Revista de la Facultad de Ciencias
Agrarias. Universidad Nacional de Cuyo. Tomo 57(1). ISSN (en línea) 1853-8665.
Año 2025.
Original article
Impact
of Cry1Ac soybean (Glicine max) on biological and reproductive cycles
and herbivory capacity of Spodoptera
cosmioides and Spodoptera eridania
(Lepidoptera: Noctuidae)
Impacto
de la soja (Glicine max) Cry1Ac sobre el ciclo biológico, reproductivo y
la capacidad herbívora de Spodoptera
cosmioides y Spodoptera eridania
(Lepidoptera: Noctuidae)
Verónica Eugenia
Ruiz2,
María Cecilia Curis1,
Melina Soledad
Buttarelli3,
Pablo Daniel
Sánchez1,
Roberto Ricardo
Scotta1
1 Universidad Nacional del Litoral. Facultad de Ciencias
Agrarias. Departamento de Producción Vegetal. Kreder 2805 (3080). Esperanza.
Santa Fe. Argentina.
2 Universidad Nacional del Litoral. Facultad de Ciencias
Agrarias. ICiAgro Litoral. CONICET, Esperanza. Argentina.
3 Estación Experimental Agropecuaria Rafaela (INTA E.E.A.
Rafaela). Área de producción Vegetal. Ruta 34 km 227 (2300). Rafaela. Santa Fe.
Argentina.
* alutz@fca.unl.edu.ar
Abstract
Increasing
populations of Spodoptera cosmioides (Walker) and Spodoptera eridania
(Stoll) have recently been detected in soybean crops in central Argentina.
Besides being polyphagous, these species tolerate the Cry1Ac insecticidal
toxin, expressed by genetically modified Bt soybean (MON89788 x
MON87701). Consequently, when facing big populations, farmers often apply
insecticides. This study aimed to determine the effects of Bt soybean on
the consumption, biological cycle, and reproduction of both Spodoptera species.
Larval feeding on Bt soybean led to a shorter pupal period (23% less
than control) and a decreased leaf-area consumption for S. cosmioides (14%
less than the non-Bt soybean). In S. eridania, the larval stage,
adult longevity, larva-to-adult, and oviposition periods were reduced (11, 23,
13, and 30% shorter than control, respectively). Despite these reductions, both
Lepidoptera species completed their reproductive cycles. These valuable findings
help us understand the biology of these potential pests in Bt soybean
crops in Argentina.
Keywords: Glicine max (L.), plant resistance, non-target pests, black armyworm, southern
armyworm
Resumen
En los últimos
años, las poblaciones de Spodoptera cosmioides (Walker) y Spodoptera
eridania (Stoll) se han incrementado en los cultivos de soja de la zona
central de Argentina. Además de ser polífagas, estas especies son tolerantes a
la toxina insecticida Cry1Ac expresada por la soja Bt genéticamente
modificada (MON89788 x MON87701), por lo que los agricultores deben recurrir al
control químico con insecticidas cuando se presentan altas densidades
poblacionales. Este estudio tuvo como objetivo determinar el efecto de la soja Bt
sobre el consumo, ciclo biológico y reproducción de ambas especies de Spodoptera.
La alimentación larval con soja Bt determinó una menor duración del
período pupal (23% menos que el tratamiento control) y una disminución en el
consumo de área foliar en S. cosmioides (14% menos que la soja no Bt).
Spodoptera eridania registró una menor duración del estado larval,
longevidad de adultos, período larva-adulto y del período de oviposición (11,
23, 13 y 30% menos que el tratamiento control, respectivamente). Sin embargo,
ambas especies de Lepidoptera completaron su ciclo reproductivo con éxito. Los
resultados obtenidos en este trabajo son de gran utilidad para comprender la
biología de estas especies, que tienen el potencial de convertirse en plagas
importantes en los cultivos de soja Bt en Argentina.
Palabras clave: Glicine max (L.), plantas resistentes, plagas no blanco, oruga cogollera negra,
oruga cogollera del sur
Originales: Recepción: 15/02/2024- Aceptación: 18/10/2024
Introduction
Genetically
modified (GM) crops exhibiting insect-resistance are valuable tools in
integrated pest management (IPM) systems (25). These crops
express genes derived from the entomopathogenic bacterium Bacillus
thuringiensis Berliner (Bt), producing (Cry) proteins with highly
selective insecticidal activity. Bt soybean expressing these
insecticidal toxins is effective in controlling several major lepidopteran
pests in agricultural environments, including Anticarsia gemmatalis (Hübner),
Chrysodeixis includens (Walker), Helicoverpa gelotopoeon (Dyar)
and Rachiplusia nu (Guenée) (44).
