Revista de la Facultad de Ciencias
Agrarias. Universidad Nacional de Cuyo. Tomo 56(2). ISSN (en línea) 1853-8665.
Año 2024.
Original article
Incidence
of Fusarium graminearum and DON in malting barley grains (Hordeum
vulgare L.)
Incidencia
de Fusarium graminearum y DON en granos de cebada cervecera (Hordeum
vulgare L.)
Carolina Manno1,
Maria Florencia
Martinez1,
Sebastián Alberto
Stenglein1,
1Instituto de Biología Funcional y Biotecnología
(BIOLAB)-INBIOTEC-CONICET-CICBA. Universidad Nacional del Centro de la
Provincia de Buenos Aires. Facultad de Agronomía. Buenos Aires. Argentina.
*elianacastanares@azul.faa.unicen.edu.ar
Abstract
Fusarium
graminearum is a fungal species affecting the quality and safety of malting
barley grains, one of the most important cereals worldwide. Fungal growth and
mycotoxin production vary among growing seasons and sowing locations, mainly
due to weather conditions. This work aimed to assess the incidence of F.
graminearum and the contamination with Deoxynivalenol (DON) in 40 barley
grain samples from different Buenos Aires, Argentina localities during the 2017
and 2018 growing seasons. F. graminearum was identified in 80% of the
samples. It was isolated in eight of eleven localities in the first and ten in
the second growing seasons, with a similar maximum incidence (20% and 17%,
respectively). On the other hand, all samples were contaminated with DON, and
75% exceeded the maximum limits established by The European Union (EC
1126/2007). The level of DON contamination was significantly higher in the
second growing season, which was rainier and had a higher mean temperature (an
average of 2.5 ppm in 2017 and 3.75 ppm in 2018). The results obtained in the
present study show the need to establish regulations in Argentina on maximum
limits of Fusarium mycotoxins in barley.
Keywords: Barley, Fusarium graminearum,
incidence, Deoxynivalenol, food safety
Resumen
Fusarium
graminearum es una especie fúngica que afecta la calidad e inocuidad de la cebada
cervecera, uno de los cereales más importantes a nivel mundial. Tanto el
crecimiento fúngico como la producción de micotoxinas varían año a año y por la
región de siembra, principalmente por las condiciones climáticas. El objetivo
de este trabajo fue evaluar la incidencia de F. graminearum y la
contaminación con Deoxinivalenol (DON) en 40 muestras de cebada cervecera de
distintas localidades de Buenos Aires, Argentina, durante las temporadas de
cultivo 2017 y 2018. F. graminearum fue identificado en el 80% de las
muestras. Se aisló en ocho de once localidades en la primera temporada de
cultivo y en diez localidades de la segunda temporada de cultivo, con una
incidencia máxima similar (20% y 17% respectivamente). Por otro lado, todas las
muestras estuvieron contaminadas con DON y el 75% excedieron los límites
máximos establecidos por la Unión Europea (EC 1126/2007). El nivel de
contaminación con DON fue significativamente mayor en la segunda temporada de
cultivo, la cual fue más lluviosa y tuvo una temperatura media mayor (un
promedio de 2,5ppm en 2017 y 3,75ppm en 2018). Los resultados obtenidos en este
estudio muestran la necesidad de establecer regulaciones en Argentina sobre
límites máximos para micotoxinas de Fusarium en cebada.
Palabras clave: Cebada, Fusarium
graminearum, incidencia, Deoxinivalenol, inocuidad
Originales: Recepción: 01/12/2023 - Aceptación: 12/08/2024
Introduction
Barley (Hordeum
vulgare L.) is the fourth most important cereal worldwide after wheat,
corn, and rice and is used for both human consumption and animal feed. The
world production of barley in the 2022 growing season was 152 million tons,
whereas, in Argentina, it was about 4.5 million tons, of which 3 million tons
were exported. The main importing countries were China (2,335,983
tons) and Brazil (612,295 tons) (6). The
use of barley has increased fundamentally in the brewing industry. In
Argentina, in the 2022 growing season, malting barley occupied 98.61% of the
sown area, whereas fodder barley occupied only 1.39%. Buenos Aires was the
province that presented the largest sown area (26).
