treatments also exhibited the shortest
half-life times (T1/2)
of 60 and 75 days, indicating that half of the residues had decomposed by those
time points (table 3).
Table
3. Decomposition coefficient (k) and
half-life time (T1/2) of cover crops intercropped with cocoa.
Tabla 3.
Coeficiente de descomposición (k) y tiempo de vida media (T1/2) de
cultivos de cobertura intercalados con cacao.
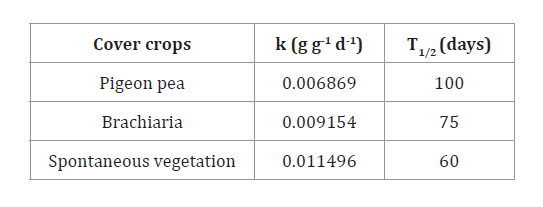
The pigeon pea
exhibited a lower decomposition coefficient (k), resulting in an estimated T1/2
of 100 days. Various factors influence the decomposition rate of
plant residues, with the chemical composition being one of these factors. The
higher the C/N ratio and higher cellulose, hemicellulose, lignin, and
polyphenols levels in the plant constituents lead to slower decomposition of
phytomass (2, 37). In a study
conducted to evaluate the decomposition rate of cover crops in the Cerrado
region during the 2001/2002 period, it was observed that the T1/2 for brachiaria was 78
days, while for pigeon pea it was 101 days (45). This finding
aligns with the results obtained in the present study. The same authors also
mention that Fabaceae plants in uncovered Cerrado soil exhibited slower
decomposition compared to grasses.
The extended
persistence of pigeon pea residues in the soil can be attributed to its
composition, characterized by a significant proportion of lignified stems that
impede rapid decomposition. This assertion is supported by the analysis
conducted on plant residues after the deposition period in the field, as
outlined in table
4.
Table 4.
Nitrogen, cellulose, and lignin concentrations in cover crop residues after 136
days deposited in the field.
Tabla
4.
Concentración de nitrógeno, celulosa y lignina en residuos de cultivos de
cobertura después de 136 días depositados en el campo.
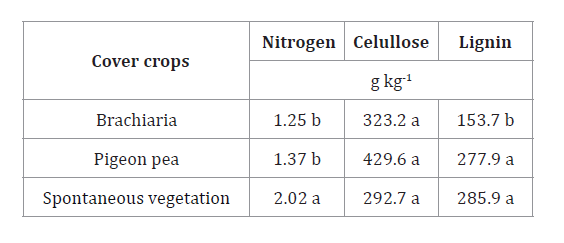
Means followed by the same letter
in the columns do not differ from each other by the Tukey’s test (p<0.05).
Los promedios seguidos por la misma
letra en las columnas no difieren entre sí por la prueba de Tukey (p<0,05).
Pigeon pea and
spontaneous vegetation exhibited the highest lignin contents, which differed
significantly from brachiaria, showing the lowest lignin contents. It is worth
noting that cover crops cut during flowering stages tend to have higher
hemicellulose and lignin concentrations (8). The same authors
also reported higher lignin concentrations during flowering for Cajanus
cajan, a species that displayed a slower decomposition rate. The same
occurred in this study for pigeon pea, which exhibited elevated lignin
concentrations (table
4)
and a reduced decomposition rate (figure 3). In contrast, the spontaneous vegetation presented high lignin
content and a rapid decomposition rate.
The chemical composition of plant residues, including lignin,
cellulose, hemicellulose, and polyphenols, as well as the C/N ratio and the
lignin: N ratio, play a significant role in the decomposition process. Lignin
presents a challenge to decomposition due to its resistance and impermeability
to microbial attack in plant tissues (7, 9).
In a study conducted by the same authors, the lignin contents of cover crops
species were assessed, with the highest concentration observed in pigeon pea cv.
mandarin. Conversely, Brachiaria ruziziensis exhibited
the lowest lignin concentration. This composition, contributes to the slower
decomposition rate of pigeon pea and the faster decomposition rate of
brachiaria. The degradation process is hindered by the presence of lignin
because only a limited number of microorganisms possess the necessary enzymes
to break down its chemical bonds (15).
In addition to
lignin, nitrogen also plays a significant role in the decomposition process.
