Revista de la Facultad de Ciencias
Agrarias. Universidad Nacional de Cuyo. Tomo 57(1). ISSN (en línea) 1853-8665.
Año 2025.
Original article
Nutritional
and morpho-anatomical characterization of Phyllostachys aurea (Poaceae,
Bambusoideae, Bambuseae) foliage for Argentine livestock systems
Caracterización
nutricional y morfo-anatómica del follaje de Phyllostachys aurea (Poaceae,
Bambusoideae, Bambuseae) para sistemas ganaderos en Argentina
María Gabriela Fernández Pepi1,
1 Universidad de Buenos Aires. Facultad de Agronomía. Dpto. de
Producción Animal. Cátedra de Nutrición Animal Av. San Martín 4453. C1417DSE.
Buenos Aires. Argentina.
2 Universidad de Buenos Aires. Facultad de Agronomía. Dpto. de
Recursos Naturales y Ambiente. Cátedra de Botánica General.
3 Laboratorio de Paleobotánica. CICYTTP (CONICET/Prov.
E.R./UADER). España 149. Diamante (E3105BWA). Entre Ríos. Argentina.
4 ConsejoNacional de Investigaciones Científicas y Técnicas
(CONICET). Buenos Aires. Argentina.
* wawrzkie@agro.uba.ar
Abstract
Bamboo cultivation
in Argentina could represent a major economic activity if its various
applications were revealed. This study characterized the anatomy and
micromorphology of leaf blades by optical and scanning electron microscopes.
Foliage leaves presented predominant parenchyma and scarce sclerenchyma.
Foliage chemical and biological composition were analyzed in 3 populations of P.
aurea sampled in two contrasting seasons of the year. The six samples
evaluated showed 13% protein, adequate for ruminant feed. Neutral detergent
fiber (aNDFom) was approximately 60% DM, a probable limiting factor for
consumption. Significant differences in ADFom (acid detergent fiber) and ADLom
(acid detergent lignin) favored spring results, with lower values than winter
results. The presence of silica in different cell types could limit digestion.
Fermentation kinetics indicated that dry matter digestibility is close to 50%,
and higher in spring given lower amounts of indigestible components. In
addition, all samples analyzed had a low content of immediately soluble
material and a high content of potentially fermentable insoluble material.
Anatomy and chemical-nutritional characterization allow P. aurea foliage
to be considered in ruminant feeding.
Keywords: woody bamboo, nutritional
value, leaf anatomy and morphology, ruminant feed
Resumen
El cultivo de los
bambúes en la Argentina no ha alcanzado la relevancia merecida, pudiendo
representar un valor económico más importante si se hicieran conocer sus
diversas aplicaciones. En el presente trabajo, se caracterizó la anatomía y
micromorfología de las láminas mediante microscopio óptico y microscopio
electrónico de barrido. Se analizó la composición química y biológica del
follaje en 3 poblaciones de P. aurea muestreadas en dos estaciones
contrastantes del año. Las hojas del follaje presentaron una predominancia de
tejido parenquimático sobre el esclerénquima, el cual fue escaso. La presencia
de sílice en diferentes tipos celulares podría limitar la digestión. El
promedio de proteína bruta fue 13% para las seis muestras analizadas y resulta
aceptable para alimentación de rumiantes en mantenimiento. A su vez, se observó
un contenido de aFDNmo (Fibra en detergente neutro) del 60%bs, lo cual puede
ser limitante para el consumo. Se identificó una diferencia significativa en
FDAmo (Fibra en detergente ácido) y LDA mo (Lignina en detergente ácido) entre
invierno y primavera, favorable a la primavera con menores valores de dichos
analitos. La cinética de fermentación indicó que la digestibilidad de la
materia seca es cercana al 50%, siendo mayor en primavera dada la menor
cantidad de componentes indigestibles. Además, para todas las muestras
analizadas, el contenido de material inmediatamente soluble fue bajo, mientras
que el material insoluble potencialmente fermentable fue elevado. Los resultados
del análisis anatómico, complementado con la caracterización químico
nutricional, permiten considerar al follaje de P. aurea en la
alimentación de rumiantes.
Palabras clave: bambú leñoso, valor
nutricional, anatomía y morfología de las hojas, alimentación para rumiantes
Introduction
The millennial
cultivation of woody bamboo in Southeast Asia has recently gained attention in
some tropical and subtropical countries of America (e.g. Colombia,
Ecuador, Bolivia, Brazil and Chile (Londoño, 2009). Parodi
(1943)
stated that bamboo cultivation in Argentina had not reached its productive and
economic potential. Its development should be encouraged by revealing its
various applications. Woody bamboo provides human feed (24), and materials for
utensils, cosmetic products, and crafts (22,
28, 30). Other uses are related to forage, paper pulp, fibers, biochar
and pyrolysis compounds (1, 2, 3, 10).
Woody bamboo has
rapid vegetative growth from vigorous rhizomes (27). Clumps can be
used after 3 to 4 years of implantation. In addition to high biomass
production, wide adaptability and evergreen leaves make them potential
candidates in forage production (40). After rational
culm cutting, bamboo gives annual harvests for 30 to 120 years, depending on
the species (17, 18). The vegetative
growth phase of Phyllostachys aurea Carrière ex Rivière & C. Rivière
lasts 15 to 30 years, followed by flowering (30,
37) with no clump death. In Argentina, this exotic species is
cultivated for ornamental purposes and its culms are used in construction and
crafts, while shoots are edible (39, 48). Bamboo foliage is
an alternative forage for domestic cattle. However, information on its
chemical-nutritional composition as feed for ruminants is scarce (29). Other countries
have identified several genotypes, growing sites, and seasons for forage
variability (6, 34, 40, 47). Biomass
production occurs in the temperate and rainy season, with growth impeded in
winter, under 10°C (19).