High efficacy of Bt
soybean crops against pest populations and the consequent reduced insecticide
use has significantly altered the agroecosystem. Consequently, the reduced
interspecific competition after controlling Bt target species has
facilitated the emergence of new phytophagous pest species; many of which could
become economically significant (19, 30, 47). Recent reports
mention increasing populations of Spodoptera cosmioides Walker and Spodoptera
eridania Stoll (Lepidoptera: Noctuidae) in Argentinean soybean crops,
including Bt cultivars (23, 29, 30, 32). Factors
contributing to these phenomena include tolerance to the Cry1Ac protein,
insecticide resistance and the ability to complete life cycles on the weed Amaranthus
sp. (2, 5, 9, 24, 26, 30). S. eridania thrives
in temperate regions like the Argentinean Pampas, with a developmental
threshold of 11.9°C and an inability to complete its life cycle above 34°C. In
contrast, S. cosmioides is adapted to warmer temperatures (from 13.2),
prevailing in soybean and cotton crops in northern Argentina (31). Although soybean
and cotton are the preferred hosts (11), caterpillars of
both species are polyphagous, and develop on weeds and grain, fruit, and
ornamental crops (14). These species
also have greater herbivorous potential than other soybean defoliators,
consuming vegetative structures, flowers, and pods (6,
21, 27, 38).
Understanding biological and reproductive pest cycles is
essential for elucidating population dynamics and predicting potential crop
populations. Most studies assessing the effects of the Cry1Ac protein on the
development and foliar consumption of lepidopteran pests (4,
5, 8, 12) have been conducted in Brazil, with almost no equivalent in
Argentina. This study hypothesized that the Cry1Ac protein affects biological
performance, reproduction, and feeding behavior of both Spodoptera species.
We investigated the impact of Bt soybean on foliar consumption and life
cycle to assess pest potential in the central soybean region of Argentina.
Materials
and methods
This study was
conducted in the breeding chamber of the Plant Production Department at the
Facultad de Ciencias Agrarias (Universidad Nacional del Litoral), in Esperanza
City, Santa Fe province, Argentina.
Insect
rearing
Spodoptera
cosmioides and S. eridania larvae were collected in February 2019
from commercial soybean fields in Santa Fe province, near Franck (31°35’00” S
60°56’00” W, 31 m a. s. l.) and Santa María Norte (31°31’00” S 61°08’00” W, 44
m a. s. l.). The caterpillars were transported to the breeding chamber in
containers with soybean leaves and identified using taxonomic keys (43,
46). They were reared under controlled temperature (24 ± 2°C),
relative humidity (60%), and photoperiod (14:10 h, light: dark) in transparent
PVC boxes (26 cm long, 17 cm wide, and 7 cm high), covered with muslin caps for
air circulation. An artificial diet consisting of corn flour, wheatgerm, yeast,
water, agar, nipagin, benzoic acid, and ascorbic acid was provided until pupation
(33). The emerged
adults were placed in oviposition cages (50 cm length, 40 cm width, and 40 cm
height), with paper sheets for oviposition. They were fed daily with an
artificial adult diet (10) provided through
soaked cotton. Eggs were collected daily and placed in 9 cm diameter Petri
dishes with artificial feed for neonate larvae. Three days after hatching,
larvae were transferred to PVC boxes for large-scale rearing with an artificial
diet. This process continued until the F2 generation, ensuring enough
population for the study.
Plant
material
Leaves for larvae
feed were obtained from soybean cultivars RA 5715 IPRO (Bt) and RA 549
(non-Bt). Both cultivars are glyphosate-tolerant, but only the former
expresses the Cry1Ac toxin. To ensure a continuous supply of leaves, both
cultivars were periodically planted in 3x2 m plots under field conditions.
Weeds were manually removed and soybean plants were kept disease-free by the
eventual application of fungicides.
Effect
of Bt soybean on the biological and reproductive cycle of S.
cosmioides and S. eridania
A second instar
(L2) larva of either S. cosmioides or S. eridania was placed on
two soybean leaflets (from Bt or non-Bt soybean plants, depending
on the treatment) inside 9 cm diameter Petri dishes lined with absorbent paper.