Malting barley is
often associated with Fusarium graminearum sensu stricto (hereafter F.
graminearum) infection, resulting in yield reduction, lower germination
capacity, lower thousand kernel weight, and protein degradation (14, 25). Besides the several economic losses, F.
graminearum can produce mycotoxins with the potential to cause adverse
effects on human and animal health. Many studies demonstrated the ability of F.
graminearum to produce deoxynivalenol (DON) (4,
7, 18, 22). Remnants of this mycotoxin have been observed after
malting and in commercial beer (3, 11, 19, 20).
DON is a trichothecene B-type which can cause vomiting, diarrhea, intestinal
inflammation, reduced feed intake, and decreased absorption of amino acids and
carbohydrates. Chronic diseases include anorexia and immunotoxicity (21). Regulations on maximum limits for DON vary
among countries. The European Union (EU) has established a maximum DON limit of
1.25 ppm in unprocessed cereals other than durum wheat, oats, and maize (EC N°
1126/2007) (28). Instead, Brazil, one of
the main importing countries in 2022, has established a maximum DON limit of 1
ppm in barley grain, malted barley, and cereal-based products (2). Argentina has established maximum DON limits
for wheat and maize (1 ppm) and cereal-based foods for infants and young
children (0.2 ppm). However, maximum DON limits for barley in Argentina have
not yet been established (8).
Barley is the main raw material in the brewing industry. Since
both fungal growth and mycotoxin production vary among growing seasons and
sowing locations, this work aimed to assess the incidence of F. graminearum as
well as the levels of contamination with DON in 40 malting barley samples from
different Buenos Aires localities, Argentina, during two consecutive growing
seasons (2017 and 2018).
Material
and methods
Barley samples were obtained from different Buenos Aires
localities, Argentina, during the 2017 and 2018 growing seasons. In the first
growing season, 20 barley samples were obtained from Tandil (3 samples),
Balcarce (3), Lobería (2), Necochea (1), Tres Arroyos (1), Coronel Pringles
(1), Olavarría (1), Guaminí (5), Bolivar (1), San Cayetano (1), and General La
Madrid (1). In the second growing season, 20 barley
samples were obtained from Tandil (5), Balcarce (1), Lobería (2), Necochea (2),
Tres Arroyos (1), Coronel Pringles (1), Olavarría (1), Guaminí (2), Bolivar
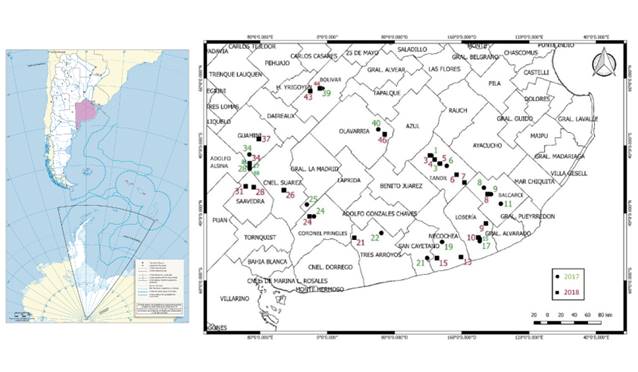
(2), Coronel Suarez (1), and Saavedra (2) (figure 1).
Figure
1. Geographical sampling points from different Buenos
Aires localities, Argentina in two consecutive growing seasons. The numbers indicate
the sample number.
Figura
1. Puntos geográficos de muestreo de
diferentes localidades de Buenos Aires, Argentina, en dos temporadas de cultivo
consecutivas. Los números indican el número de muestra.