Panizzo et al. (2017)
nutritionally evaluated the foliage of the native woody bamboo Guadua
chacoensis (Rojas Acosta) Londoño & P. M. Peterson and suggested its
use as grazing supplement. In southern Argentina, Chusquea culeou E.
Desv. (“caña colihue”) constitutes the main winter forage for bovine diet while
other grasses remain covered in snow (25).
We aimed to nutritionally and morpho-anatomically characterize P. aurea foliage
for ruminant feed.
Materials
and methods
Selection
and collection of material
We used foliage
leaves from 3 populations of the woody bamboo P. aurea: Lucien Hauman
(S: 34°35’; E: 58°28’), Arturo Ragonese (S: 34°36’; E: 58°40’) Botanical
Gardens and wild bamboo from Buenos Aires Delta (S: 34°24’; E: 58°33’). We
sampled in two contrasting production seasons, spring (October 2017) and winter
(July 2018). Nine culms were harvested at each location (i.e. 3 culms ×
3 bamboo sites per harvest). A pool of foliage leaves was used in nutritional
characterization of each population. Additionally, segments of the middle
portion of blades were selected for anatomical and micromorphological studies.
The climate was humid temperate (figure 1) (20).
Monthly rainfall and mean temperature did not restrict clumps growth of P.
aurea. However, summer in 2018 was drier with rains accumulated in May and
June.
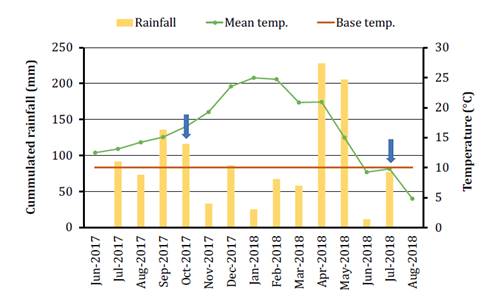
Figure 1. Average
temperatures and cumulated rainfall during the months before Phyllostachys
aurea foliage harvest (20);
and growth base temperature of bamboo species according to Halvorson et al. (2010).
Figura 1. Temperaturas
promedio y precipitaciones acumuladas durante los meses antes de la cosecha del
follaje de Phyllostachys aurea (20)
y temperatura base de crecimiento de especies de bambú según Halvorson et al. (2010).
Morphological
and anatomical studies
The species’ growth
habit and leaf size, consistency, and indumentum followed McClure
(1966)
and Judziewicz
et al. (1999) terminology. Foliage leaf blades collected in spring were
dehydrated in alcohol series and embedded in paraffin following traditional
anatomical techniques (12). Twenty μm-thick
sections were cut with a rotary microtome and stained with safranine-Fast
green. The following anatomical traits were considered: midrib and keel
(position, vascular bundles, adaxial and abaxial sclerenchyma), abaxial and
adaxial epidermis, bulliform cells, arm cells and intercellular spaces [sometimes
referred to as “fusoid cells” (42), described in
accordance to Ellis
(1976, 1979) and Clark (2005). Observations were made with a Nikon®
Microphot FXA optical microscope (Tochigi, Japan). Blade micromorphology was
studied with a Scanning Electron Microscope (SEM, Phillips XL 30 Microscope
(Phillips, The Netherlands). To describe abaxial and adaxial epidermal traits,
small blade fragments were cleaned in xylene for 1.5 h with an ultrasonic
cleaner (Cleanson, model CS 1106, Argentina). The material was air-dried,
mounted, and coated with a gold-palladium (40%-60%) alloy by a Thermo
VGScientific, then observed using a Phillips XL 30 Scanning Electron Microscope
(MACN-CONICET, Argentina). Papillae pattern in long cells follows (49). Foliar anatomy
and micromorphology related content of sclerenchyma, silica cells, macro and
micro hairs, prickle and hooks, with possible forage acceptability. To quantify
phytoliths and describe their association in each period and locality,
articulated (i.e., more than two elements) and non-articulated elements
(i.e.; a single element per morphotype), were considered (15). Morphotype
description involved an ad hoc classification based on the proposals of Neumann
et al. (2019).
Chemical-nutritional
evaluation
Determinations
included: dry matter (DM) by oven-drying at 105°C, ashes by incineration at
550°C (Cen; AOAC,
1990,
No. 942.05), crude protein
(CP; AOAC,
1990,
No. 984.15), neutral and
acid detergent fibers (42) using α-amylase
and ash free (aNDFom and aADFom, respectively), and ash free acid detergent
lignin (ADLom). Silica was determined by calcination technique (Si; Labouriau,
1983).
The in vitro evaluation
of gas production (GP) followed the recommendations of Wawrzkiewicz
et al. (2005). Measurements were made at regular intervals (i.e. 2, 4,
6, 8, 12, 16, 20, 24, 36, 48, 60, and 72 h). In addition, samples were
incubated for up to 48 h to recover undigested residues in ANKOM® F57 filter
bags determining DM digestibility (DMDiv)
and NDF (NDFDiv).
Cumulative net gas production (CNGP) and digestibility were corrected for blank
(i.e. bottles without substrate) and incubated dry matter.
GP kinetics were
adjusted to the equation CNGP = a + b x (1 - e-ct)
(35). CNGP (ml/g DM)
was determined at 12, 24, 48 and 72 h, parameters a, b and c, maximum hourly
rate of GP (RGPMax;
ml/g DM.h) and the time at which it occurs (TRGPmax;
h) and average rate gas production (RGPAv).
Statistical
analysis
Comparisons were
made between spring and winter considering the three populations as replicates.