Petioles were wrapped in cotton saturated with distilled water, maintaining
humidity. Bt and non-Bt soybean leaflets were harvested at V6-V8
vegetative stage before anthesis, according to phenology by Fehr et
al. (1977). The V6-V8 vegetative stage corresponds to maximum Cry1Ac
expression in the Bt cultivar (45). Food and
absorbent papers were renewed daily until pupation. We defined sex by observing
the terminal portion of pupae (7) using a
stereomicroscope set (Lancet Instruments, China) at 30× magnification. Thirty
replicates were performed for each treatment (Bt and non-Bt soybean)
and species (S. cosmioides and S. eridania). Once adults emerged,
one couple was placed per oviposition container (17 cm height, 11 cm upper
diameter, and 7 cm lower diameter), covered with a muslin cap facilitating air
circulation and preventing adult escape. The same diet used for rearing was
supplied to adults with soaked cotton (10). Fecundity was
determined by daily collecting egg masses laid by females after mating. Egg
masses were photographed using an Olympus SZ40 stereomicroscope (Olympus
Corporation, Tokyo, Japan) at 40X for egg counting, considering any overlapping
or superimposed eggs. Each egg mass was placed in a separate Petri dish (9 cm
diameter) lined with absorbent paper and food for emerging neonates. Fertility
(viable eggs) was estimated by the number of viable larvae hatched from each
egg mass. The assays were conducted with 11 and 10 couples of S. cosmioides and
14 and 10 couples of S. eridania for the Bt and non-Bt soybean
treatments, respectively.
The following variables were recorded: duration (in days) of
larval, pupal and adult stages, the larva-to-adult period, pupal weight (g)
using an OHAUS-PIONNER precision scale (± 0.0001 g), fecundity (number of
eggs/female), fertility (number of hatched eggs), pre-oviposition (days from
adult emergence to first egg laying), oviposition (days from the first to the
last egg laying), and post-oviposition (days from last egg laying to death).
Effect of Bt soybean on leaf consumption by larvae of S.
cosmioides and S. eridania
Leaf
area consumption (cm2) was determined using the same larvae and
soybean cultivars (species and treatments) as when assessing the impact of Bt
soybean on the biological and reproductive cycle. Fresh leaflets were
provided daily as food, and the remaining unconsumed portions were scanned
using an HP Deskjet F4280 multifunction printer. The consumed leaf area was
quantified by image analysis with ImageJ® software (1).
Adjusted leaf area loss due to dehydration was based on data from soybean
leaflets not exposed to larvae.
Statistical analysis
Bioassays
for each lepidopteran species were conducted independently under a completely
randomized experimental design. Since the duration of larva, pupal, and adult
stages, as well as the larva-to-adult period did not meet normality,
non-parametric Kruskal-Wallis test (α ≤ 0.05) was performed. Pupal weight was
analyzed by ANOVA and Tukey test (α ≤ 0.05). Foliar consumption
means were compared using an independent
samples T-test (α ≤ 0.05). All statistical analyses were conducted using
InfoStat software (13).
Results
Effect of Bt soybean on the biological and reproductive
cycle of S. cosmioides and S. eridania
Feeding
S. cosmioides larvae with Bt soybean leaves did not significantly
affect larval duration compared to control, with 20.46 and 19.48 days,
respectively (H= 0.64; p= 0.4160) (table 1).
However, in S. eridania, significant differences were evidenced for
larval length of 28.13 days when fed with Bt soybean leaves and 31.69
days when supplied with non-Bt soybean leaves (H= 7.43; p= 0.0062) (table
1).
Significant differences in the S. cosmioides pupal stage
duration showed 10.65 and 13.96 days (H= 14.72; p= 0.0001), with Bt and
non-Bt soybean, respectively. In S. eridania, differences were
not significant (H= 3.77; p= 0.0434) (table 1). Regarding the adult stage, no
significant differences were found in S. cosmioides (H= 0.04; p=
0.8485). However, S. eridania adults lived significantly longer on non-Bt
soybean leaves (12.87 days), compared to Bt soybean (9.90 days) (H=
11.70; p= 0.0005) (table
1).
Table
1. Days of larval, pupal, and adult stages,
larva-to-adult period, and pupae weight (g) (Mean ± SD) of Spodoptera
cosmioides and S. eridania, fed Bt and non-Bt soybean
leaves under controlled conditions.
