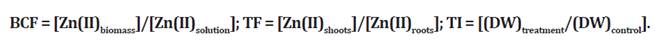Revista de la Facultad de Ciencias
Agrarias. Universidad Nacional de Cuyo. Tomo 56(2). ISSN (en línea) 1853-8665.
Año 2024.
Original article
Morphophysiological
and biochemical responses of Schedonorus arundinaceus to Zinc (II)
excess: insights from biomarkers and elemental accumulation
Respuestas
morfofisiológicas y bioquímicas de Schedonorus arundinaceus al exceso de
zinc (II): Perspectivas sobre biomarcadores y acumulación elemental
Valeria Bernardo1,
Sebastián Garita1,
Josefina Plaza
Cazón2,
Cecilia Arango1,
1Instituto de Fisiología Vegetal (INFIVE-CCT La Plata). Diagonal
113 N° 495 (B1900) La Plata, Buenos Aires. Argentina.
2Centro de Investigación y Desarrollo en Fermentaciones
Industriales (CINDEFI-CCT La Plata). Calle 50 N° 227 (B1900) La Plata. Buenos
Aires. Argentina.
3Universidad Nacional de La Plata. Facultad de Ciencias Agrarias
y Forestales. Laboratorio de Morfología Comparada de Espermatofitas (LAMCE).
Cátedra de Morfología Vegetal. Avenida 60 y 119 (B1900) La Plata. Buenos Aires.
Argentina.
4Universidad Nacional de La Plata. Museo de Ciencias Naturales.
División Plantas Vasculares. Paseo del Bosque S/N° (B1900) La Plata. Buenos
Aires.
5Universidad Nacional del Noroeste de la Provincia de Buenos
Aires. Departamento de Ciencias Básicas y Experimentales. Roque Sáenz Peña 456
(B6000) Junín. Buenos Aires. Argentina.
*magonzalez921994@gmail.com
Abstract
Excessive levels of
zinc have detrimental effects on plant physiology and morphology, hindering
growth and development. This study aimed to elucidate the morphophysiological
and biochemical mechanisms of Schedonorus arundinaceus in response to
high concentrations of zinc exposure and to investigate the correlation between
these parameters to identify potential stress biomarkers in this species.
Plants were exposed to seven zinc concentrations
(0-500-1000-1500-2000-2500-3000 μM) for 50 days. The results showed decreased
dry weight, root area, photosynthetic pigments, root soluble proteins and
stomatal conductance with increasing zinc concentrations. Conversely, proline,
malondialdehyde and leaf-soluble protein content increased. Histological
observations revealed altered stomata size and abnormalities in root tissue.
Zinc accumulation exceeded phytotoxic thresholds (100-400 mg kg-1)
even at lower concentrations, reaching a maximum of 3432 mg kg-1 in shoots. Visible Zn-P
crystals were observed on leaf surfaces at the highest zinc treatment. These
results suggest that S. arundinaceus possesses a notable capacity to
bioaccumulate zinc, particularly in the roots. Furthermore, the strong
correlation between proline levels and zinc biomass concentration suggests its potential
use as a stress biomarker for zinc-induced stress in this species.
Keywords: zinc phytotoxicity,
stress adaptations, root damage, physiological response
Resumen
Los niveles
excesivos de zinc afectan negativamente la fisiología y morfología de las
plantas, dificultando su crecimiento y desarrollo. Este estudio tuvo como
objetivo dilucidar los mecanismos morfofisiológicos y bioquímicos de Schedonorus
arundinaceus en respuesta a altas concentraciones de zinc y explorar la
correlación entre estos parámetros para identificar posibles biomarcadores de
estrés. Las plantas fueron expuestas a siete concentraciones de zinc
(0-500-1000-1500-2000-2500-3000 μM) durante 50 días. Se observó una disminución
del peso seco, área radicular, pigmentos fotosintéticos, proteínas solubles en
raíz y conductancia estomática con el incremento de zinc. No obstante, aumentó
el contenido de prolina, malondialdehído y proteínas solubles en hojas. Las
observaciones histológicas revelaron alteraciones en el tamaño de los estomas y del tejido radicular. La acumulación de zinc
superó los umbrales fitotóxicos (100-400 mg kg-1),
alcanzando un máximo de 3432 mg kg-1 en hojas. Se observaron
cristales de Zn-P en la superficie de las hojas en las máximas concentraciones.
Estos resultados sugieren que S. arundinaceus posee una notable
capacidad para bioacumular zinc, particularmente en las raíces. Además, la
fuerte correlación observada entre la prolina y la acumulación de zinc sugiere
su potencial uso como biomarcador de estrés inducido por el exceso de zinc en
esta especie.
Palabras clave: fitotoxicidad por
zinc, adaptación al estrés, daño radical, respuestas fisiológicas
Originales: Recepción: 17/11/2023 - Aceptación: 04/07/2024
Introduction
Recently, heavy
metal (HM) contamination has emerged as a pressing environmental issue. These
non-biodegradable and toxic substances can accumulate in water bodies, soil,
sediments, and the food chain, posing a significant risk to human health (10). The extensive use of mineral fertilizers and
pesticides (38), coupled with the release
of untreated urban and industrial effluents (55),
has contributed to soil and water pollution (2).
In Argentina, this environmental concern is particularly important in the
“green belts” -peri-urban areas where commercial fresh vegetable production for
urban centers is concentrated. The green belts of La Plata, Florencio Varela
and Berazategui are especially significant, accounting for 82% of horticultural
farms and 81% of cultivated land. These areas are crucial for fresh food
production and significantly impact the local economy (32). The land use in the peri-urban area of La
Plata is diverse, including residential areas, heavy industry, wastelands,
intensive farmland, forests, cattle-raising areas and quarries. With urban
planning lagging, this transitional zone faces competition among urbanization,
industrialization and horticultural activities, leading to increased pollution
and ecosystem degradation (32). Studies
on HM contamination, particularly Zn, in these areas are scarce but indicate
that Zn can exceed normal or toxic levels due to industrial and motor emissions
(7, 13). High concentrations of Zn, Pb,
Cr and Cu, often exceeding toxic limits, owe to excessive pesticide and
fertilizer use in horticultural peri-urban areas (11,
41). This evidence suggests that intensive agricultural practices and
urban-industrial emissions, waste production and disposal could elevate HM
levels in these areas, threatening environmental health and food security (21). While certain HMs are essential for plant
growth and development, their accumulation at high levels can be phytotoxic (3). Among these, zinc [Zn(II)]
plays a crucial role in normal plant development. It activates multiple enzymes,
participates in the synthesis and metabolism of important biomolecules and
serves as a key component of the transcription factor family known as “zinc
fingers”, which regulates cell proliferation and differentiation processes (49). However, excessive levels result in
structural and functional abnormalities negatively impacting plant performance (46). Symptoms include chlorosis, necrosis,
reduced growth due to inhibited cell division and elongation, reduced CO2
fixation and carbohydrate transport and alterations in cellular
membrane permeability (28).