The data were analyzed with SAS® statistical program. Seasons were compared by
a completely randomized one-way design, with no interactions. A Tukey test compared
means at p < 0.05.
GP rates were
compared via a model considering time (T) as a repeated measure and the
interaction with season (S × T) with a completely randomized two-way design
with interaction. A Tukey test compared means at p < 0.05.
Results
Morphological
studies
The studied clumps were 2-9 m tall (figure 2A).

A:
hábito. B: turión. C: nudos y entrenudos de la parte media de las cañas. D:
nudos, entrenudos basales y hojas del follaje. Escalas: A: 2 m; B: 3 cm; C: 12
cm; D: 1,5 cm.
Figure
2 / Figura 2. Phyllostachys aurea.
Rhizome axillary buds formed edible shoots (figure 2B)
and produced aerial culms. These were woody, hollow, 1-5 cm in diameter, with
grooved internodes on the side of the bud, upper nodes spaced apart, and the
lower ones asymmetric and proximate (figure 2C
and figure
2D). Bamboos have culm leaves and foliage leaves. The formers have
a protective function (figure 2B)
and at maturity are deciduous, constituting abundant litter on the soil
surface. Foliage leaves are photosynthetic and have perennial lanceolate leaf
blades, 5-16 × 1-2 cm (figure 2D).
Anatomical
studies
In cross-section,
foliage leaf blades present characteristic C3 anatomy. Well-developed
midrib, keel projecting abaxially, with a first-order vascular bundle
surrounded by a double sheath consisting of an outer parenchyma sheath and
inner mestome sheath, locked towards both surfaces (figure 3A).
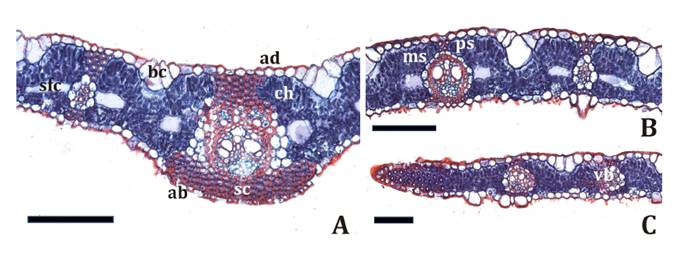
A: keel. B: arm. C:
margin. ad:
adaxial epidermis; ab: abaxial epidermis; bc: bulliform cells; ch:
chlorenchyma; ms: mestome sheath; ps: parenchyma sheath; sc: sclerenchyma; sfc:
spaces between fusoid cells; vb: vascular bundle. Scale bars: 100 μm.
A:
costilla central. B: ala. C: margen. ad: epidermis
adaxial; ab: epidermis abaxial; bc: células buliformes; ch: clorénquima; ms:
vaina mestomática; ps: vaina parenquimática; sc: esclerénquima; sfc: espacios
entre células fusoides; vb: haz vascular. Escalas: 100 μm.
Figure
3. Foliage leaf blade cross-section in Phyllostachys
aurea.
Figura
3. Lámina de Phyllostachys aurea en
transcorte.
The adaxial
epidermis exhibited a single layer of smooth-walled cells and groups of
fan-shaped bulliform cells. The abaxial epidermis was papillose and formed by
various cell types (i.e., long cells, suberous cells, silica cells, and
hooks, (figure
3A, 3B and 3C).
Chlorenchyma was diffuse and exhibited large intercellular
spaces between fusoid cells, and on both sides of the vascular bundles in
contact with parenchyma sheath. Arm cells are thin-walled and asymmetrically
invaginated on the abaxial side. Scarce sclerenchyma. Girders associated with
primary and secondary vascular bundles and strands at blade edges (figure
3C). In superficial view, leaf blades with SEM showed marginal
hooks, smooth adaxial surface (figure 4A and 4B)
and abaxial epidermis formed by a diversity of cell types among which were long
cells with short papillae, stomatal complex slightly sunken with elongated
papillae of the long contiguous cells overarching the stomata (subtype IV),
silica cells, suberous cells, bicellular microhairs, hooks and prickles (figure
4C and 4D).
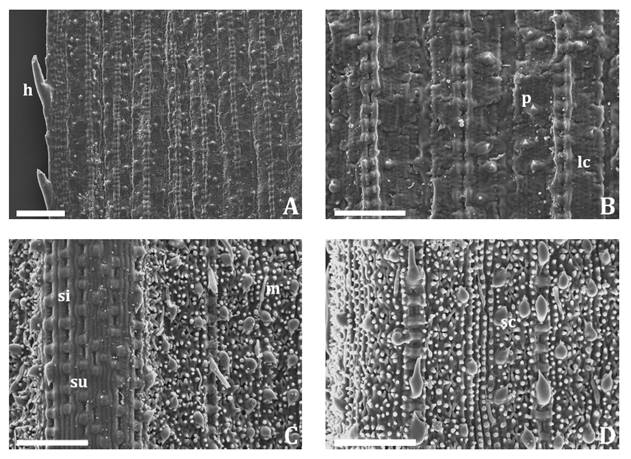
A-B: adaxial
epidermis. C-D: abaxial epidermis. h:
hook; lc: long cell; m: bicellular microhair; p: prickle; sc: stomatal complex;
si: silica cell; su: suberous cell. Scale bars: A: 200 μm; B-D: 100 μm.
A-B, epidermis
adaxial. C-D, epidermis abaxial. h, aguijón; lc,
célula larga; m, micropelo bicelular; p, gancho; sc, aparato estomático; si,
célula silícea; su, célula suberosa. Escalas: A, 200 μm; B-D, 100 μm.
Figure
4. Leaf blade of Phyllostachys aurea observed
with a Scanning Electron Microscope.