Tabla
1. Duración (días) de los estadios
larval, pupal y adulto, el período de larva a adulto y el peso de las pupas (g)
(Media ± DE) de Spodoptera cosmioides y S. eridania, alimentadas
con hojas de soja Bt y no-Bt en condiciones controladas.
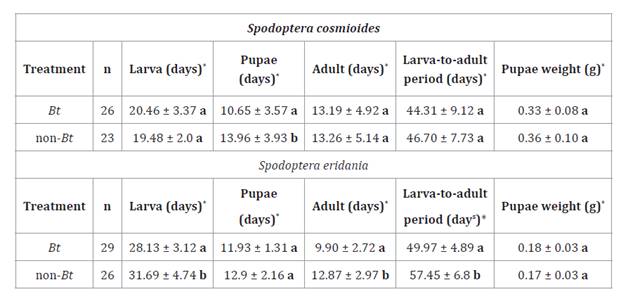
*
Different letters indicate significant differences among treatments. (Test:
Kruskal Wallis, α ≤ 0.05). Pupal weight (Test: Tukey, α ≤ 0.05).
*Diferentes letras en las columnas
indican diferencias significativas entre tratamientos (Prueba: Kruskal Wallis,
α ≤ 0,05). Peso de pupa (Prueba: Tukey, α ≤ 0,05).
The
larva-to-adult period for S. cosmioides was 44.31 days on Bt soybean
and 46.70 days on non-Bt soybean, with no significant differences (H=
0.48; p= 0.4881). In contrast, S. eridania had a larva-to-adult period
significantly longer on non-Bt soybean (57.45 days) compared to Bt soybean
(49.97 days) (H= 13.89; p= 0.0002) (table 1).
Considering
pupal weight, no significant differences were found for either species: S.
cosmioides (F= 0.95; p= 0.3359) and S. eridania (F= 3.77; p= 0.0577)
(table
1).
Larval
feeding did not affect average number of eggs per female in either species. S.
cosmioides had 3291.82 eggs on Bt soybean leaves and 3049.0 on non-Bt
soybean leaves (H= 0.08; p= 0.7782). S. eridania had lower fecundity
than S. cosmioides, with 841.79 eggs on Bt soybean and 830.70
eggs on non-Bt soybean (H= 0.01; p= 0.9068) (table 2).
The Cry1Ac protein ingested by larvae did not affect fecundity in the studied
species (table
2). Similarly, no significant differences were observed in
fertility (percentage of hatched eggs) between treatment species (H= 0.04; p=
0.8327 for S. cosmioides and H= 2.14; p= 0.1432 for S. eridania)
(table
2).
Table
2. Fecundity (number of eggs) and Fertility
(% of hatched eggs) (Mean ± SD) of Spodoptera cosmioides and S. eridania
fed Bt and non-Bt soybean leaves under controlled conditions.
Tabla
2. Fecundidad (número de huevos) y
fertilidad (% de huevos eclosionados) (Media ± DE) de Spodoptera cosmioides y
S. eridania alimentadas con hojas de soja Bt y no-Bt en
condiciones controladas.
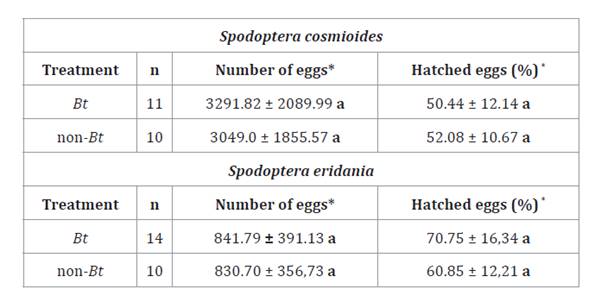
*Different
letters in columns indicate significant differences between treatments. (Test:
Kruskal Wallis α ≤ 0.05).
* Diferentes letras en las columnas
indican diferencias significativas entre tratamientos (Prueba: Kruskal Wallis α
≤ 0,05).
Pre
and post-oviposition periods were similar for both species under both larval
feeding treatments (table 3),
(H= 1.32; p= 0.2395 and H= 0.85; p= 0.34 for S. cosmioides, respectively;
H= 0.51; p= 0.4460 and H= 2.25; p= 0.1238 for S. eridania,
respectively). However, significant differences were found in the oviposition
period for S. eridania, with 4.29 and 6.13 days in adults emerging from
larvae fed Bt and non-Bt soybean leaves, respectively (H= 4.62;
p= 0.0293).