Within the Poaceae
family, several species have demonstrated resilience in harsh environments,
quickly colonizing contaminated areas (38).
Schedonorus arundinaceus (=Festuca arundinacea), commonly known
as tall fescue, is the most widely used temperate perennial grass throughout
the Argentina Humid Pampa region (covering most of Buenos Aires and parts of
Santa Fe Cordoba and La Pampa provinces) due to its broad ecological adaptation
and naturalization (45). It has been
observed to thrive in soils contaminated with various heavy metals (HMs),
including Pb, Cd, Cu, Zn, Ni and Cr (1, 39).
Our previous studies have shown that this species can grow normally with
elevated Zn(II) concentrations, indicating its potential
for phytoremediation (23). Investigating
the morpho-physiological and biochemical responses of Zn(II)
excess on S. arundinaceus in this region is crucial, as it could provide
an effective solution for mitigating Zn(II) contamination, thereby improving
soil quality and ensuring food security in the area. To effectively manage
species, it is crucial to investigate not only the uptake rate of HMs but also
the underlying mechanisms that regulate stress response (26). Also, establishing correlations between
plant absorption kinetics and key physiological and biochemical parameters can
provide valuable insights for identifying biomarkers to assess specific plant
stress responses (26). The objective of
this study was to elucidate the morpho-physiological and biochemical mechanisms
of S. arundinaceus that are elicited in response to excessive exposure
to high Zn(II) concentrations. Furthermore, we aimed
to investigate the correlation between these parameters in order to identify
potential stress biomarkers for this species. These findings could offer
promising insights into the application of S. arundinaceus in
phytotechnologies such as phytoremediation, biofortification and phytomining.
Materials
and methods
Growth
conditions and treatments
This experiment was
conducted between August and October in a greenhouse located in La Plata City,
Buenos Aires, Argentina. S. arundinaceus seeds were disinfected with
NaClO (10%) for 5 minutes, rinsed with sterile water and placed in plastic plug
trays with 72 cells containing a perlite-vermiculite mixture (1:1 v/v). Weekly
applications of half-strength Hoagland nutrient solution (25) were provided. After 14 days, the seedlings
were transplanted into hydroponic containers filled with a complete Hoagland
nutrient solution (pH 6.2 ± 0.5). Following a 15-day acclimatisation period to
the hydroponic conditions, increasing concentrations of Zn(II) (provided as
ZnSO4 7H2O)
were added to obtain seven treatments: control (1μM of Zn(II) (0.065 mg L-1), equivalent to
the Zn(II) concentration in the Hoagland nutrient solution), 500 μM (32.69 mg L-1), 1000 μM (65.38
mg L-1), 1500 μM
(98.07 mg L-1),
2000 μM (130.76 mg L-1),
2500 μM (163.45 mg L-1)
and 3000 μM (196.14 mg L-1)
of Zn(II). These various concentrations were selected based on a previous
experiment (22) to screen for responses
that might not be discernible at lower concentrations and to determine the
maximum tolerance limit for this species. The pH of the solutions was
maintained at 6.2 ± 0.5 using 0.1 N NaOH/HCl and aerated using aeration pumps.
The solutions were changed every two weeks throughout the experiment. S.
arundinaceus plants were harvested 50 days after the initial application of
the Zn(II) solutions.
Morpho-physiological
and biochemical parameters measured
The dry weight (DW) of roots and shoots was determined at
harvest by oven-drying at 80°C until constant weight was achieved. Leaf
relative water content (LRWC) was calculated based on the measurements of dry
weight (DW), fresh weight (FW) and turgid weight (TW) of 1 cm diameter leaf
discs (49). Maximum root length (RL) and
root area (RA) were calculated using RhizoVision Explorer v2.0.3 (43, 44). Total chlorophyll and carotenoid
contents were determined from a 0.5 cm diameter leaf disk; absorbance measurements
were taken at 647, 664, and 480 nm (52).
Soluble protein content was measured from 0.1 g of fresh leaves and roots;
absorbance was read at 595 nm and protein concentration was calculated using a
standard curve prepared with different concentrations of bovine serum albumin
(BSA) (SiFMa Chemical Co.) (9).
Malondialdehyde (MDA) content was measured from 0.2 g of fresh leaves and roots
by reaction with thiobarbituric acid (24).
Proline content was measured from 0.1 g of fresh leaves and roots; absorbance
was read at 520 nm to calculate proline content per unit of fresh weight (4). Absorbance measurements for the
aforementioned parameters were determined using a Shimadzu UV-160
spectrophotometer (Kyoto, Japan).
Stomatal density
(StD) was calculated through image capture and digitalization using a Gemalux
XSZ-H microscope equipped with a Motic camera and Motic Image Plus 2.0 software
(15). Stomatal counting was conducted
using a Leitz SM lux optical microscope with a clear drawing camera. Adaxial
and abaxial stomatal conductance (StC) were determined using a Decagon SC-1
Porometer between 4 p.m. and 6 p.m. on a sunny day, in the middle portion of
fully expanded and non-senescent leaves. For histological observations, roots
were decolorized with 50% sodium hypochlorite and 5% chloral hydrate. The
tissues were stained with an 80% safranin O alcoholic solution and mounted in
gelatin-glycerin (14).
Zn(II) tolerance assessment
and bioconcentration analysis
Dried root and shoot samples (0.5 g) were ground and digested
using a mixture of HNO3 and H2SO4
(4:1 ratio). The absorbance was read using a Shimadzu AA6650F
Atomic Absorption Spectrophotometer (Japan) (8).
The bioaccumulation factor (BCF), translocation factor (TF) and tolerance index
(TI) were calculated using the following equations:
Crystalline
formations resembling salt crystals were exclusively observed on the leaf
surfaces of plants exposed to the highest concentration of Zn(II)
(3000 μM). The composition of these crystals was analyzed using
energy-dispersive X-ray analysis (EDX) and examined under scanning electron
microscopy (SEM).
Experimental
design and data analysis
The experimental
design was fully randomized, consisting of 7 treatments with 10 replicates per
treatment. The following variables were analyzed in all treatments using 10
replicates per treatment: dry weight, LWRC, root length, root area, total
chlorophyll and carotenoids, soluble protein, MDA, proline, Zn content,
bioaccumulation and translocation factors and tolerance index. The following
variables were analyzed only in the control and maximum Zn concentration (3000
μM) using 10 replicates per treatment: stomatal density and conductance and
histological observations. The data were analyzed using analysis of variance
(ANOVA), and means were compared using the least significant difference (LSD)
test at a significance level of 5% (p<0.05) (16).
Pearson correlations, involving 24 variables, were determined using R version
4.3.1 software. All results were expressed as mean values with corresponding
standard deviations.