Figura 4. Lámina
de Phyllostachys aurea observada con Microscopio Electrónico de Barrido.
The phytolytic association of all analyzed materials presented a
greater abundance of morphotypes accompanying leaf epidermis: silica cells,
suberous cells, microhairs, hooks, prickles, and fan-shaped bulliform cells (figure
5A, 5B and 5C). To a lesser extent, prismatic morphotypes with wavy edges
derived from long cells and short cylindrical elements (figure 5A and 5B).
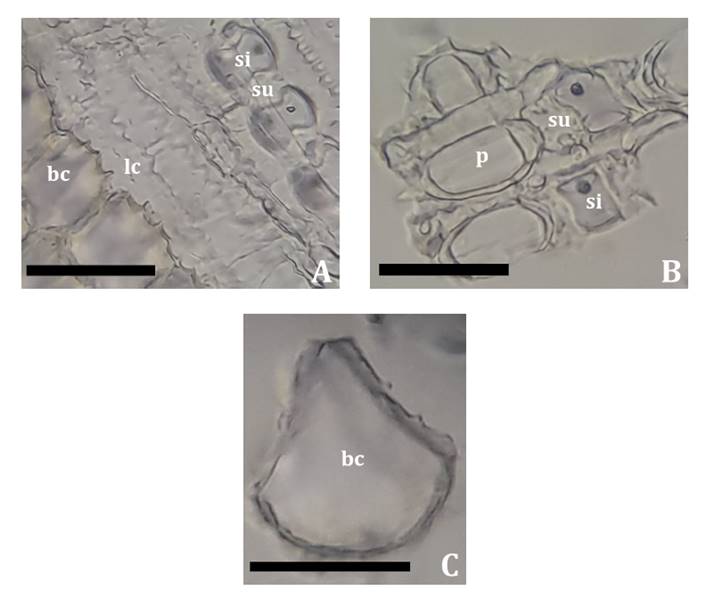
A: Fragment of leaf
blade adaxial epidermis, general view. B: Prickles, silica and suberous cells,
detail. C: Fan-shaped bulliform cell. bc,
bulliform cell; lc, long cell; p, prickle; si, silica cell; su, suberous cell.
Scale bars: 50 μm.
A: Fragmento de
epidermis adaxial de la lámina foliar, vista general; B: Detalle de aguijones,
células silíceas y suberosas. C: Célula buliforme con forma de abanico. bc, célula buliforme; lc, célula larga; p, gancho; si,
célula silícea; su, célula suberosa. Escala: 50 μm.
Figure
5 / Figura 5. Phyllostachys aurea.
Chemical-nutritional
evaluation
P. aurea did not present differences between
seasons in DM, CP and aNDFom. Averages exceeded 500 g/kg HM and 133 and 623
g/kg DM of CP and aNDFom, respectively (table 1).
The inorganic fraction reached 193 g/kg DM while 81% of leaf blades constituted
organic matter. Structural carbohydrates predominated with a low concentration
of water-soluble non-structural carbohydrates (i.e. 623 and 26 g/kg DM,
aNDFom and WSC, respectively) and moderate CP contents (table 1).
The high silica content (i.e. 82% of total ash) suggests the presence of
indigestible material and lower organic matter for animal feed.
Table 1. Chemical
composition of P. aurea leaf blades in winter and spring.
Tabla 1. Composición
química de láminas de P. aurea en dos épocas del año.
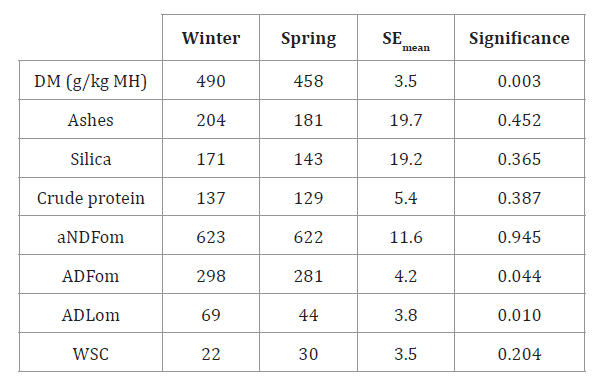
DM: dry matter; aNDFom: insoluble
fiber in neutral detergent with α-amylase and free of ash; ADFom: ash-free acid
detergent fiber insoluble fiber; ADLom: lignin in acid detergent in sulfuric
acid and free of ashes; WSC: water-soluble carbohydrates; SEmean: standard error of the mean. Data
are expressed in g/kg DM, unless indicated.
MS: materia seca; aFDNmo: fibra
insoluble en detergente neutro con α-amilasa y libre de cenizas; FDAmo: fibra
insoluble en fibra detergente ácida sin cenizas; LDAmo: lignina en detergente
ácido en ácido sulfúrico y libre de cenizas; CS: carbohidratos solubles en
agua; EEmedia:
error estándar de la media. Los datos se encuentran expresados en g/kg MS,
excepto que se indique lo contrario.
Phyllostachys aurea
presented
lower moisture content and higher concentration of ADFom and ADLom in winter
than in spring, with increases of 7, 6 and 57% (P < 0.05), respectively (table 1). ADLom
accumulation in the cell wall of winter leaf blades coincides with a 9%
decrease in DMDin in winter compared to
summer harvest (P < 0.05; table 2).
Table 2. In
vitro nutritional evaluation of P. aurea leaf
blades in winter and spring.
Tabla
2. Evaluación nutricional in vitro para dos
estaciones del año de láminas de P. aurea.