The spontaneous vegetation treatment presented the highest nitrogen contents,
which differed from the other treatments, as indicated in table 4. Higher nitrogen
concentrations in plant tissues enable microorganisms to oxidize amide bonds
(NH2) of organic
molecules. This process provides energy for microbial growth and facilitates
decomposition (3). The presence of
higher nitrogen levels in the spontaneous vegetation treatment justifies the
observed remaining dry mass at the end of the study, which was similar to that
of the brachiaria treatment, despite having similar lignin contents to pigeon
pea. The increased nitrogen levels in spontaneous vegetation promoted greater
microbial activity in the plant tissue, thereby facilitating decomposition even
in the presence of lignin levels.
Conclusions
The cover crops investigated in this study are regarded as
promising options for intercropping with cocoa, as they exhibit an average dry
mass production of 10 Mg ha-1.
This value falls within the desired range for conservationist systems. When
selecting species for intercropping, it is crucial to consider the
decomposition rates these plants. This consideration ensures that the soil
surface remains covered for an extended duration, leading to enhanced
conservation and improvement of the soil’s physical, chemical, and biological
properties. Soil conservation can be achieved by choosing cover crop species
with slower decomposition rates, in turn contributing to the overall health and
quality of the soil.
Acknowledgments
The authors would like to acknowledge CEPLAC (Comissão Executiva
do Plano da Lavoura Cacaueira) for granting the experimental area and technical
support in the development of the study; CNPq for financial support (Process
Number: 427047/2018-8); and the PQ Fellowship (Processes number:
307027/2020-1).
1. Alvarenga, R.
C.; Cabezas, W. A. L.; Cruz, J. C.; Santana, D. P. 2001. Plantas de cobertura
de solo para sistema plantio direto. Informe Agropecuário, Belo Horizonte. 22:
25-36.
2. Araújo, L. da.
S.; da Cunha, P. C. R.; Silveira, P. M.; de Sousa Netto, M.; de Oliveira, F. C.
2015. Potencial de cobertura do solo e supressão de tiririca (Cyperus rotundus) por resíduos culturais
de plantas de cobertura. Revista Ceres. 62(5): 483-488.
https://doi.org/10.1590/00347 37X201562050009
3. Assis, R. L.;
Boer, C. A.; Pacheco, L. P.; Braz, A. J. B. R.; Costa K. A. P.; Torres, J. L.
R. 2016. Produção e impacto de biomassa de plantas de cobertura cultivadas na
primavera. Revista Energia na Agricultura. 31(4): 32333.
https://doi.org/10.17224/EnergAgric.2016v31n4p328-333
4. Associação
Nacional das Indústrias Processadoras de Cacau (AIPC). 2021. Estatísticas:
Recebimento. Online. http://aipc.com.br/estatisticas/recebimento/. (Data da
consulta: 08/12/2022).
5. Azpilicueta, C.
V.; Aruani, M. C.; Reeb, P. 2023. Cover crops in pear (Pyrus communis)
orchards: effects on soil nematode assemblage. Revista de la Facultad de
Ciencias Agrarias. Universidad Nacional de Cuyo. Mendoza. Argentina. 55(2):
85-96. DOI: https://doi.org/10.48162/ rev.39.111
6. Brito, L. C. R. A.; Souza, H. A.; Araújo Neto, R. B.;
Azevedo, D. M. P.; Sagrilo, E.; Vogado, R. F.; Carvalho, S. P.; Ferreira, A. C.
M.; Cavigelli, M. A. 2023. Improved soil fertility, plant nutrition and grain
yield of soybean and millet following maize intercropped with forage grasses
and crotalaria in the Brazilian savana. Crop Pasture Sci. doi:
10.1071/CP22251
7. Carvalho, A. M.;
Dantas, R. A.; Coelho, M. C.; Lima, W. M.; Souza, J. P. S.; Fonseca, O. P.;
Guimarães Junior, R. 2010. Teores, celulose e lignina em plantas de cobertura
para sistema plantio direto no Cerrado. Planaltina, DF: Embrapa Cerrados. 15p.
Boletim de Pesquisa e Desenvolvimento.
8. Carvalho, A. M.
D.; Souza, L. L. P. D.; Guimarães Júnior, R.; Alves, P. C. A. C.; Vivaldi, L.