Results
Morpho-physiological
and biochemical parameters
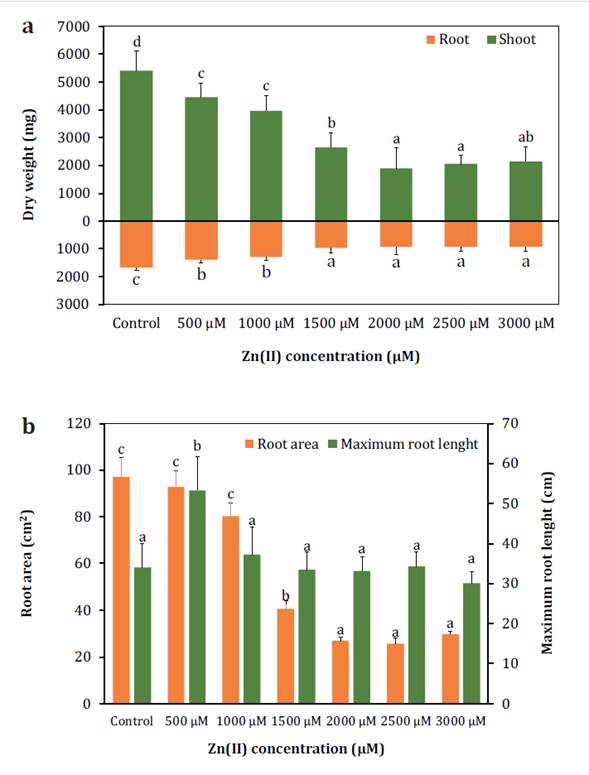
The DW significantly decreased as the concentrations of Zn(II) increased, with shoots and roots experiencing a
reduction of 60% and 45% respectively, at the maximum concentration (figure 1a).
Columns
represent mean values of 10 replications, and vertical bars show standard
deviation. Columns sharing different letters indicate significant differences
(p < 0.05).
Las columnas
representan los valores medios de 10 repeticiones y las barras verticales
muestran la desviación estándar. Las columnas que comparten letras diferentes
indican diferencias significativas (p < 0,05).
Figure
1. Effect of Zn(II)
concentrations on the dry weight (a), root area and maximum root length (b) of S.
arundinaceus.
Figura 1. Efecto
de las concentraciones de Zn(II) sobre el peso seco
(a), el área y la longitud radical máxima (b) de S. arundinaceus.
The RA also showed a significant decrease starting from 1000 μM,
while the RL exhibited a significant increase at lower concentrations (figure 1b) but remained unchanged at higher concentrations,
indicating a potential reduction in secondary root formation. The LRWC remained
stable, suggesting a relatively consistent water status in response to Zn(II) exposure (table 1).
Table 1. Effect
of Zn(II) concentrations on physiological and
biochemical parameters of S. arundinaceus.
Tabla 1. Efecto
de las concentraciones de Zn(II) sobre los parámetros
fisiológicos y bioquímicos de S. arundinaceus.
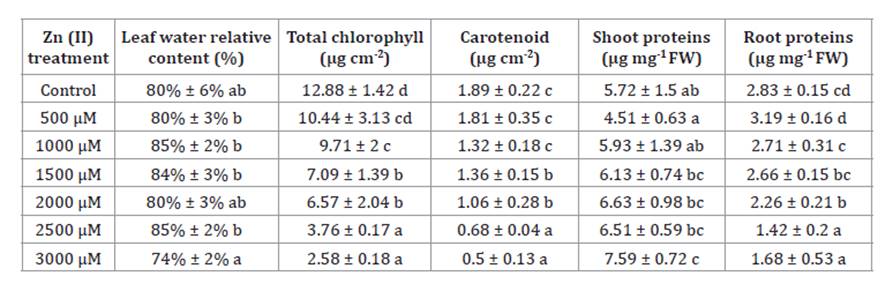
Data
given in the table are means of 10 replications ± standard deviation. For each
parameter, data followed by different letters indicate significant differences
(p < 0.05).
Los datos
indicados en la tabla son la media de 10 réplicas ± desviación estándar. Para
cada parámetro, los datos seguidos de letras diferentes indican diferencias
significativas (p < 0,05).
Total chlorophyll and carotenoid contents decreased with
increasing Zn(II) concentrations, reaching values 80%
and 74% lower than the control at the highest concentration (table
1). Significant changes were also observed in soluble protein content.
Leaves exhibited a slight increase, being 24% higher under 3000 μM compared to
the control, while roots showed a decrease of 40% under the same concentration
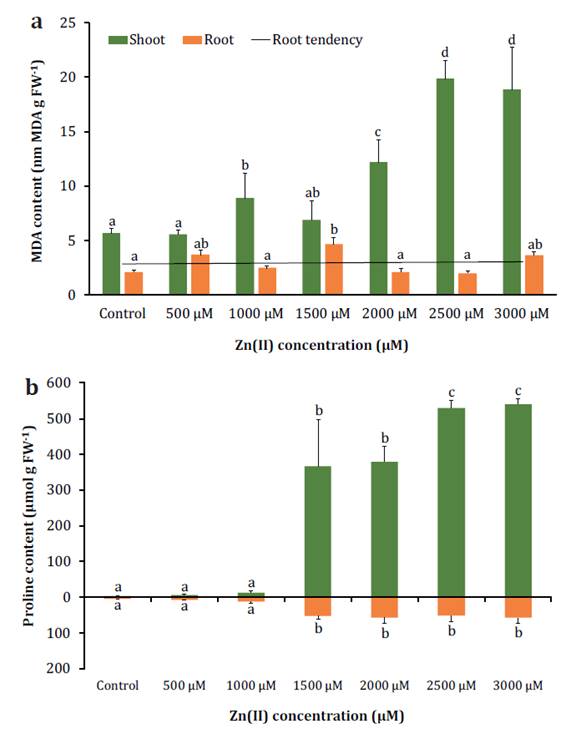
(table 1). The levels of MDA increased in leaves under high
Zn(II) stress, reaching values three times higher than
the control at 3000 μM. In contrast, root MDA levels remained lower and showed
no significant differences among treatments (figure 2a).
Columns
represent mean values of 10 replications, and vertical bars show standard
deviation. Columns sharing different letters indicate significant differences
(p < 0.05).
Las columnas
representan los valores medios de 10 repeticiones y las barras verticales
muestran la desviación estándar. Las columnas que comparten letras diferentes
indican diferencias significativas (p < 0,05).
Figure
2. Effect of Zn(II)
concentrations on S. arundinaceus MDA (a) and proline (b) contents.
Figura 2. Efecto
de las concentraciones de Zn(II) en los contenidos de
MDA (a) y prolina (b) de S. arundinaceus.
Proline showed a significant increase in both leaves and roots
under Zn(II) concentrations of 1500 μM and higher,
reaching values in shoots that were 162 times higher than the control, while in
roots, it was 13 times higher (figure 2b). These findings
could indicate the activation of stress-related mechanisms in response to Zn(II) excess, as proline is believed to function as a
scavenger of reactive oxygen species (ROS) or as an osmolyte, aiding in the
defense against oxidative stress.