Table 3. Pre-oviposition,
oviposition and post-oviposition periods of Spodoptera cosmioides and S.
eridania (Mean ± SD) fed Bt and non-Bt soybean leaves under
controlled conditions.
Tabla 3. Períodos
de preoviposición, oviposición y postoviposición de Spodoptera cosmioides y
S. eridania (Media ± DE) alimentados con hojas de soja Bt y no Bt
en condiciones controladas.
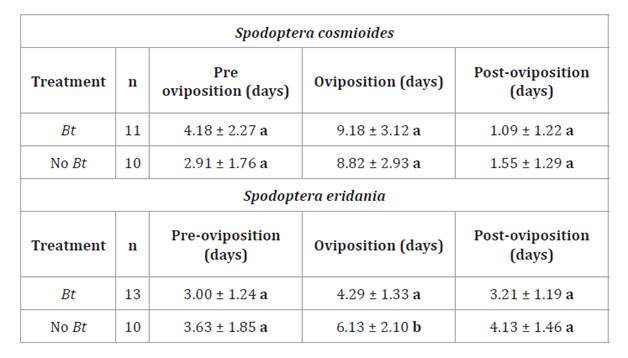
* Different letters in columns
indicate significant differences between treatments. (Test: Kruskal Wallis α ≤
0.05).
*
Diferentes letras en las columnas indican diferencias significativas entre
tratamientos (Prueba: Kruskal Wallis α ≤ 0,05).
Effect of Bt soybean on leaf consumption by S.
cosmioides and S. eridania
Total leaf area consumption by S. cosmioides was lower
when larvae were fed with Bt soybean (T= -2.77; p= 0.0081). In contrast,
S. eridania showed no significant differences in leaf area consumption
between Bt and non-Bt soybean leaves (T= 0.05; p= 0.9585) (table 4).
Table
4. Leaf area consumption of Spodoptera
cosmioides and S. eridania (Mean ± SD) fed Bt and non-Bt soybean
leaves under controlled conditions.
Tabla
4. Consumo de área foliar de Spodoptera
cosmioides y S. eridania (Media ± DE) alimentados con hojas de soja Bt
y no Bt en condiciones controladas.
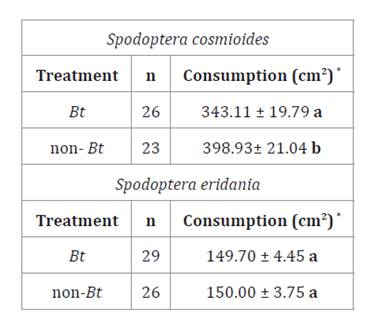
*Different
letters in columns indicate significant differences between treatments. (Test:
T, α ≤ 0.05).
* Diferentes letras en las columnas
indican diferencias significativas entre tratamientos. (Test: T, α ≤ 0,05).
Discussion
gM crops expressing
Cry proteins are crucial for pest control. Besides killing susceptible species,
these crops can have sublethal effects on tolerant species, through direct or
indirect exposure, leading to broader ecological changes (40). Spodoptera
cosmioides and S. eridania exhibit tolerance against the Cry1Ac
protein (2) due to the type
and quantity of receptor proteins in larval midgut membranes, low receptor
affinity, or rapid protein degradation (35). Thus, insect
exposure to stress factors like Cry1Ac protein expressed by Bt soybean
may enhance fitness of the exposed population (17,
18). This explains why S. eridania individuals exhibited
shorter durations in both larval and adult stages, and a reduced larva-to-adult
period when fed soybean leaves expressing the Cry1Ac protein. In contrast, S.
cosmioides only experienced a decrease in the pupal period when fed on
insect-resistant GM soybeans (Bt).
Regarding
larval cycle, our results for S. cosmioides agree with Bernardi
et al. (2014) and Silva et al. (2019),
who observed a similar duration for the last larval stage under the same
treatments. In contrast, S. eridania showed significant differences
between treatments with an average duration of 28.13 days on Bt soybeans
and 31.69 days on non-Bt soybeans. These results are consistent with
those reported by Bortolotto et al. (2014)
and Rabelo et al. (2020),
who observed a significant reduction of 2 days in the larval stage of S.
eridania when fed GM soybeans expressing Cry1Ac.