J. 2011. Cover plants with potential use for crop-livestock integrated systems
in the Cerrado region. Pesquisa Agropecuária Brasileira. 46:1200-1205.
https://doi.org/10.1590/ S0100204X2011001000012
9. Carvalho, A. M.
D.; Coser, T. R.; Rein, T. A.; Dantas, R. D. A.; Silva, R. R.; Souza, K. W.
2015. Manejo de plantas de cobertura na floração e na maturação fisiológica e
seu efeito na produtividade do milho. Pesquisa Agropecuária Brasileira. 50:
551-561.
10. Carvalho, J. E.
B.; Xavier, F. A. S; Santos, N. S. 2021. Decomposição e liberação de nutrientes
por diferentes plantas de cobertura em um pomar de laranjeira. Cruz das Almas,
BA: Embrapa Mandioca e Fruticultura. 26. Boletim de Pesquisa e Desenvolvimento.
11. Crusciol, C. A.
C.; Cottica, R. L.; Lima, E. V.; Andreotti, M.; Moro, E.; Marcon, E. 2005.
Persistência de palhada e liberação de nutrientes do nabo forrageiro no plantio
direto. Pesquisa Agropecuária Brasileira, Brasília. 40(2): 161-168.
https://doi.org/10.1590/ S0100204X2005000200009.
12. Dalla Rosa, J.;
Mafra, A. L.; Medeiros, J. C.; Albuquerque, J. A.; Miquelluti, D. J.; Nohatto,
M. A.; Ferreira, E. Z.; Oliveira, O. L. P. 2013. Soil physical properties and
grape yield influenced by cover crops and management systems. Revista
Brasileira de Ciência do Solo. 37(5): 1352-1360.
13. Detmann, E.; E.
Silva, L. F. C.; Rocha, G. C.; Palma, M. N. N.; Rodrigues, J. P. P. 2021.
Métodos para análise de alimentos: INCT-Ciência Animal. 2° ed. Minas Gerais:
Visconde do Rio Branco. 350 p.
14. International
Cacao Organization. Statistics (ICCO). 2022. Production.
https://www.icco.org/wp-content/uploads/Production_QBCS-XLVIII-No.-2.pdf. (Data
da consulta: 16/01/ 2023).
15. Janusz, G.;
Pawlik, A.; Sulej, J.; Świderska-Burek, U.; Jarosz-Wilkołazka, A.; Paszczyński,
A. 2017. Lignin degradation: microorganisms, enzymes involved, genomes analysis
and evolution. FEMS microbiology reviews. 41: 6.941-962.
16. Kliemann, H.
J.; Braz, A. J. P. B.; Silveira, P. M. da. 2006. Taxas de decomposição de resíduos
de espécies de cobertura em Latossolo Vermelho distroférrico. Pesquisa
Agropecuária Tropical. 36: 21-28.
17. Läderach, P.;
Martinez-Valle, A.; Schroth G.; Castro, N. 2013. Predicting the future climatic
suitability for cocoa farming of the world’s leading producer countries, Ghana
and Côte d’Ivoire. Clim Chang. 119(3-4): 841-854.
https://doi.org/10.1007/s10584-013-0774-8
18. Landsberg, J.
J.; Gower, S. T. 1997. Applications of physiological ecology to forest
management. New York. Academic Press.
19. Lucena, C. C.;
Carvalho, J. E. B.; Xavier, F. A. S. 2017. Manejo de coberturas vegetais em
pomares de citros nos tabuleiros costeiros. Cruz das almas, BA: Embrapa
Mandioca e Fruticultura. 48p.
20. Maia, A. H.;
Souza, V. S.; Souza, M. E. 2019. Produtividade de bananeiras BRS princesa
consorciada com adubos verdes em Nova Xavantina, Mato Grosso, Brazil. Brazilian
Journal of Development. 5: 29772-29785. https://doi.org/10.34117/bjdv5n12-120
21. Malavolta, E.;
Vitti, G. C.; Oliveira, A. S. 1997. Avaliação do estado nutricional das
plantas: principios e aplicações. 2° ed. Piracicaba, Potafós. 319 p.
22. Menezes, L. A.
S.; Leandro, W. M. 2004. Avaliação de espécies de coberturas do solo com
potencial de uso em sistema de plantio direto. Pesquisa Agropecuária Tropical.
Goiânia. 34(3): 173-180.