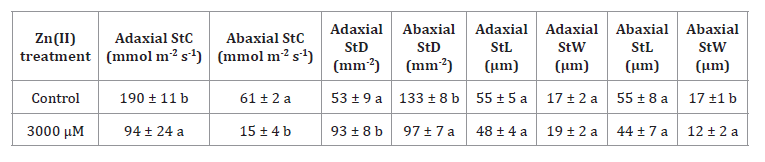
Significant differences were observed in adaxial and abaxial
stomatal density (StD), conductance (StC), length (StL) and width (StW)
exclusively between the control and 3000 μM treatments. Hence, the subsequent
results will focus solely on these specific treatments. Both adaxial and
abaxial StC decreased, reaching values 2 and 4 times lower than the control
under 3000 μM. Adaxial StD was significantly higher, while abaxial StD
exhibited the opposite trend. However, total StD did not vary significantly.
Although there were no significant differences in adaxial and abaxial StL, a
noticeable decrease in abaxial StW was observed (table 2).
Table 2. Adaxial
and abaxial stomatal density (StD), conductance (StC), length (StL), and width
(StW) of S. arundinaceus.
Tabla 2. Densidad
estomática adaxial y abaxial (StD), conductancia (StC), longitud (StL) y
latitud (StW) de S. arundinaceus.
Data
given in the table are the mean of 10 replications ± standard deviation. For
each trait, data followed by different letters indicate significant differences
(p < 0.05).
Los datos
indicados en la tabla son la media de 10 repeticiones ± desviación estándar.
Para cada parámetro, los datos seguidos de letras diferentes indican
diferencias significativas (p < 0,05).
This suggests that under higher Zn(II)
concentrations, smaller and rounder stomata were present. Also, neither
non-glandular trichomes nor salt glands were observed on the leaves of plants
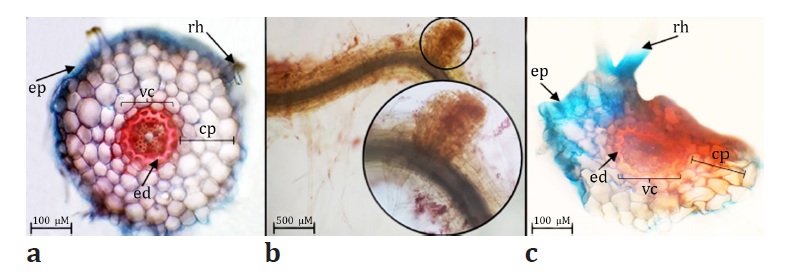
from all treatments. In terms of root anatomy, the transversal section analysis
of control roots revealed a concentric cortical parenchyma composed of orderly
to slightly disordered cells, with a vascular cylinder surrounded by endodermis
(figure 3a).
Figure
3. Control roots transversal sections (a), root
protuberances observed under 3000 μM (b) and 3000 μM treated roots transversal
sections (c) (ep: epidermis, rh: root hair, cp: cortical parenchyma, vc:
vascular cylinder, ed: endodermis).
Figura 3. Secciones
transversales de las raíces del control (a), protuberancias radiculares
observadas bajo 3000 μM (b) y secciones transversales de raíces tratadas con
3000 μM (c) (ep: epidermis, rh: pelo radical, cp: parénquima cortical, vc:
cilindro vascular, ed: endodermis).
In
contrast, roots exposed to 3000 μM exhibited morphological changes, including
protuberances (figure 3b), dorsoventral compression of
cortical parenchyma, endodermis and vascular
bundles as well as signs of disintegration and medullary proliferation (figure 3c).
Zn(II) tolerance assessment
and bioaccumulation analysis
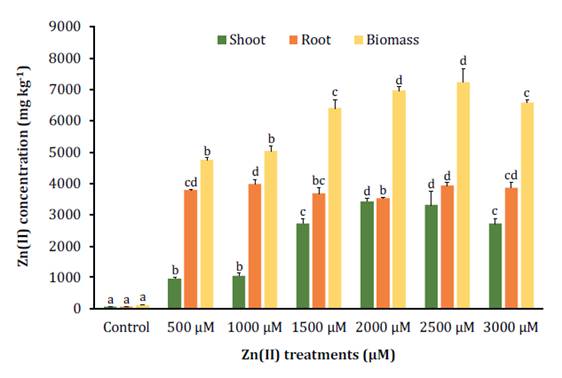
S. arundinaceus exhibited significant
accumulation of Zn(II), with levels reaching 50 times
higher than the control under the 3000 μM treatment, but the highest biomass
concentration (7244 mg kg-1 DW)
was observed under the 2500 μM treatment. Zn(II)
distribution showed that roots accumulated more Zn(II) than shoots in the lower
concentrations, but this difference became three times smaller after reaching
the 1500 μM concentration (figure 4).
Columns
sharing different letters indicate significant differences (p < 0.05).
Columns
represent mean values of 10 replications, and vertical bars show standard
deviation.
Las
columnas que comparten letras diferentes indican diferencias significativas (p
< 0,05).
Las columnas
representan los valores medios de 10 repeticiones y las barras verticales
muestran la desviación estándar.
Figure
4. Zn(II)
accumulation in shoots, roots, and biomass of S. arundinaceus.
Figura 4. Acumulación
de Zn(II) en hojas, raíces y biomasa de S.
arundinaceus.
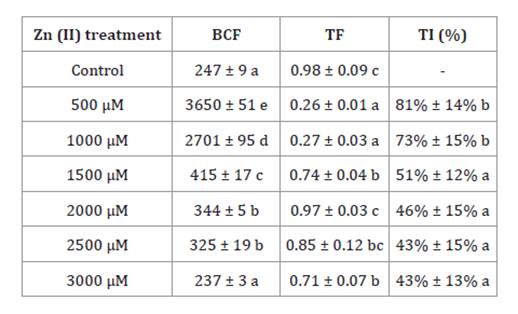
BCF values were only higher than 1000 under the 500 μM and 1000
μM treatments, while the TF values remained below 1 in all treatments. The TI
exhibited a decrease from 81% to 43% as Zn(II)
concentration increased from 500 μM to 3000 μM, respectively (table
3).
Table 3. Bioconcentration
factor (BCF), translocation factor (TF) and tolerance index (TI).
Tabla 3. Factor
de bioconcentración (BCF), factor de translocación (TF) e índice de tolerancia
(TI).
Data
given in the table are mean of 10 replications ± standard deviation. For each
trait, data followed by different letters indicate significant differences (p
< 0.05).
Los datos que
figuran en la tabla son la media de 10 repeticiones ± desviación estándar. Para
cada rasgo, los datos seguidos de letras diferentes indican diferencias
significativas (p < 0,05).
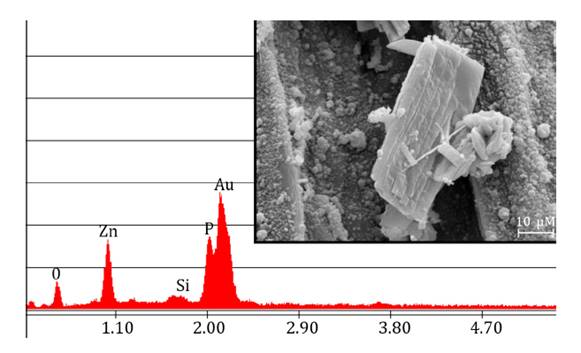
Crystalline formations resembling salt crystals were exclusively
observed on the leaf surfaces of plants exposed to 3000 μM. SEM analysis
revealed distinct crystalline structures on the leaf surfaces and EDX analysis
confirmed the predominant composition of Zn(II) and P
(figure 5).