Meteorological data
from each sampled locality was collected during the 2017 and 2018 growing
season. In particular, accumulated precipitation and mean temperature were
analyzed to evaluate the eventual influence of climatic conditions on the
incidence of F. graminearum and the levels of contamination with DON in
barley grains. Meteorological data was obtained and analyzed from the Giovanni
online data system, developed and maintained by the NASA GES DISC and the QGis
software (v.3.16) (1). Data was collected
during the period of flowering (November) in which barley is susceptible to Fusarium
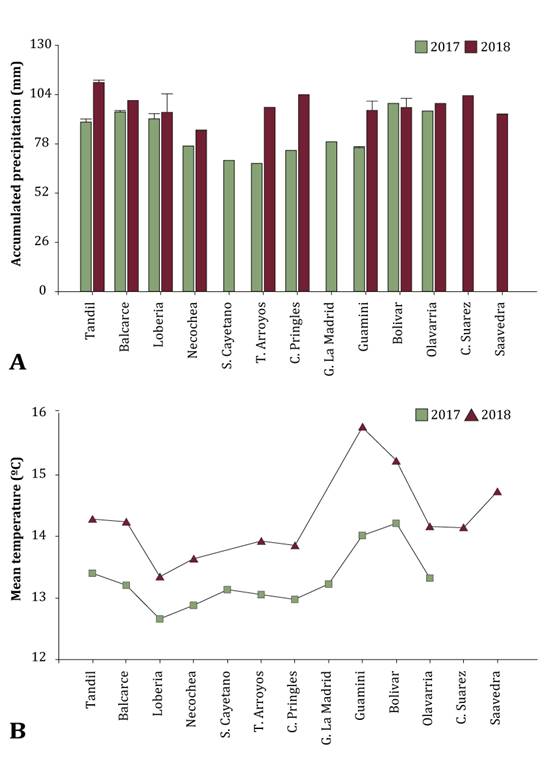
infection (figure 2).
Figure
2.
Accumulated precipitation (A) and mean temperature (B) from sampling localities
during November in the two growing seasons. The lack of standard deviation in
some localities is because only one data was obtained.
Figura
2. Precipitaciones acumuladas (A) y temperatura media (B) de
las localidades muestreadas durante noviembre en las dos temporadas de cultivo.
La falta de desviaciones estándar en algunas localidades se debe a que solo se
obtuvo un dato.
Grain samples (500 g) were reduced with a grain divider,
surfaced disinfected by washing with sodium hypochlorite 5% and ethanol 70%,
subsequently, for 2 min, and washed twice with sterile distilled water for 2
min. One hundred grains were randomly plated (10 grains/ plate) on potato
dextrose agar 2% (PDA) with 0.25 g chloramphenicol/L and incubated at 25 °C for
4-7 days, under a 12 h light/dark cycle. Potential F. graminearum colonies
(mycelia from white to pale orange to yellow and with red pigments in the agar)
were subcultured onto PDA and carnation leaves agar (CLA) and incubated at 25
°C for 15 days, under a 12 h light/dark cycle for identification (12). To confirm
morphological results, a F. graminearum-specific PCR was performed for a
representative subgroup using primers Fg16F and Fg16R (16). Monosporic cultures of previously identified
F. graminearum isolates were cultured for 6 days on PDA plates at 25 °C.
Genomic DNA was extracted using the cetyltrimethylammonium bromide (CTAB)
method (27). Polymerase chain reaction
(PCR) mixture (25 μL) contained 10-20 ng of genomic DNA, 10X reaction buffer,
2.5 mM MgCl2,
1 μM of each primer, 30 μM of dNTPs (Genbiotech S.R.L., Argentina), 1 U of Taq
DNA Polymerase (Inbio-Highway, Argentina), 0.014% of Cresol Red solution
(Sigma-Aldrich Co. St Louis, MO), 0.0005% Tween 20®, 0.0005% Nonidet P40®. The
amplification of DNA was performed in an XP Thermal Cycler (Bioer Technology
Co., China) (16). After electrophoresis
separation in 5X TBE buffer at 80 V on 1.5% agarose gel containing 3-4 μL of
GelRedTM, the PCR products (≈ 400 base pairs) were visualized under UV light
(Biotium, Hayward, USA). The isolate 1-1 was used as positive control (7).
The protein content
of grain samples was measured with a NIT analyzer with a double-face
monochromator (Agricheck, Bruins Instruments, Germany) to evaluate their
relationship with the incidence of F. graminearum.
DON contamination was analyzed in a representative subsample (20
g) by enzyme-linked immunosorbent assay (ELISA) following the manufacturers’
specifications (AgraQuant DON, RomerLabs) with a detection limit of 0.2 ppm.
The plate was scanned using an automatic plate reader Rayto RT-6000.