23. Nunes, U. R.;
Andrade Júnior, V. C.; Silva, E. D. B.; Santos, N. F.; Costa, H. A. O.;
Ferreira, C. A. 2006. Produção de palhada de plantas de cobertura e rendimento
do feijão em plantio direto. Pesquisa Agropecuária Brasileira. 41: 943-948.
24. Pacheco, L. P.;
Leandro, W. M.; de Almeida Machado, P. L. O.; de Assis, R. L.; Cobucci, T.,
Madari, B. E.; Petter, F. A. 2011. Produção de fitomassa e acúmulo e liberação
de nutrientes por plantas de cobertura na safrinha. Pesquisa Agropecuária
Brasileira. 46(1): 17-25.
25. Pacheco, L. P.;
Monteiro, M. M. S.; Petter, F. A; Nóbrega, J. C. A.; Santos, D. S. 2017.
Biomass and nutrient cycling by cover crops in Brazilian Cerrado in the state
of Piaui. Revista Caatinga. 30: 13-23.
26. Piasentin, F.
B.; Saito, C. H. 2012. Caracterização do cultivo de cacau na Região Econômica
Litoral Sul, Sudeste da Bahia. Estudo & Debate, Lajeado. 19(2): 63-80.
27. Pires, M. D. F.
M.; Medeiros, J. C.; Souza, H. A. D.; Rosa, J. D.; Boechat, C. L.; Mafra, A.
L.; Rocha, A. G. D. 2020. Conservation system improves soil microbial quality
and increases soybean yield in the Northeastern Cerrado. Bragantia. 7927,(4): 599-611.
28. Pissinati, A.;
Moreira, A.; Santoro, P. H. 2018. Yield components and nutrients content in
summer cover plants used in crop rotation in no-tillage system. Communications
in Soil Science and Plant Analysis. 14: 1-13.
https://doi.org/10.1080/00103624.2018.1474899
29. Quaresma, M. A.
L.; Oliveira, F. L.; Silva, D. M. N.; Coelho, R. I.; Costa, E. C. 2015.
Desempenho de bananeiras cultivar “nanicão” sobre cobertura viva de solo no
Semiárido. Revista Caatinga. 28(04): 110-115.
https://doi.org/10.1590/1983-21252015v28n412rc
30. Quaresma, M. A. L.; Oliveira, F. L.; Silva, D. M. N. 2017. Leguminous
cover crops for banana plantations in Semi-Arid Regions. Revista Caatinga,
Mossoró. 30(3): 614-621. https://doi. org/10.1590/1983-21252017v30n309rc
31. R-Development
Core Team. 2019. A Language and Environment for Statiscal Computing: Foundation
for Statiscal Compunting: Vienna, Austria.
32. Redin, M.;
Recous, S.; Aita, C.; Chaves, B.; Pfeifer, I. C.; Bastos, L. M.; Giacomini, S.
J. 2018. Contribuição de raízes e parte aérea para aportes de carbono e
nitrogênio na camada superficial do solo em sistemas de plantio direto sob
condições subtropicais. Revista Brasileira de Ciência do Solo. 42.
33. Sanches, G. C.
S. 2019. Análise de viabilidade econômica dos principais modais de produção de
cacau no Sul da Bahia: Cabruca e SAF-Cacau Seringueira. 94 f. Dissertação
(Pós-Graduação em Desenvolvimento econômico). Instituto de Economia,
Universidade Estadual de Campinas, Campinas, SP. 2019.
34. Santana, E. N.;
Kuhlcamp, K.; do Monte, F. D. M.; Souza, L.; Gouvea, R.; Santos, A.; Pire, J.
L. 2021. Ocorrência do peco fisiológico em genótipos de cacaueiro no sistema
alternativo de cultivo (pleno sol) no norte capixaba. In: Congresso Capixaba de
Pesquisa Agropecuária. 1: Vitória, ES.
https://biblioteca.incaper.es.gov.br/digital/handle/item/4156. (Data de consulta
05/01/2023).
35. Santana, S. O.;
Santos, R. D.; Gomes, I. A.; Jesus, R. M.; Araujo, Q. R.; Mendonça, J. R.;
Calderano, S. B.; Faria Filho, A. F. 2002. Solos da região Sudeste da Bahia:
atualização da legenda de acordó com o sistema brasileiro de classificação de
solos Ilhéus: Ceplac; Rio de Janeiro: Embrapa Solos. Boletim de Pesquisa e
Desenvolvimento, 16.