Figure
5. EDX spectra and scanning electron microscopy (SEM)
image of the crystals observed on S. arundinaceus leaf surfaces exposed
to 3000 μM.
Figura 5. Análisis
EDX e imagen de microscopía electrónica de barrido (SEM) de los cristales
observados en las superficies de las hojas de S. arundinaceus expuestas
a 3000 μM.
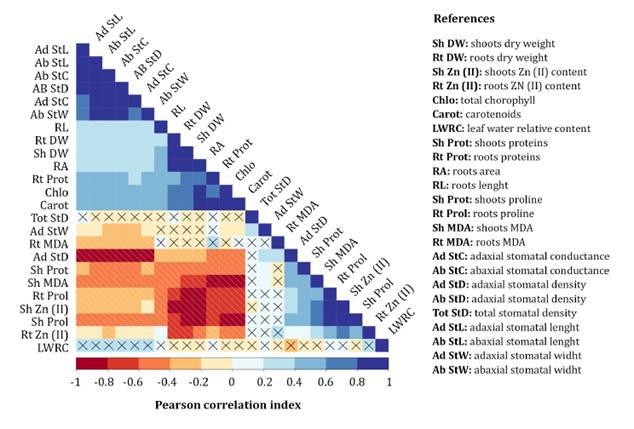
Correlation
analysis
Higher correlations were observed between Zn(II)
concentrations in leaves and the examined parameters compared to Zn(II)
concentrations in roots. The results revealed highly significant positive
correlations between shoot Zn(II) content and MDA
levels in roots, as well as proline content in shoots and roots. Conversely,
shoot and root DW, photosynthetic pigments content, shoot phenolic compounds
content and RA exhibited high negative correlations. Root Zn(II)
content showed significantly high positive correlations with shoot and root
proline content and negative correlations with shoot and root dry weight, shoot
phenolics content and RA (figure 6).
Crossed
correlations indicate non-significant correlation (p >0.05%).
Las
correlaciones con cruces indican correlaciones no significativas (p >0,05%).
Figure
6. Pearson correlation matrix of various
morpho-physiological and biochemical parameters of S. arundinaceus exposed
to Zn(II) stress.
Figura 6. Matriz
de correlación de Pearson de varios parámetros morfofisiológicos y bioquímicos
de S. arundinaceus expuestos a estrés por Zn(II).
Proline content
demonstrated a potential association with a stress threshold bioconcentration
of Zn(II) in S. arundinaceus. Under the 1500 μM
treatment, proline content increased by 27 times in shoots and 5 times in roots
compared to the preceding treatment. This significant increase was specific to
proline, while other biochemical and physiological parameters displayed gradual
changes with increasing Zn(II) concentrations. At 1500
μM, S. arundinaceus bioaccumulated 6408 mg kg-1
DW, which could be considered a stress threshold limit, as
various growth, physiological and biochemical parameters displayed significant
alterations beyond this point, indicating a higher stress condition.
Furthermore, after this treatment, the shoot concentration of Zn(II) reached similar levels to that of the roots,
suggesting that defensive mechanisms were incapable of preventing excessive
translocation of Zn(II) to the shoots.
Discussion
S. arundinaceus bioaccumulated high
concentrations Zn(II), with a maximum of 7244 ± 424
(SD) mg kg-1 DW at 2500 μM Zn(II).
Shoot Zn(II) concentrations (970-3432 mg kg-1) exceeded
phytotoxic levels, even at lower treatments, as shoot concentrations above
100-400 mg kg-1 DW are considered
phytotoxic (42). Similar findings were
reported in 5-month-old S. arundinaceus plants in the vegetative stage,
reaching Zn(II) values of 432 and 1099 mg kg-1
in shoots and roots, respectively (54).
This difference in accumulation observed in comparison to our study could be
related to the different substrates (Haplargids soil) or genotypes used in this
experiment. Concerning this, other research reported higher concentrations in
shoots and roots, reaching up to 6000 and 9000 mg kg-1
DW respectively, indicating the presence of a possible ecotype of
S. arundinaceus and variations in Zn(II)
tolerance within the same species (12).
Our study suggests
that S. arundinaceus acted as an accumulator and phytostabilizer
species, exhibiting BCF values exceeding 1000 at lower concentrations but TF
values never surpassing 1. After 1500 μM, the distribution pattern of Zn(II) changed significantly, approaching TF values closer
to 1. Crystalline formations were observed on the leaves of plants treated with
3000 μM Zn(II), suggesting a potential excretion
mechanism. However, it has not been reported that S. arundinaceus produce
visible Zn(II) crystals through leaf excretion. A
study found that S. arundinaceus excreted Cd through guttation fluid (18). Some halophyte grasses have salt glands or
glandular trichomes, which they use to excrete salts in large quantities, which
can also excrete HMs (53). However, S.
arundinaceus exhibited non-glandular trichomes and lacked salt glands.
We hypothesized
that high Zn(II) concentrations caused crystal
formation through guttation fluid with Zn-P salts, serving as a tolerance
mechanism. This is also correlated with the decrease in shoots Zn(II) content observed between the 2500 μM and 3000 μM
treatments. However, further investigation is needed to understand the
formation and implications of these Zn-P crystals in Zn(II)
tolerance.
Excessive levels of
Zn(II) can have negative effects on plant physiology
and morphology, including inhibition of cell division and elongation, increased
production of ROS and decreased photosynthesis, nutrient uptake and water
absorption (28). Our results showed a
decrease in general DW, as well as RA reduction with increasing Zn(II) treatments. Moreover, TI values indicated that Zn(II) inhibited growth, reaching values below 60% at 1000
μM and higher concentrations. Higher TI values suggest plant tolerance to HMs
without significant growth inhibition (48).
Additionally, morphological and anatomical abnormalities, such as small
protuberances, were observed in roots treated with 3000 μM Zn(II).
It was found that Zn(II)-treated Brassica napus root
epidermal cells exhibited distortion, smaller size, shrinkage and irregular
alignment, which was associated with growth inhibition, reduced nutrient
absorption and damage to the root apex (30).
Accumulation of HMs
in root cell walls diminishes their elasticity, leading to alterations in water
uptake. However, our experiment did not show significant differences in LRWC,
suggesting that the observed morphological changes were insufficient to affect
root water uptake. Similar findings have been reported for Psidium guajava exposed
to high Ni levels, where enhanced vacuole volume was proposed as a contributing
factor (6). Furthermore, a decrease in
stomatal conductance and the presence of smaller stomata were observed at 3000
μM Zn(II), as reported in Citrus reticulata (47), suggesting that these alterations may
represent an adaptive response aimed at mitigating excessive water loss. Zn(II) excess leads to a deficiency in carbonic anhydrase,
which affects HCO3-
concentration in the guard cells and K+
uptake, consequently resulting in alterations in guard cell
morphology and stomatal shape (37). S.
arundinaceus exhibited a reduction in photosynthetic pigments content.