Our
results showed that S. eridania adults from Bt soybeans live 3
days less than those from the control group. In contrast, Silva
(2013) and Bortolotto et al. (2014)
reported a significant 3 days-increase in longevity of S. eridania males
when reared on Bt soybean leaves. This discrepancy suggests that Cry1Ac
might induce asynchronous adults’ emergence between the two cultivars,
potentially reducing mating chances in natural conditions. According to Jakka
et al. (2014) and Murúa et al. (2019),
the non-simultaneous emergence of adults in both cultivars could compromise the
refuge strategy to avoid or delay resistance emergence. On the other hand, we
found a shortened life cycle of S. eridania (7.48 days) when larvae were
fed soybeans expressing Cry1Ac, as seen by Ramírez &
Gómez (2010), who reported an average life cycle of 51.72 days for S.
eridania with artificial diet.
Several
studies have demonstrated that noctuid pupae weight can vary with temperature,
host plants, and exposure to sublethal insecticide concentrations or Bt crop
toxins (22).
However, our results indicate that Bt protein did not affect pupal
weight of either species. Additionally, feeding larvae with Bt soybean
leaves did not affect the reproductive capacity of either Spodoptera species,
as observed by Silva et al. (2016)
and Sosa et al. (2020),
in S. cosmioides for larvae fed with Bt soybean leaves. However, Páez
Jerez et al. (2022) reported more eggs per female in S.
cosmioides individuals fed Bt soybean. According to Specht
& Roque-Specht (2019), fecundity in S. cosmioides is
highly variable, with females capable of producing up to 5000 eggs/female,
higher than for S. eridania, S. albula, S. frugiperda and S.
littoralis (26, 27).
In our study, average egg number per S. eridania female is consistent
with Silva (2013), who reported similar
fecundity in females reared on both Bt and non-Bt soybeans during
larval stage, with averages of 881.35 and 911.85 eggs per female, respectively.
We
found that exposure to the insecticidal protein Cry1Ac during larval stage
shortened the oviposition period in S. eridania. Although the literature
lacks specific data on the oviposition period of S. eridania fed on Bt
cultivars, previous studies have reported variable oviposition periods
ranging from 4.2 days to 6. 75 days when larvae were reared on non-Bt soybean
leaves (14).
Biological
fitness is the ability of an organism to compete successfully, pass on its
genes to subsequent generations and influence population density and the
potential to become a pest. However, insecticide exposure can have variable
effects, enhancing or reducing performance, potentially leading to adverse
impacts on survival, developmental rate, reproduction, and adult longevity (3).
This phenomenon has been documented in several Lepidoptera species exposed to Bt
protein (16).
In our study, we observed a reduced pupal period in S. cosmioides and
shortened larval, adult, and larval-to-adult cycles and oviposition periods in S.
eridania when fed Bt soybean leaves.
Food quantity and quality directly influence host plant
preference affecting biological, physiological, and behavioral features (11). While some
studies have found no effects of Cry toxins on foliar consumption in
lepidopterans (11), other research
reports less leaf consumption due to Cry proteins in corn (5), as we found for S.
cosmioides. According to Zurbrügg et al. (2010),
glyphosate-resistant soybeans expressing the Cry1Ac toxin have more
carbohydrates and lower protein content than non-transgenic cultivars. This
variation in nutritional composition may influence insect food preference as
seen in S. cosmioides when fed on Cry1Ac-expressing soybean.
Conclusion
Transgenic crops expressing Cry insecticidal proteins are
valuable tools for controlling susceptible pests. However, they may also induce
changes in life cycles, population dynamics, reproductive stages, feeding behavior,
or longevity of non-target species. Understanding developmental and
reproductive parameters of these non-target pests is essential for predicting
population growth and species dynamics within agricultural systems. Our
findings shed light on the biology of S. cosmioides and S. eridania in
Bt soybean crops in Argentina, considering foliar consumption and
herbivorous capacity. Since our experiments were conducted under controlled
conditions, these investigations should further assess actual field damage
caused by Spodoptera species in soybean crops.
1.
Abràmoff, M. D., Magalhães, P. J.; Ram, S. J. 2004. Image processing with
ImageJ. Biophotonics International. 11(7): 36-42.
2.
Bernardi, O.; Sorgatto, R. J.; Barbosa, A. D.; Domínguez, F. A.; Dourado, P.
M.; Carvalho, R. A.; Martinelli, S.; Head, G. P.; Omoto, C. 2014. Low
susceptibility of Spodoptera cosmioides, Spodoptera eridania and Spodoptera
frugiperda (Lepidoptera: Noctuidae) to genetically-modified soybean
expressing Cry1Ac protein. Crop Protection. 58: 33-40. https://doi.
org/10.1016/j.cropro.2014.01.001
3.