Statistical
analyses were done using RStudio version 2022 (24).
Normal distribution of the data was evaluated using the Shapiro–Wilks test.
Analysis of variance (ANOVA) was performed to evaluate differences in F.
graminearum incidence and DON contamination levels between growing seasons
and localities. Pearson’s Correlation analysis was performed between the
incidence of F. graminearum and protein content, the incidence of F.
graminearum and DON contamination levels, and the DON contamination levels
and climatic conditions.
Results
and discussion
Our results showed that 32 out of the 40 samples (80%) were
contaminated with F. graminearum. A total of 236 isolates were
morphologically identified, and the selected isolates amplified fragments of ≈
400 base pairs, which confirmed the morphological observations. The number of
samples contaminated by F. graminearum was greater than in other studies
carried out in Buenos Aires (18), central
Italy (4), and Southern Brazil (22, 23). A recent study has demonstrated that
warm nights increase the incidence of F. graminearum in barley, so it is
important to consider that the incidence observed in this study may increase in
the coming years (15). The incidence
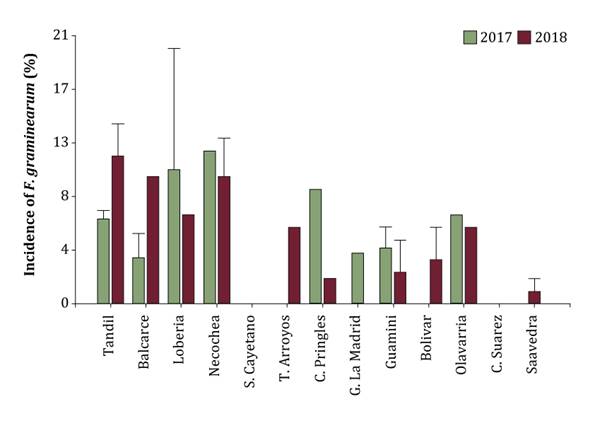
(percentage of infected grains per sample) varied from 0 to 20% in the first
growing season, with an average incidence of 5.3%, and varied from 0 to 17% in
the second growing season, with an average incidence of 6.5% (figure
3).
Figure
3. Incidence of F. graminearum (average of
grains infected per sample) in malting barley samples grown in different Buenos
Aires localities, in two consecutive growing seasons. The lack of standard
deviation in some localities is because only one data was obtained.
Figura 3. Incidencia
de F. graminearum (porcentaje de granos infectados por muestra) en
muestras de cebada cervecera en diferentes localidades de Buenos Aires, en dos
temporadas de cultivo consecutivas. La falta de desviaciones estándar en
algunas localidades se debe a que solo se obtuvo un dato.
A similar incidence was
observed in wheat samples from different Buenos Aires localities (13). Regarding distribution by locality, F.
graminearum was isolated from eight localities in 2017 and ten localities
in 2018 (figure 3). The greatest number of isolates was
obtained in Necochea and Tandil in the first and the second growing seasons
respectively, whereas the lowest number of isolates was obtained in Balcarce and
Saavedra in the first and the second growing seasons respectively (figure
3). F. graminearum was not isolated from Bolivar, San Cayetano, and
Tres Arroyos in the first growing season, and from Coronel Suarez in the
second. Correspondent analyses showed non-significant differences in the
incidence of F. graminearum in barley between different seasons and
different localities. However, we did not have the same number of samples per
locality and the same localities per growing season. Besides, an association
with the favorable climatic conditions in each locality was not found.
Protein content
analysis showed that 67.5% of the samples did not meet the regulation (5), two samples exceeded the maximum limit of
protein content (13%) and 25 samples did not reach the minimum limit of protein
content (9.5%). Although the incidence of F. graminearum was slightly
higher in the second growing season, 90% of the samples of the first growing
season and 45% of the second did not meet the regulation. Pearson’s Correlation
analysis was non-significant and showed a low correlation between protein
content and the incidence of F. graminearum (r = 0.226, p >0.05). In
other studies, protein content was evaluated after inoculation with Fusarium
species, and no effects were observed, attributing the fluctuation in
protein content to environmental conditions during crop development (9), and genotype evaluated (14).