Similar results were reported in other plant species exposed to high Zn(II) concentrations (33).
This decrease can be attributed to the inhibition of chlorophyll synthesis
caused by Zn(II)-induced deficiencies of Mg(II) and
Fe(II) (29).
Excessive Zn(II) bioaccumulation triggers ROS overproduction, leading
to oxidative stress and membrane damage (51).
Our study revealed an increase in shoot MDA levels, while root levels did not show
significant differences. This finding is consistent with the observations in
other plant species, exposed to high Zn(II)
concentrations (17, 51). ROS
overproduction disrupts the integrity of chloroplast membranes and impairs
photosynthetic performance (50), which is
consistent with the negative correlation observed between shoot MDA content and
photosynthetic pigment levels in our experiment. Contrarily, root MDA levels
suggest the activation of antioxidant defense mechanisms, indicating the
ability of the root system to mitigate ROS damage (27).
The accumulation of
organic osmolytes, including total soluble proteins and proline, serves as
crucial indicator of stress adaptation in plants. Shoots soluble protein
content of S. arundinaceus increased with higher Zn(II)
doses, while the opposite was observed in the roots. Similar findings were
reported in T. aestivum, where HM stress led to a significant increase
in shoot-soluble protein content compared to roots (35).
This could be attributed to the synthesis of stress proteins or chelators, such
as glutathione, phytochelatins, metallothioneins, proline or histidine, which
aid in stress tolerance and HM detoxification through compartmentalization in
vacuoles (19). Reduction in protein
content under Zn(II) stress has also been attributed
to the increased activity of proteases or catabolic enzymes induced by HM
stress (28). Additionally, in our
experiment, a significant increase in proline content was observed in shoots
and roots at concentrations of 1500 μM and higher.
Similarly, a higher
increase in proline content in the shoots of Solanum lycopersicum compared
to the roots under Zn(II) stress was reported (4). Proline plays a significant role in
osmoregulation, osmoprotection and ROS detoxification to maintain cellular
homeostasis (31, 47). Additionally, it
acts as a chemical chaperone and a biomembrane protector against oxidative
damage, thereby stabilizing protein structure (36).
Thus, proline accumulation serves as a strategy for plants to defend against
oxidative stress (40). However, the
positive correlation observed between shoot MDA and proline contents in S.
arundinaceus suggests that proline increase may not fully compensate for
oxidative stress. Furthermore, findings indicated that the extent of proline
accumulation and its effectiveness as an osmotic adjuster are species/cultivar
specific and depend on the severity and duration of the stress (34). Biomarkers have emerged as valuable tools
for environmental analysis and phytoremediation programs, complementing
traditional soil chemical analysis (28).
Proline content demonstrated promising characteristics as a potential biomarker
for Zn(II) stress in S. arundinaceus, as it
exhibited a strong correlation with Zn(II) bioaccumulation. Also, proline
determination is a cost-effective, simple and non-destructive method that can
be employed at different stages of the analysis process. However, proline
levels can also be influenced by other types of abiotic stresses, such as
salinity and drought, resulting in varying responses depending on the plant
species (20).
Conclusion
High Zn(II) concentrations affected
growth, biochemical and morpho-physiological parameters of S. arundinaceus (principally
the dry weight, photosynthetic pigments, soluble proteins, MDA and proline
content). However, this species exhibited a remarkable ability to bioaccumulate
high levels of Zn(II) (3432 mg kg-1 in shoots), surpassing
phytotoxic thresholds (100-400 mg kg-1)
by approximately 9 times and activating stress tolerance mechanisms such as the
accumulation of proline and proteins, which could act as ROS inhibitors and HM
chelators. Morphological adaptations, such as smaller stomata, played a role in
maintaining a stable water content. S. arundinaceus performed better
under 1000 and 1500 μM Zn(II), demonstrating high
biomass production and Zn(II) bioaccumulation. Proline holds potential as a
possible biomarker for monitoring the status of S. arundinaceus in response
to Zn(II) stress. However, further studies are
necessary to determine if similar responses occur with other HMs in the same
species.
Acknowledgments
The authors would like to thank Laura Wahnan (INFIVE-CONICET)
and Cecilia Bernardelli (CINDEFI-CONICET) for technical assistance. This study
was financially supported by Agencia Nacional de Promoción Científica y
Tecnológica of Argentina (PICT-2016-2535), Universidad Nacional de La Plata
(UNLP) (A316) and Universidad Nacional del Noroeste de la Provincia de Buenos
Aires (UNNOBA) (0597/2019).
1. Albornoz, C. B.;
Larsen, K.; Landa, R.; Quiroga, M. A.; Najle, R.; Marcovecchio, J. 2016. Lead
and zinc determinations in Festuca arundinacea and Cynodon dactylon collected
from contaminated soils in Tandil (Buenos Aires province, Argentina). Environ
Earth Sci. 75(9): 1-8. https:// doi.org/10.1007/s12665-016-5513-9
2. Alcalá Jáuregui,
J. A.; Rodríguez Ortiz, J. C.; Filippini, M. F.; Martínez Carretero, E.;
Hernández Montoya, A.; Rojas Velázquez, Á. N.; Méndez Cortés, H.; Beltrán
Morales, F. 2022. Metallic elements in foliar material and fruits of three tree
species as bioindicators. Revista de la Facultad de Ciencias Agrarias.
Universidad Nacional de Cuyo. Mendoza. Argentina. 54(2): 61-72. DOI:
https://doi.org/10.48162/rev.39.083
3. Anjum, N. A.;
Singh, H. P.; Khan, M. I.; Masood, A.; Per, T. S.; Negi, A.; Batish, D. R.;
Khan, N. A.; Duarte, A. C.; Pereira, E.; Ahmad, I. 2015. Too much is bad-an
appraisal of phytotoxicity of elevated plant-beneficial heavy metal ions.
Environ Sci Pollut Res. 22(5): 3361-3382. https://doi.
org/10.1007/s11356-014-3849-9
4. Badiaa, O.;
Yssaad, H. A. R.; Topcuoglu, B. 2020. Effect of heavy metals (Copper and Zinc)
on proline, polyphenols and flavonoids content of Tomato (Lycopersicon
esculentum Mill.). Plant Arch. 20: 2125-2137.