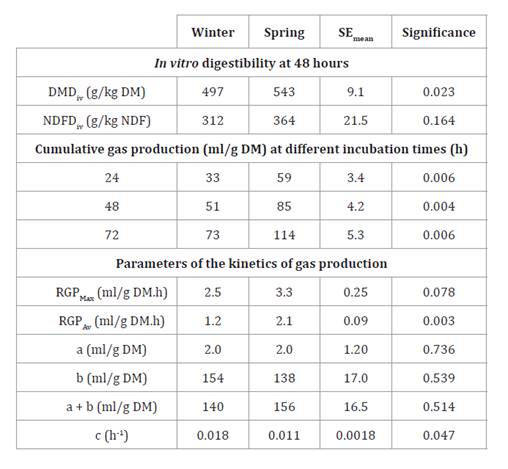
SEmean: mean standard error; DMD, dry
matter digestibility; NDFD: insoluble fiber in neutral detergent digestibility;
RGPMax:
maximum rate gas production; RGPAv, average rate gas production; a: GP of
soluble fraction; b: GP of potentially degradable fraction; c: b degradation
rate (CNGP = a + b x (1 - e-ct); Ørskov and McDonald, (1979). Digestibility and kinetics of
gas production.
EEmedia: error estándar de la media; DMS:
digestibilidad de la materia seca; DFDN: digestibilidad de la fibra insoluble
en detergente neutro; TPGMax: tasa máxima de producción de gas;
TPGProm:
tasa promedio de producción de gas; a: PG de fracción soluble; b: PG de
fracción potencialmente degradable; c: tasa de degradación de b (PGNA = a + b x
(1 - e-ct); Ørskov y McDonald, (1979). Digestibilidad y cinética de producción
de gas.
However, increases
in ADF and ADL did not translate into statistically significant changes in
IVNDF (p = 0.16), possibly given the greater SEmean magnitude concerning DMDiv
(9.1 and 21.5 SEmean for
DMDiv and NDFDiv,
respectively). Additionally, digestibility determined at 48 h, corresponding to
potential values, could promote difference fading. P. aurea high cell
wall content and greater winter lignification promoted digestibility not
exceeding 54% and 36% for DM and aNDFom, respectively.
Leaf blade CP
content was slightly below the threshold (13%DM) recommended for the ruminant
diet. Moreover, since average soluble carbohydrates were 26 g/kg DM and aNDFom
was over 600 g/kg DM, P. aurea foliage can be classified as medium-quality
feed.
GP kinetics
coincided with that described for ADFom, ADLom and digestibilities, being RGPAv
1.8 times higher in spring and 1.7 in the PGAN at 24, 48 and 72
h, compared to winter (table
2;
P < 0.05). This could be due to 36% less lignin of summer leaf blades, and
89% more organic matter not associated with the cell wall (i.e. 1000 -
(Ash + CP + aNDFom) = 68 vs 36 g/kg DM; table 1). This hypothesis
was supported by the greater DMDiv,
although no significant differences were detected for DNFDiv.
Although the c rate was higher in spring leaf blades, the
adjusted rate was 39% lower than in winter (i.e. 0.011 and 0.018 h-1, respectively; table
2; P = 0.047). The model describing GP kinetics may overestimate c
when the curve does not reach the plateau (figure 6).
However, spring foliage showed GP net rates of 3.2 fold and twice higher at
hour 1 and 18, respectively (i.e. E × T; P = 0.034; figure 6).
In all cases, TRGPmax was 3 hours after
incubation. Higher GP rates at 1 and 3 hours could be due to WSC fermentation,
while the later reduced rate is given by the lower availability of cell-wall
carbon skeletons.
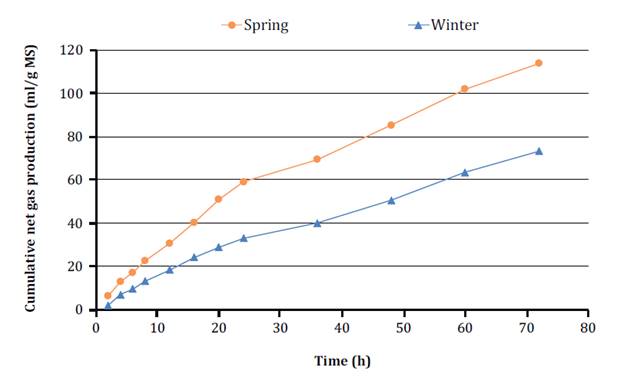
Figure 6. Cumulative net gas production
(ml/g DM incubated) as a function of incubation hours of P. aurea leaf
blades in spring and winter.
Figura 6. Producción de
gas acumulada neta (ml/g MS incubada) en función de las horas de incubación de
las láminas de P. aurea en dos estaciones del año.
Discussion
Morpho-anatomically,
P. aurea foliage leaves presented predominant parenchyma and scarce
sclerenchyma, desirable traits for forage use. As foliage matures, silica is
deposited in bulliform cells, microhairs, hooks, and prickles, and to a lesser
extent in suberous, long, and subsidiary cells in the stomatal complex (50). This study
coincides with our anatomical and nutritional results showing that silica in
different cell types could limit digestion. Silica in epidermis, trichomes,
cell walls or lumen of grasses acts as a structural inhibitor of microbial
digestion leading to lower acceptability and DMD (26). Thus, low DMDiv
and NDFDiv could be explained by high epidermal Si contents preventing wall
enzymatic degradation.
Chemical-nutritional
composition of P. aurea leaf blades was statistically different between
spring and winter harvests, as previously found (9,
19, 47). However, these differences did not modify productive
implications in ruminant feeding, describing a forage of medium to low
nutritional value (i.e. CP,133; aDFNom, 623; ADFom, 290; ADLom, 57; DMDiv, 520; NDFDiv, 338 g/kg DM).