All the samples analyzed were contaminated with DON in different
concentrations. In barley studied in Buenos Aires (18),
central Italy (4), and Southern Brazil (23), the number of contaminated samples was lower
than in this study. In contrast, in wheat samples studied in Buenos Aires, the
number of contaminated samples was similar to the number observed (13). Significant differences (p <0.05) were
found only between growing seasons. The 2018 growing season samples showed
higher DON concentrations than the 2017. The second growing season was rainier
and showed higher mean temperature than the first; however, only a moderate
correlation was observed between DON content and accumulated precipitations
(r= 0.365, p <0.05). A similar association was observed by Gonzalez et al. (2008) and Piacentini
et al. (2015). In the present study, 30 out of 40 samples (75%)
exceeded the maximum limit established by the EU (1.25 ppm) and 32 out of 40
(80%) exceeded the maximum limit established by Brazil (1 ppm). These values
warn of the need to establish control measures to guarantee the production of
safe grains, suitable for commercialization and industrialization. As observed
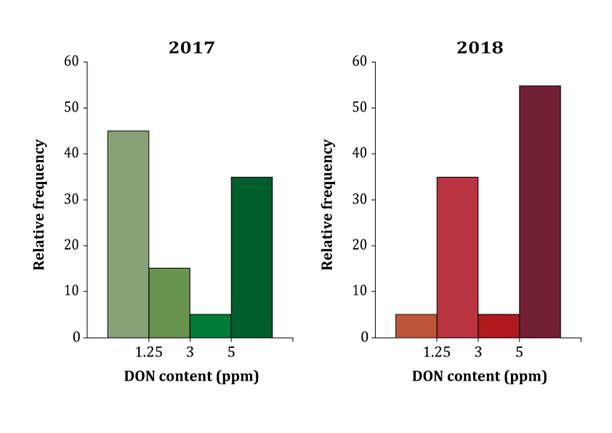
in figure 4, DON contamination levels greater than 5 ppm were
observed in 35% of the samples in the first growing season and 55% in the
second growing season, whereas DON contamination levels lower than 1.25 ppm were
observed in 45% of the samples in the first growing season and 5% in the second
growing season.
Figure
4. Relative frequency of DON content in malting barley
samples grown in two consecutive growing seasons.
Figura 4. Frecuencia
relativa del contenido de DON en muestras de cebada cervecera de dos temporadas
de cultivo consecutivas.
In other studies carried
out in Buenos Aires (18) and Brazil (23) DON contamination levels were similar to
those observed in this study. However, some authors obtained DON contamination
levels lower than our results, and even, none samples (4) and only one sample (17), exceeded the maximum limit established by
the EU.
Pearson’s
Correlation analysis was performed between the incidence of F. graminearum and
DON contamination levels, showing a statistically significant correlation
between variables (r = 0.467, p <0.05). In the first growing season, Tandil,
one of the localities with the highest DON contamination level, had an
incidence of F. graminearum higher than average (7%). In contrast, in
Bolivar, the locality with the lowest DON contamination level, F.
graminearum was not isolated. These results may indicate the presence of
other DON-producing Fusarium species (13,
17, 18) or that F. graminearum was not obtained with the
isolation method used (13). In the second
growing season, Tandil, one of the localities with the highest DON
contamination level, had the highest F. graminearum incidence. In
contrast, Guaminí, the locality with the lowest DON contamination level, had an
incidence of F. graminearum lower than average (3%). The correlation
between the incidence of F. graminearum and DON contamination levels was
also demonstrated in other studies (4, 13, 22).
Conclusion
In conclusion, our results demonstrate that 80% of malting
barley samples from Buenos Aires, Argentina were contaminated with F.
graminearum in the 2017 and 2018 growing seasons. The incidence of F.
graminearum observed was greater than in other studies in Buenos Aires in
barley. The incidence of F. graminearum and precipitations influenced
DON contamination levels. All the samples analyzed were contaminated with this
mycotoxin. The results obtained in the present study show the need to establish
regulations in Argentina on maximum limits of Fusarium mycotoxins in
barley and to carry out continuous monitoring to prevent the negative impact on
consumers’ health.