Bird, L. J.; Akhurst, R. J. 2004. Relative fitness of Cry1A-resistant and
-susceptible Helicoverpa armigera (Lepidoptera: Noctuidae) on
conventional and transgenic cotton. Journal of Economic Entomology. 97:
1699-1709. https://doi.org/10.1603/0022-0493- 97.5.1699
4.
Bortolotto, O.; Silva, G. V.; De Freitas Bueno, A.; Pomari, A. F.; Martinelli,
S.; Head, G. P.; Carvahlo, R. A.; Barbosa, G. C. 2014. Development and
reproduction of Spodoptera eridania (Lepidoptera: Noctuidae) and its egg
parasitoid Telenomus remus (Hymenoptera: Platygastridae) on the
genetically modified soybean (Bt) MON 87701×MON 89788. Bulletin of Entomological
Research. 104(6): 724-730. https://doi.org/10.1017/S0007485314000546
5.
Bortolotto, O. C.; de Freitas Bueno, A.; de Queiroz, A. P.; Vieira Silva, G.;
Caselato Barbosa, G. 2015. Larval development of Spodoptera eridania (Cramer)
fed on leaves of Bt maize expressing Cry1F and Cry1F + Cry1A.105 + Cry2Ab2
proteins and its Non-Bt isoline. Revista Brasileira de Entomologia. 59(3):7-11.
https://doi.org/10.1016/j.rbe.2014.12.001
6.
Bueno, R. C. O. F.; Bueno, A. F.; Moscardi, F.; Parra, J. R. P.; Hoffmann-Campo,
C. B. 2011. Lepidopteran larva consumption of soybean foliage: basis for
developing multiple-species economic thresholds for pest management decisions.
Pest Management Science. 67: 170-174. https://doi.org/10.1002/ps.2047
7.
Butt, B.; Cantu, E. 1962. Sex determination of lepidopterous pupae. Washington.
D.C: US Department of Agriculture. 33 p.
8.
Carpane, P. D.; Llebaria, M.; Nascimento, A. F.; Vivan, L. 2022. Feeding injury
of major lepidopteran soybean pests in South America. Plos one. 17(12).
https://doi.org/10.1371/journal. pone.0271084
9.
Coradin, J.; Braga Pereira Braz, G.; Guerra da Silva, A.; de Oliveira Procópio,
S.; Sales Vian, G.; Leão Lima Chavaglia, P. V.; Rodrigues Goulart, M. A.; de
Freitas Souza. 2023. Selectivity of latifolicides associated with glyphosate
applied in postemergence on soybean (Glycine max) cultivars. Revista de
la Facultad de Ciencias Agrarias. Universidad Nacional de Cuyo. Mendoza.
Argentina. 55(1): 86-97. DOI: https://doi.org/10.48162/rev.39.098
10.
Curis, M. C.; Bertolaccini, I.; Lutz, A. 2017. Efecto de dietas en adultos de Spodoptera
cosmioides (Lepidoptera: Noctuidae) sobre la fertilidad, fecundidad y
longevidad del adulto. Revista FAVE–Ciencias Agrarias. 16(2): 17-24.
10.14409/fa.v16i2.7015
11.
da Silva, D. M.; de Freitas Bueno, A.; dos Santos Stecca, C.; Andrade, K.;
Neves, P. M. O. J.; de Oliveira, M. C. N. 2017. Biology of Spodoptera
eridania and Spodoptera cosmioides (Lepidoptera: Noctuidae) on
different host plants. Florida Entomologist. 100(4): 752-760. https://doi.
org/10.1653/024.100.0423
12.
de Sousa Ramalho, F.; Azeredo, T. L.; Bezerra de Nascimento, A. R.; Sales
Fernandes, F.; Nascimento Júnior, J. L.; Malaquias, J. B.; da Vieira Silva, C.
A. D.; Cola Zanuncio, J. 2011. Feeding of fall armyworm, Spodoptera
frugiperda, on Bt transgenic cotton and its isoline. Entomologia
Experimentalis et Applicata. 139(3): 207-214. https://doi.org/10.1111/ j.1570-
7458.2011.01121.x
13.