Other authors reported similar results (4, 40) and Bhardwaj
et al. (2019) studying Jersey cows, characterized P. aurea with lower
palatability and nutritional value than other bamboos like Dendrocalamus
hamiltonii Nees & Arn. ex Munro, D. asper (Schult.
& Schult. f.) Backer ex K. Heyne, Melocanna baccifera (Roxb.) Kurz, Phyllostachys
bambusoides Siebold & Zucc. and P.
pubescens (Pradelle) Mazel ex J. Houz. In contrast, Panizzo
et al. (2017) described Guadua chacoensis leaf blades as better
nutritional forage, with 220 g CP/kg DM, 541 g aNDFom/kg DM and 64%
degradability at 48 hours.
Asaolu et
al. (2010) and Halvorson et al. (2010) mentioned that
bamboo is suitable for animals under maintenance conditions. The advantage of P.
aurea is that leaf foliage blades are perennial and available when other
forage species become scarce (4, 8, 9, 34). Mekuriaw
et al. (2011), documented using bamboo foliage as feed for cattle, sheep,
goats, and chickens in Ethiopia. In addition, our average aNDFmo content of 623
g/kg DM (table
1)
could limit dry matter intake as for other forages (i.e., aNDFom >
600 g/kg DM; Mertens,
1973).
Compared to other forage species, P. aurea low GP and CNGP rates suggest
lower rumen fermentation and a consequent negative effect on potential
consumption (16, 45). However, its CP
content over 120 g/kg DM would not limit rumen digestion or requirements of
ruminant categories in maintenance (7).
P. aurea foliage presented
lower nutritional value than most C3 grasses and legumes grown
in the temperate region of Argentina, both fresh and hayed or ensiled (i.e.,
oats, barley, fescue, ryegrass, red clover and alfalfa; Jaurena
and Danelón, 2006; Wawrzkiewicz
et al., 2019). On the other hand, compared with C4 (i.e., rhodes
grass, gatton panic, honey grass; Fernández Pepi et al.,
2018)
grasses, bamboo contributed more CP and a similar or lower cell wall content.
For this reason, P. aurea would be an alternative to C4 grasses, given its
constant biomass production, nutritional quality and availability in winter or
dry seasons.
Yayota et
al. (2009) mention that low nutritional quality of bamboo cannot be
completely explained by cell-wall quantity and composition. Factors like
tannins or Si hinder microbiologic accessibility, slowing digestion (46).
Our results evidence
foliar homogeneity of P. aurea throughout the year, for the temperate
region of Argentina, and strong environmental adaptability while keeping stable
chemical-nutritional characteristics. This allows considering P. aurea as
an ingredient for ruminant supplementation in times of forage scarcity,
increasing effective fiber intake. The use of foliage leaves, otherwise
discarded after culms are used in construction and crafts, transforming a
byproduct into an additional source of feed for ruminants.
Conclusions
Phyllostachys aurea presented morphological
characteristics, growth habit, propagation form and vegetative cycle allowing
high capacity for establishment and development in diverse environments. The C3
leaf anatomy with abundant parenchyma and scarce sclerenchyma
along with its chemical-nutritional composition suggest its value as feed for
ruminants. Although cell wall content estimated as NDF limits potential
consumption, bamboo could be used as supplement in unfavorable times and ensure
fiber contribution. Finally, P. aurea presents homogeneous traits
throughout seasons and sites (low phenotypic plasticity) constituting a food
source throughout the year.
Acknowledgments
UBACyT
20020190100206BA Project.
To the directors and staff of Lucien Hauman (FAUBA) and Arturo
Ragonese (INTA Castelar) Botanical Gardens, and to Mr. Diego Regnicoli (Delta
del Tigre).
1. Alchouron, J.;
Navarathna, C.; Chludil, H. D.; Dewage, N. B.; Perez, F.; Hassan, E. B.;
Pittman Jr., C. U.; Vega, A. S.; Mlsna, T. E. 2020. Assessing South American Guadua
chacoensis bamboo biochar and Fe3O4 nanoparticle dispersed analogues for
aqueous arsenic (V) remediation. Science of the Total Environment. 706: 135943.
https://doi.org/10.1016/j.scitotenv.2019.135943
2. Alchouron, J.;
Navarathna, C.; Rodrigo, P. M.; Snyder, A.; Chludil, H. D.; Vega, A. S.; Bosi,
G.; Perez, F.; Mohan, D.; Pittman Jr., C. U.; Mlsna, T. E. 2021. Household
arsenic contaminated water treatment employing iron oxide/bamboo biochar
composite: An approach to technology transfer. Journal of Colloid and Interface
Science. 587: 767-779. https://doi.org/10.1016/j.
jcis.2020.11.036
3. Alchouron, J.;
Bursztyn Fuentes, A. L.; Musser, A.; Vega, A. S.; Mohan, D.; Pittman Jr., C.
U.; Mlsna, T.; Navarathna, C. 2022. Arsenic removal from household drinking
water utilizing biochar and biochar composites: a focus on scale up. In: Mohan,
D.; Pittman Jr., C. U.; Mlsna, T. (Eds.). Sustainable biochar for water and
wastewater treatment. Netherlands. Elsevier. 277-320.
4. Andriarimalala,
J. H.; Kpomasse, C. C.; Salgado, P.; Ralisoa, N.; Durai, J. 2019. Nutritional
potential of bamboo leaves for feeding dairy cattle. Pesquisa Agropecuária
Tropical. V. 49: e54370. https://doi.org/10.1590/1983-40632019v4954370
5. AOAC.
International Association of Official Analytical Chemists. 1990. Official
Methods of Analysis. Arlington.