Acknowledgments
This work was supported by CONICET [PIP 11220200101213] and
UNCPBA.
1. Acker, J. G.;
Leptoukh G. 2007. Online analysis enhances use of NASA Earth science data. Eos,
Trans. AGU. 88(2): 14-17. http://doi.org/10.1029/2007EO020003
2. Agência Nacional
de Vigilância Sanitària (ANVISA). 2022. Instrução normativa - IN Nº 160. Limites
máximos tolerados para micotoxinas em alimentos. Diário Oficial da União.
3. Bauer, J. I.;
Gross, M.; Gottschalk, C.; Usleber, E. 2016. Investigations on the occurrence
of mycotoxins in beer. Food Control, 63: 135-139.
http://dx.doi.org/10.1016/j.foodcont.2015.11.040
4. Beccari, G.;
Caproni, L.; Tini, F.; Uhlig, S.; Covarelli, L. 2016. Presence of Fusarium species
and other toxigenic fungi in malting barley and multi-mycotoxin analysis by
liquid chromatography high-resolution mass spectrometry. Journal of
Agricultural and Food Chemistry, 64(21): 4390-4399.
https://doi.org/10.1021/acs.jafc.6b00702
5. Bolsa de
Comercio de Rosario. 2013. Norma de calidad para la comercialización de cebada
cervecera, NORMA V - ANEXO A - Resolución SENASA 27/2013.
6. Bolsa de
Comercio de Rosario. 2022. Anuario estadístico 2022.
7. Castañares, E.;
Albuquerque, D. R.; Dinolfo, M. I.; Pinto, V. F.; Patriarca, A.; Stenglein, S.
A. 2014. Trichothecene genotypes and production profiles of Fusarium
graminearum isolates obtained from barley cultivated in Argentina.
International Journal of Food Microbiology, 179: 57-63.
https://doi.org/10.1016/j.ijfoodmicro.2014.03.024
8. Código
Alimentario Argentino (CAA). 2023. Ley 18.284. Capítulo III – De los Productos
Alimenticios. Artículo 156 quater – Resolución Conjunta SRYGS y SAB N°22/2019.
Actualizado al 05/2023.
9. Geißinger, C.;
Whitehead, I.; Hofer, K.; Heß, M.; Habler, K.; Becker, T.; Gastl, M. 2018.
Influence of Fusarium avenaceum infections on barley malt: Monitoring
changes in the albumin fraction of barley during the malting process.
International Journal of Food Microbiology, 293: 7-16.
https://doi.org/10.1016/j.ijfoodmicro.2018.12.026
10. Gonzalez, H.
H.; Molto, G. A.; Pacin, A.; Resnik, S. L.; Zelaya, M. J.; Masana, M.; Martínez,
E. J. 2008. Trichothecenes and mycoflora in wheat harvested in nine locations
in Buenos Aires province, Argentina. Mycopathologia. 165(2): 105-114.
https://doi.org/10.1007/s11046- 007-9084-x
11. Laitila, A.
2015. Toxigenic fungi and mycotoxins in the barley-to-beer chain. Brewing
Microbiology, 107-139. https://doi.org/10.1016/B978-1-78242-331-7.00006-X
12. Leslie, J. F.;
Summerell, B. A. 2006. The Fusarium Laboratory Manual, Blackwell
Publishing, Ames. Iowa, USA.
13. Martínez, M.; Castañares, E.; Dinlofo, M. I.; Pacheco, W.
G.; Moreno, M. V.; Stenglein, S. A. 2014. Presencia de Fusarium graminearum en
muestras de trigo destinado al consumo humano. Revista Argentina de
Microbiología. 46(1): 41-44.
14. Martínez, M.;
Ramirez Albuquerque, L. D.; Dinolfo, M. I.; Biganzoli, F.; Fernández Pinto, V.;
Stenglein, S. A. 2020. Effects of Fusarium graminearum and Fusarium
poae on disease parameters, grain quality and mycotoxin contamination in
barley (part II). Journal of the Science of Food and Agriculture. 100(7):
3182-3191. https://doi.org/10.1002/jsfa.10354
15. Martínez, M.;
Biganzoli, F.; Arata, A.; Dinolfo, M. I.; Rojas, D.; Cristos, D.; Stenglein, S.