5. Bates, L. S.;
Waldren, R. P.; Teare, I. D. 1973. Rapid determination of free proline for
water-stress studies. Plant Soil. 39(1): 205-207. https://doi.org/10.1007/bf00018060
6. Bazihizina, N.;
Redwan, M.; Taiti, C.; Giordano, C.; Monetti, E.; Masi, E.; Azzarello, E.;
Mancuso, S. 2015. Root based responses account for Psidium guajava survival
at high nickel concentration. J Plant Physiol. 174: 137-146. https://doi.org/10.1016/j.jplph.2014.10.011
7. Bilos, C.;
Colombo, J. C.; Skorupka, C. N.; Presa, M. R. 2001. Sources, distribution and
variability of airborne trace metals in La Plata City area, Argentina. Environ
Pollut. 111(1): 149-158. https://doi.org/10.1016/S0269-7491(99)00328-0
8. Bonfranceschi,
B. A.; Flocco, C. G.; Donati, E. R. 2009. Study of the heavy metal
phytoextraction capacity of two forage species growing in a hydroponic
environment. J Hazard Mater. 165(1-3): 366-371. https://doi.org/10.1016/j.jhazmat.2008.10.024
9. Bradford, M. M.
1976. A rapid and sensitive method for the quantitation of microgram quantities
of protein utilizing the principle of protein-dye binding. Anal Biochem.
72(1-2): 248-254. https://doi.org/10.1016/0003-2697(76)90527-3
10. Briffa, J.;
Sinagra, E.; Blundell, R. 2020. Heavy metal pollution in the environment and
their toxicological effects on humans. Heliyon. 6(9): e04691. https://doi.org/10.1016/j. heliyon.2020.e04691
11. Brutti, L. N.;
Beltran, M. J.; García de Salamone, I. 2018. Biorremediación de los recursos
naturales. Ediciones INTA. Argentina.
12. Cao, A.;
Cappai, G.; Carucci, A.; Muntoni, A. 2004. Selection of plants for zinc and
lead phytoremediation. J Environ Sci Health. 39(4): 1011-1024. https://doi:
10.1081/ ESE120028410
13. Chaparro, M.
A.; Gogorza, C. S.; Chaparro, M. A.; Irurzun, M. A.; Sinito, A. M. 2006. Review
of magnetism and heavy metal pollution studies of various environments in
Argentina. Earth Planets Space. 58(10): 1411-1422. https://doi.org/10.1186/BF03352637
14. D’Ambrogio de
Argüeso, A. 1986. Manual of techniques in plant histology. Hemisferio Sur.
Buenos Aires.
15. de
Strittmatter, C. D. 1973. New diaphanization technique. Bol Soc Argent Bot. 15:
126-129.
16. Di Rienzo, J.
A.; Casanoves, F.; Balzarini, M. G.; González, L.; Tablada, M.; Robledo, C.W.
InfoStat Versión 2020. Grupo InfoStat, FCA; Universidad Nacional de Córdoba,
Argentina. http:// www.infostat.com.ar
17. Dobrikova, A.;
Apostolova, E.; Hanć, A.; Yotsova, E.; Borisova, P.; Sperdouli, I.; Adamakis,
I. S.; Moustakas, M. 2021. Tolerance mechanisms of the aromatic and medicinal
plant Salvia sclarea L. to excess zinc. Plants 10(2):194.
https://doi.org/10.3390/plants10020194
18. Dong, Q.; Fei,
L.; Wang, C.; Hu, S.; Wang, Z. 2019. Cadmium excretion via leaf hydathodes in
tall fescue and its phytoremediation potential. Environ Pollut. 252: 1406-1411.
https://doi. org/10.1016/j.envpol.2019.06.079
19. Emamverdian,
A.; Ding, Y.; Mokhberdoran, F.; Xie, Y.; 2015. Heavy metal stress and some mechanisms
of plant defense response. Sci World J. 2015: 1-18.
https://doi.org/10.1155/2015/756120
20. Ghosh, U. K.; Islam, M. N.; Siddiqui, M. N.; Cao, X.; Khan,
M. A. R. 2022. Proline, a multifaceted signalling molecule in plant responses
to abiotic stress: understanding the physiological mechanisms. Plant Biol
(Stuttg). 24(2): 227-239. https://doi.org/10.1111/plb.13363
21. Giuffré, L.;
Romaniuk, R. I.; Marbán, L.; Ríos, R. P.; Torres, T. G. 2012. Public health and
heavy metals in urban and periurban horticulture. Emir J Food Agric. 24(2):
148-154.
22. Gonzalez, M.
A.; Ruscitti, M. F.; Plaza Cazón, J. D. C.; Arango, M. C. 2021. Bioaccumulation
and physiological responses of Festuca arundinacea (Poaceae) to Zn(II) excess. Agronomía y Ambiente. 41(1): 13-21.
23. Hasanuzzaman,
M.; Nahar, K.; Fujita, M. 2018. Plants under metal and metalloid stress:
Responses, tolerance and remediation. Springer, Singapore.
24. Heath, R. L.;
Packer, L. 1968. Photoperoxidation in isolated chloroplasts. Arch Biochem
Biophys. 125(3): 850-857. https://doi.org/10.1016/0003-9861(68)90523-7
25. Hoagland, D.
R.; Arnon, D. I. 1950. The water-culture method for growing plants without soil.
Circular. California agricultural experiment station. 347(2).
26. Htwe, T.;
Chotikarn, P.; Duangpan, S.; Onthong, J.; Buapet, P.; Sinutok, S. 2022.
Integrated biomarker responses of rice associated with grain yield in
copper-contaminated soil. Environ Sci Pollut Res. 29(6): 8947-8956.
https://doi.org/10.1007/s11356-021-16314-y
27. Jan, S.;
Parray, J. A. 2016. Approaches to heavy metal tolerance in plants. Springer.
Singapore.
28. Kaur, H.; Garg,
N. 2021. Zinc toxicity in plants: A review. Planta. 253(6): 129. https://doi.
org/10.1007/s00425-021-03642-z
29. Khan, M. I. R.;
Jahan, B.; Alajmi, M. F.; Rehman, M. T.; Khan, N. A. 2019. Exogenously-sourced
ethylene modulates defense mechanisms and promotes tolerance to zinc stress in
mustard (Brassica juncea L.). Plants. 8(12): 540.
https://doi.org/10.3390/plants8120540
30. Kouhi, S. M.;
Lahouti, M.; Ganjeali, A.; Entezari, M. H. 2016. Anatomical and ultrastructural
responses of Brassica napus after long-termexposure to excess zinc. Turk
J Biol. 40: 652-660. https:// doi.org/10.3906/biy-1411-13
31. Li, X.; Yang,
Y.; Jia, L.; Chen, H.; Wei, X. 2013. Zinc-induced oxidative damage, antioxidant
enzyme response and proline metabolism in roots and leaves of wheat plants.
Ecotoxicol Environ Saf. 89: 150-157. https://doi.org/10.1016/j.ecoenv.2012.11.025
32. López, I.;
Rotger, D. V. 2020. Expansión urbana, humedales y evolución en los usos del
suelo en el Gran La Plata. Biología Acuática. (35): 017.
https://doi.org/10.24215/16684869e017
33. Majdoub, N.;
el-Guendouz, S.; Rezgui, M.; Carlier, J.; Costa, C.; Kaab, L. B. B.; Miguel, M.
G. 2017. Growth, photosynthetic pigments, phenolic content and biological
activities of Foeniculum vulgare Mill., Anethum graveolens L. and
Pimpinella anisum L. (Apiaceae) in response to zinc. Ind Crops Prod.