6. Asaolu, V. O.;
Odeyinka, S. M.; Akinbamijo, O. O.; Sodeinde, F. G. 2010. Effects of moringa
and bamboo leaves on groundnut hay utilization by West African Dwarf goats.
Livestock Research for Rural Development. Volume 22, Article #12
http://www.lrrd.org/lrrd22/1/asao22012. htm
7. Astibia, O. R.;
Cangiano, C. A.; Cocimano, M. R.; Santini, F. J. 1984. Utilización del
nitrógeno por el rumiante. Revista Argentina de Producción Animal. 4(4):
373-384.
8. Bhandari, M.;
Kaushal, R.; Banik, R. L.; Tewari, S. K. 2015. Genetic evaluation of
nutritional and forage quality of different bamboo species. Indian Forester.
141(3): 265-274.
9. Bhardwaj, D. R.;
Sharma, P.; Bishist, R.; Navale, M. R.; Kaushal, R. 2019. Nutritive value of
introduced bamboo species in the northwestern Himalayas, India. Journal of
Forestry Research. 30(6): 2051-2060. https://doi.org/10.1007/s11676-018-0750-2
10. Campos Roasio,
J.; Peñaloza Wagenknecht, R.; Kahler González, C.; Poblete W. H.; Cabrera
Perramón, J. 2003. Bambú en Chile. Parte 1. Corporación de Investigación
Tecnológica de Chile, Universidad Austral de Chile, FONDEF. Santiago, Chile.
143 p. https://doi. org/10.52904/20.500.12220/690
11. Clark, L. 2005.
Bamboo biodiversity. Iowa State University. https://www.eeob.iastate.edu/
research/bamboo/index.html
12. D´Ambrogio de
Argüeso. 1986. Manual de técnicas en histología vegetal. Hemisferio Sur. Buenos
Aires. 83 p.
13. Ellis, R. P.
1976. A procedure for standardizing comparative leaf anatomy in the Poaceae. I.
The leaf-slide as viewed in transverse section. Bothalia. 12: 65-109.
14. Ellis, R. P.
1979. A procedure for standardizing comparative leaf anatomy in the Poaceae.
II. The epidermis as seen in surface view. Bothalia. 12: 641-671.
15. Fernández Pepi,
M. G. 2013. Estudios fitolíticos de las comunidades vegetales del ecotono
fueguino como una herramienta para reconocer sus variaciones de composición en
el pasado reciente (Tesis Doctoral). Facultad de Ciencias Exactas y Naturales.
Universidad de Buenos Aires. Argentina.
16. Fernández Pepi,
M. G.; Todarello, M. (ex-aequo); Schrauf, G.; Wawrzkiewicz, M; Jaurena,
G. 2018. Variación de la calidad forrajera asociada a eventos transgénicos de Paspalum
dilatatum Poir. Revista Argentina de Producción Animal. 37 (Supl. 1): 313.
17. Guerreiro, C.
2014. Flowering cycles of woody bamboos native to southern South America.
Journal of Plant Research. 127(2): 307-313.
https://doi.org/10.1007/s10265-013-0593-z
18. Guerreiro C.;
Vega, A. S. 2021. Bamboo flowering in South America: What the past tells about
the future. p. 353-377. In: Ahmad, Z.; Ding, Y.; Shahzad, A. (Eds.).
Biotechnological advances in bamboo. Singapore. Springer.
https://doi.org/10.1007/978-981-16-1310-4_15
19. Halvorson, J.
J.; Cassida, K. A.; Turner, K. E.; Belesky, D. P. 2010. Nutritive value of
bamboo as browse for livestock. Renewable Agriculture and Food Systems. 26(02):
161-170. https://doi. org/10.1017/S1742170510000566
20. INTA. CIRN
(Centro de Investigación en Recursos Naturales). Sistema de información y
gestión agroecológica. http://siga.inta.gob.ar/#/; consultado el 17 de marzo de
2023.
21. Jaurena, G.;
Danelón, J. L. 2006. Tabla de composición de alimentos para rumiantes de la
región Pampeana argentina. Buenos Aires, Hemisferio Sur. 62 p.
22. Judziewicz, E.
J.; Clark, L. G.; Londoño, X.; Stern, M. J. 1999. American bamboos. Washington,
D. C. Smithsonian Institution Press. 392 p.
23. Labouriau, L.
G. 1983. Phytolith work in Brazil: a minireview. The Phytolitharien. 2: 6-10.
24. Londoño, X. 2009. Usos y potencialidades de los bambúes en
Sur América. Botânica Brasileira: futuro e compromissos: 236-243. 60° Congreso
Nacional de Botánica. Feira de Santana. BA. Brasil.
25. Martínez, L. V.
2008. Evaluación del espacio para la ganadería extensiva sustentable y la
conservación del huemul (Hippocamelus bisulcus), en el Parque Nacional
Los Alerces, provincia de Chubut, Argentina. APRONA Boletín Científico. 41:
45-67.
26. Massey, F. P.;
Ennos, A. R.; Hartley, S. E. 2007. Herbivore specific induction of silica-based
plant defences. Oecologia. 152(4): 677-683.
27. McClure, F. A.
1938. Notes on Bamboo culture with special reference to southern China. Reprint
from the Hong Kong Naturalist. 9(1-2): 1-18.
28. McClure, F. A.
1953a. Bamboo as a building material. Contribution of the Foreign Agricultural
Service, United States Department of Agriculture. Washington. D. C.
29. McClure, F. A.
1953b. Bamboo as a source of forage. Proceedings of the 8th Pacific
Scientific Association Congress: 609-644.
30. McClure, F. A.
1966. The bamboos. A fresh perspective. Harvard University Press. Cambridge.
Massachusetts.