A. 2022. Warm nights increase Fusarium Head Blight negative impact on barley
and wheat grains. Agricultural and Forest Meteorology. 318 p.
https://doi.org/10.1016/j.agrformet.2022.108909
16. Nicholson, P.;
Simpson, D. R.; Weston, G.; Rezanoor, H. N.; Lees, A. K.; Parry, D. W.; Joyce,
D. 1998. Detection and quantification of Fusarium culmorum and Fusarium
graminearumin cereals using PCR assays. Physiological and Molecular Plant
Pathology. 53(1): 17-37. https://doi. org/10.1006/pmpp.1998.0170
17. Nielsen, L. K.;
Cook, D. J.; Edwards, S. G.; Ray, R. V. 2014. The prevalence and impact of
Fusarium head blight pathogens and mycotoxins on malting barley quality in UK.
International Journal of Food Microbiology. 179(100): 38-49. https://doi.org/10.1016/j.ijfoodmicro.2014.03.023
18. Nogueira, M.
S.; Decundo, J.; Martinez, M.; Dieguez, S. N.; Moreyra, F.; Moreno, M. V.;
Stenglein, S. A. 2018. Natural contamination with mycotoxins produced by Fusarium
graminearum and Fusarium poae in malting barley in Argentina. Toxins
(Basel). 10(2). https://doi. org/10.3390/toxins10020078
19. Oliveira, P.
M.; Mauch, A.; Jacob, F.; Waters, D. M.; Arendt, E. K. 2012. Fundamental study
on the influence of Fusarium infection on quality and ultrastructure of
barley malt. International Journal of Food Microbiology, 156(1): 32-43. https://doi.org/10.1016/j. ijfoodmicro.2012.02.019
20. Pascari, X.;
Rodriguez-Carrasco, Y.; Juan, C.; Manes, J.; Marin, S.; Ramos, A. J.; Sanchis,
V. 2019. Transfer of Fusarium mycotoxins from malt to boiled wort. Food
Chemistry. 278: 700-710. https://doi.org/10.1016/j.foodchem.2018.11.111
21. Payros, D.;
Alassane-Kpembi, I.; Pierron, A.; Loiseau, N.; Pinton, P.; Oswald, I. P. 2016.
Toxicology of deoxynivalenol and its acetylated and modified forms. Archives of
Toxicology. 90(12): 2931-2957. https://doi.org/10.1007/s00204-016-1826-4
22. Piacentini, K.
C.; Savi, G. D.; Pereira, M. E.; Scussel, V. M. 2015. Fungi and the natural
occurrence of deoxynivalenol and fumonisins in malting barley (Hordeum
vulgare L.). Food Chemistry. 187: 204-209.
https://doi.org/10.1016/j.foodchem.2015.04.101
23. Piacentini, K.
C.; Rocha, L. O.; Savi, G. D.; Carnielli-Queiroz, L.; De Carvalho Fontes, L.;
Correa, B. 2019. Assessment of toxigenic Fusarium species and their
mycotoxins in brewing barley Grains. Toxins (Basel). 11(1).
https://doi.org/10.3390/toxins11010031
24. R Core Team.
2022. R: A language and environment for statistical computing. R Foundation for
Statistical Computing, Vienna, Austria. https://www.R-project.org/
25. Sarlin, T.;
Laitila, A.; Pekkarinen, A.; Haikara, A. 2018. Effects of three Fusarium species
on the quality of barley and malt. Journal of the American Society of Brewing
Chemists. 63(2): 43-49. https://doi.org/10.1094/ASBCJ-63-0043
26. Sistema de
Información Simplificado Agrícola (SISA). Cebada 2022-2023.
27. Stenglein, S.
A.; Balatti, P. A. 2006. Genetic diversity of Phaeoisariopsis griseola in
Argentina as revealed by pathogenic and molecular markers. Physiological and Molecular
Plant Pathology. 68(4-6): 158-167. https://doi.org/10.1016/j.pmpp.2006.10.001
28. The European
Union. 2007. Commission Regulation (EC) N° 1126/2007. Setting maximum levels
for certain contaminants in foodstuff. Official Journal of the European Union.