109: 627-636. https://doi.org/10.1016/j.indcrop.2017.09.012
34. Mansour, M. M.
F.; Ali, E. F. 2017. Evaluation of proline functions in saline conditions.
Phytochemistry. 140: 52-68. https://doi.org/10.1016/j.phytochem.2017.04.016
35. Mohammadi, A.;
Mansour, S. N.; Najafi, M. L.; Toolabi, A.; Abdolahnejad, A.; Faraji, M.; Miri,
M. 2022. Probabilistic risk assessment of soil contamination related to
agricultural and industrial activities. Environ Res. 203: 111837.
https://doi.org/10.1016/j.envres.2021.111837
36. Mohsenzadeh,
S.; Moosavian, S. S. 2017. Zinc sulphate and nano-zinc oxide effects on some
physiological parameters of Rosmarinus officinalis. Am J Plant Sci.
8(11): 2635-2649. https://doi.org/10.4236/ajps.2017.811178
37. Mukhopadhyay,
M.; Mondal, T. K. 2015. Effect of Zinc and Boron on growth and water relations
of Camellia sinensis (L.) O. Kuntze cv. T-78. Natl Acad Sci Lett. 38(3):
283-286. https:// doi:10.1007/s40009-015-0381-5
38. Patra, D. K.;
Acharya, S.; Pradhan, C.; Patra, H. K. 2021. Poaceae plants as potential
phytoremediators of heavy metals and eco-restoration in contaminated mining
sites. Environ Technol Innov. 21: 101293.
https://doi.org/10.1016/j.eti.2020.101293
39. Peng, H.;
Liang, K.; Luo, H.; Huang, H.; Luo, S.; Zhang, A.; Xu, H.; Xu, F. 2021. A
bacillus and Lysinibacillus Sp. bio-augmented Festuca arundinacea phytoremediation
system for the rapid decontamination of chromium influenced soil. Chemosphere.
283: 131186. https:// doi.org/10.1016/j.chemosphere.2021.131186
40. Reddy, S. H.;
Al-kalbani, H.; Al-Qalhati, S.; Al-Kahtani, A. A.; Al Hoqani, U.; Al Azmi, S.
N.; Kumar, A.; Kumar, S.; Settaluri; V. S. 2024. Proline and other
physiological changes as an indicator of abiotic stress caused by heavy metal
contamination. Journal of King Saud University Science. 103313.
https://doi.org/10.1016/j.jksus.2024.103313
41. Reyzábal, L.;
Andrade, L.; Marcet, P.; Montero, M. J. 2000. Effect of long-term cultivation
on zinc and copper contents in soils from the Bahía Blanca horticultural belt
(Argentina). Commun Soil Sci Plan. 31(9-10): 1155-1167.
https://doi.org/10.1080/00103620009370504
42. Saxena, G.;
Kumar, V.; Shah, M. P.; 2020. Bioremediation for environmental sustainability:
Toxicity, mechanisms of contaminants degradation, detoxification and
challenges. Elsevier. Amsterdam.
43. Seethepalli,
A.; York, L. M. 2020. RhizoVision Explorer-Interactive software for generalized
root image analysis designed for everyone (Version 2.0.3). Zenodo.
http://doi.org/10.5281/ zenodo.4095629
44. Seethepalli, A.; Dhakal, K.; Griffiths, M.; Guo, H.;
Freschet, G. T.; York, L. M. 2021. RhizoVision Explorer: Open-source software
for root image analysis and measurement standardization. AoB PLANTS. 13(6):
plab056. https://doi.org/10.1093/aobpla/plab056
45. Soto, M. B.; de
las Mercedes Echeverría, M.; Lúquez, J.; San Martino, S.; Assuero, S. G.;
Petigrosso, L. R. 2022. Tolerancia a la salinidad de festuca alta, naturalizada
y comercial, libre e infectada con endófitos durante la germinación. Revista de
la Facultad de Agronomía. 121(1): 084. https://doi.org/10.24215/16699513e084
46. Sturikova, H.;
Krystofova, O.; Huska, D.; Adam, V. 2018. Zinc, zinc nanoparticles and plants.
J Hazard Mater. 349: 101-110.
47. Subba, P.;
Mukhopadhyay, M.; Mahato, S. K.; Bhutia, K. D.; Mondal, T. K.; Ghosh, S. K.
2014. Zinc stress induces physiological, ultra-structural and biochemical
changes in mandarin orange (Citrus reticulata Blanco) seedlings. Physiol
Mol Biol Plants. 20(4): 461-473. https://doi. org/10.1007/s12298-014-0254-2
48. Tablang, J. O.;
Temanel, F. B.; Campos, R. P. C.; Ramos, H. C. 2021. Bioaccumulation of lead by
pepper elder (Peperomia pellucida (L.) Kunth) in a lead-contaminated
hydroponic system. Environ Nat Resour J. 19(4): 282-291. https://doi.org/10.32526/ennrj/19/2021010
49. Villalobos, E.;
Umaña, C.; Sterling, F. 1990. Determinación del contenido relativo de agua en
progenies de palma aceitera (Elaeis guineensis), durante la época seca
en Quepos, Costa Rica. Agronomía Costarricense. 14: 73-8.
50. Waszczak, C.;
Carmody, M.; Kangasjärvi, J. 2018. Reactive oxygen species in plant signaling.
Annu Rev Plant Biol. 69: 209-236. https://doi.org/10.1146/annurev-arplant-042817-040322
51. Wei, C.; Jiao,
Q.; Agathokleous, E.; Liu, H.; Li, G.; Zhang, J.; Fahad, S.; Jiang, Y. 2022.
Hormetic effects of zinc on growth and antioxidant defense system of wheat
plants. Sci Total Environ. 807: 150992. https://doi.org/10.1016/j.scitotenv.2021.150992
52. Wellburn, A. R.
1994. The spectral determination of chlorophylls a and b, as well as total
carotenoids, using various solvents with spectrophotometers of different
resolution. J Plant Physiol. 144(3): 307-313. https://doi.org/10.1016/s0176-1617(11)81192-2
53. Yuan, F.; Leng,
B.; Wang, B. 2016. Progress in studying salt secretion from the salt glands in
recretohalophytes: how do plants secrete salt? Front Plant Sci. 7: 977.
https://doi. org/10.3389/fpls.2016.00977
54. Zamani, N.;
Sabzalian, M. R.; Khoshgoftarmanesh, A.; Afyuni, M. 2015. Neotyphodium
Endophyte changes Phytoextraction of zinc in Festuca arundinacea and Lolium
perenne. Int J Phytoremediation. 17(5): 456-463.
https://doi.org/10.1080/15226514.2014.922919
55. Zamora-Ledezma, C.; Negrete-Bolagay, D.; Figueroa, F.;
Zamora-Ledezma, E.; Ni, M.; Alexis, F.; Guerrero, V. H. 2021. Heavy metal water
pollution: A fresh look about hazards, novel and conventional remediation
methods. Environ Technol Innov. 22: 101504. https://doi. org/10.1016/j.eti.2021.101504