31. Mekuriaw, Y.;
Urge, M.; Animut, G. 2011. Role of indigenous Bamboo species (Yushania
alpina and Oxytenanthera abyssinica) as ruminant feed in
northwestern Ethiopia. Livestock Research for Rural Development. 23: Article
#185. http://www. lrrd.org/lrrd23/9/meku23185. htm.htm
32. Mertens, D. R.
1973. Application of theoretical mathematical models to cell wall digestion and
forage intake in ruminants. Ph.D. thesis. Cornell Univ. Ithaca. NY.
33. Neumann, K.;
Strömberg, C. A. E.; Ball, T.; Albert, R. M.; Vrydaghs, L.; Cummings, L. S.
2019. International Code for Phytolith Nomenclature (ICPN) 2.0. Annals of
Botany. 124(2): 189-199.
34. Ocheja, J. O.;
Ayoade, J. A.; Okwori, A. I.; Abu, A.; Oyibo, A. 2013. Nutritive quality of
bamboo leaves as feed resource for herbivorous animals. Journal of Agricultural
Production and Technology. 2(2): 61-65.
35. Ørskov, E. R.;
McDonald, I. 1979. The estimation of protein degradability in the rumen from
incubation measurements weighed according to rate of passage. Journal of
Agricultural Science. 92: 499-503.
36. Panizzo, C. C.;
Fernández, P. V.; Colombatto, D.; Ciancia, M.; Vega, A. S. 2017. Anatomy,
nutritional value and cell wall chemical analysis of foliage leaves in Guadua
chacoensis (Poaceae, Bambusoideae, Bambuseae), a promising source of
forage. Journal of Science of Food and Agriculture. 97(4): 1349-1358.
https://doi.org/10.1002/jsfa.7873
37. Parodi, L. R.
1937. La floración de Phyllostachys aurea en la Argentina. Revista
Argentina de Agronomía. 4: 307-308.
38. Parodi, L. R.
1943. Los Bambúes cultivados en la Argentina. Revista Argentina de Agronomía.
10(2): 89-110.
39. Rúgolo, Z. E.
2016. Phyllostachys. In: Rúgolo, Z. E. (Ed.). Bambúes leñosos nativos y
exóticos de la Argentina. Hurlingham, Ed. Trama. 160-166.
40. Sahoo, A.;
Ogra, R. K.; Sood, A.; Ahuja, P. S. 2010. Nutritional evaluation of bamboo
cultivars in sub- Himalayan region of India by chemical composition and in
vitro ruminal fermentation. Grassland Science. 56: 116-125.
https://doi.org/10.1111/j.1744-697X.2010.00183.x
41. Satya, S.; Bal,
L. M.; Singhal, P.; Naik, S. N. 2010. Bamboo shoot processing: food quality and
safety aspect (a review). Trends in Food Science and Technology. 21: 181-189.
https://doi. org/10.1016/j.tifs.2009.11.002
42. Van Soest, P.
J.; Robertson, J. B.; Lewis, B. A. 1991. Methods for dietary fiber, neutral
detergent fiber, and nonstarch polysaccharides in relation to animal nutrition.
Journal of Dairy Science. 74: 3583-3597.
43. Vega, A. S.;
Castro, M. A.; Guerreiro, C. 2016. Ontogeny of fusoid cells in Guadua species
(Poaceae, Bambusoideae, Bambuseae): evidence for transdifferentiation and
possible functions. Flora. 222: 13-19.
44. Wawrzkiewicz,
M.; Danelón, J. L.; Grigera, J. J. 2005. Desaparición in vitro de
forraje ensilado sometido a distintos procesamientos de laboratorio. Archivos
Latinoamericanos de Producción Animal. 13(4): 191-214.
45. Wawrzkiewicz,
M.; Álvarez Ugarte, D. H.; Fernández Pepi, M. G.; Jaurena, G. 2019.
Desaparición de sustrato, ambiente ruminal y producción de metano in vitro de
dietas con heno y burlanda seca de maíz. 42° Congreso Argentino de Producción
Animal (AAPA). Bahía Blanca, Argentina. 42 (Supl.1): 32.
46. Wilson, J. R.
1994. Cell wall characteristics in relation to forage digestion by rumiants.
Journal of Agriculture Science. 122: 173182.
47. Yayota, M.;
Karashima, J.; Kouketsu, T.; Nakano, M.; Ohtani, S. 2009. Seasonal changes in
the digestion and passage rates of fresh dwarf bamboo (Pleioblastus
argenteostriatus f. glaber) in sheep. Animal Feed Science and
Technology. 149(1-2): 89-101. https://doi. org/10.1016/j.
anifeedsci.2008.05.006
48. Zelaya Alvarez,
V. M.; Fernández, P. V.; Vega, A. S.; Mantese, A. I.; Federico, A. A.; Ciancia,
M. 2017. Glucuronoarabinoxylans as major cell walls polymers from young shoots
of the woody bamboo Phyllostachys aurea. Carbohydrate Polymers. 167:
240-249. https://doi. org/10.1016/j.carbpol.2017.03.015
49. Zhang, Y. X.; Zeng, C. X.; Li, D. Z. 2014. Scanning electron
microscopy of the leaf epidermis in Arundinarieae (Poaceae: Bambusoideae):
evolutionary implications of selected micromorphological features. Botanical
Journal of the Linnean Society. 176(1): 46-65.
50. Zucol, A. F.; Brea, M. 2005. Sistemática de fitolitos,
pautas para un sistema clasificatorio. Un caso en estudio en la Formación
Alvear (Pleistoceno inferior), Entre Ríos, Argentina. Ameghiniana. 42(4):
685-704.