36. Santos, F. C.
D.; Albuquerque Filho, M. R. D.; Vilela, L.; Ferreira, G. B.; Carvalho, M. D.
C. S.; Viana, J. H. M. 2014. Decomposição e liberação de macronutrientes da
palhada de milho e braquiária, sob integração lavoura-pecuária no cerrado
baiano. Revista Brasileira de Ciência do Solo. 38(6): 1855-1861.
https://doi.org/10.1590/S010006832014000600020
37. Santos, R.;
Siqueira, R.; Lima, C.; Almeida, A.; Pedrosa, A.; Oliveira, C. 2009.
Decomposição e liberação de nitrogênio de duas espécies de adubos verdes
manejados no período seco em cafezal. Revista Brasileira de Agroecologia. 4:
1342-1345.
38. Silva, M. L.
N.; Curi, N.; Blancaneaux, P.; Lima, J. M.; Carvalho, A. M. 1997. Rotação adubo
verde-milho e adsorção de fósforo em Latossolo Vermelho-Escuro. Pesq. Agropec.
Bras. 32: 649-654.
39. Silva, M. P.;
Arf, O.; Sá, M. E. DE; Abrantes, F. L.; Berti, L. F.; Souza, L. C. D.; Arruda,
N. 2014. Palhada, teores de nutrientes e cobertura do solo por plantas de
cobertura semeadas no verão para semeadura direta de feijão. Revista Agrarian.
Dourados. 7(24): 233-243.
40. Silveira, P.
M.; Cunha, P. C. R.; Stone, L. F.; Santos, G. G. 2010. Atributos químicos de
solo cultivado com diferentes culturas de cobertura. Pesquisa Agropecuária
Tropical. 40: 283-290.
41. Sousa, D. C.;
Medeiros, J.C.; Lacerda, J. J. J.; Dalla Rosa J.; Boechat, C. L.; Souza, M. N.
G.; Rodrigues, P. C. F.; Oliveira Filho, E. G.; Mafra, A. L. 2019. Dry Mass
Accumulation, Nutrients and Decomposition of Cover Plants. Journal of
Agricultural Science. 11: 152-160. https://doi. org/10.5539/jas.v11n5p152
42. Sousa, I. R.
L.; Pauletto, D.; Lopes, L. S. S.; Rode, R.; Peleja, V. L.; Freitas, B. B.2020.
Taxa de decomposição foliar de espécies utilizadas em sistemas agroflorestais.
Revista Verde de Agroecologia e Desenvolvimento Sustentável. 15(2): 118-126.
43. Teodoro, R. B.;
Oliveira, F. L. D.; Silva, D. M. N. D.; Fávero, C.; Quaresma, M. A. L. 2011.
Aspectos agronômicos de leguminosas para adubação verde no Cerrado do Alto Vale
do Jequitinhonha. Revista Brasileira de Ciência do Solo. 35(2): 635-640.
https://doi.org/10.1590/S0100- 06832011000200032
44. Thomas, R. J.;
Asakawa, N. M. 1993. Decomposition of leaf litter from tropical forage grasses
and legumes. Soil Biology & Biochemistry. 25: 1351-1361.
45. Torres, J. L.
R.; Pereira, M. G.; Fabian, A. J. 2008a. Produção de matéria seca por plantas
de cobertura e mineralização de seus resíduos em plantio direto. Pesq. Agropec.
Bras. 43: 421-428. https://doi.org/10.1590/S0100-204X2008000300018.
46. Torres, J. L.
R.; Pereira, M. G. 2008b. Dinâmica do potássio nos resíduos vegetais de plantas
de cobertura no Cerrado. Revista Brasileira de Ciência do Solo. Viçosa. 32(4):
1609-1618. https://doi.org/10.1590/S0100-06832008000400025.
47. Urbano, C. N.;
Simonete, M. A.; Ernani, P. R.; Chaves, D. M.; Moro, L. 2018. Aporte de
serapilheira e nutrientes ao solo em povoamentos jovens de Eucalyptus no
planalto catarinense. Revista Ecologia e Nutrição Florestal. 6(2): 33-44.
doi.org/10.5902/2316980X27068
48. Van Soest, P. J.; Wine, R. H. 1968. The determination of
lignin and cellulose in acid-detergent fibre with permanganate. Journal of the
Association of Official Analytical Chemists, Bethesda. 51: 780-785.