Di Rienzo, J. A.; Casanoves, F.; Balzarini, M. G.; Gonzalez, L.; Tablada, M.;
Robledo, C. W. 2014. InfoStat versión 2014. Córdoba, Argentina: Grupo InfoStat.
Facultad de Ciencias Agropecuarias. Universidad Nacional de Córdoba. Argentina.
http://www.infostat.com.ar
14. Dos Santos, K. B.; Meneguim, A. M.; Neves, P. M. O. J. 2005.
Biologia de Spodoptera eridania (Cramer) (Lepidoptera: Noctuidae) em
diferentes hospedeiros. Neotropical Entomology. 34: 903-910.
https://doi.org/10.1590/S1519-566X2005000600005
15.
Fehr, W. R.; Caviness, C. E.; Burmood, D. T.; Pennington, J. S. 1971. Stage of
development descriptions for soybeans, Glycine Max (L.) Merrill 1. Crop
Science. 11(6): 929-931. https://doi.
org/10.2135/cropsci1971.0011183X001100060051x
16.
Gassmann, A. J.; Carrière, Y.; Tabashnik, B. E. 2009. Fitness costs of insect
resistance to Bacillus thuringiensis. Annual Review of Entomology.
54(1): 147-163. 10.1146/annurev. ento.54.110807.090518
17.
Guedes, R. N. C.; Cutler, G. C. 2014. Insecticide-induced hormesis and
arthropod pest management. Pest Management Science. 70: 690- 697.
https://doi.org/10.1002/ps.3669
18.
Guedes, R. N. C.; Walse, S. S.; Throne, J. E. 2017. Sublethal exposure,
insecticide resistance, and community stress. Current Opinion in Insect Science.
21: 47-53. https://doi.org/10.1016/j. cois.2017.04.010
19.
Horikoshi, R. J.; Dourado, P. M.; Berger, G. U.; de S. Fernandes, D.; Omoto,
C.; Willse, A.; Martinelli, S.; Head, G. P.; Corrêa, A. S. 2021. Large-scale
assessment of lepidopteran soybean pests and efficacy of Cry1Ac soybean in
Brazil. Scientific Reports. 11(1): 15956.
https://doi.org/10.1038/s41598-021-95483-9
20.
Jakka, S. R. K.; Knight, V. R.; Jurat-Fuentes, J. L. 2014. Fitness costs
associated with field-evolved resistance to Bt maize in Spodoptera
frugiperda (Lepidoptera: Noctuidae). Journal of Economic Entomology.
107(1): 342-351. https://doi.org/10.1603/EC13326
21.
Justus, C. M.; Paula-Moraes, S. V.; Pasini, A.; Hoback, W. W.; Hayashida, R.;
de Freitas Bueno, A. 2022. Simulated soybean pod and flower injuries and
economic thresholds for Spodoptera eridania (Lepidoptera: Noctuidae)
management decisions. Crop Protection. 155: 105936. https://
doi.org/10.1016/j.cropro.2022.105936
22.
Liu, Z.; Gong, P.; Li, D.; Wei, W. 2010. Pupal diapause of Helicoverpa
armigera (Hübner) (Lepidoptera: Noctuidae) mediated by larval host plants:
pupal weight is important. Journal of Insect Physiology. 56: 1863-1870.
https://doi.org/10.1016/j.jinsphys.2010.08.007
23.
Lutz, A. L.; Bertolaccini, I.; Scotta, R. R.; Mantica, F.; Magliano, M. F.;
Sanchez, P. D.; Curis, M. C. 2019. Primer reporte de Spodoptera eridania (Stoll)
(Lepidoptera: Noctuidae) en el Centro de la Provincia de Santa Fe. Revista FAVE
- Ciencias Agrarias. 18(2): 7-12. https://doi. org/10.14409/fa.v19i2.8781
24.
Machado, E. P.; dos S Rodrigues Junior, G. L.; Somavilla, J. C.; Führ, F. M.;
Zago, S. L.; Marques, L. H.; Santos, A. C.; Nowatzki, T.; Dahmer, M. L.; Omoto,
C.; Bernardi, O. 2020. Survival and development of Spodoptera eridania, Spodoptera
cosmioides and Spodoptera albula (Lepidoptera: Noctuidae) on
genetically‐modified soybean expressing Cry1Ac and Cry1F proteins. Pest
Management Science. 76 (12): 4029-4035. https://doi. org/10.1002/ps.5955
25.
Martins-Salles, S.; Machado, V.; Massochin-Pinto, L.; Fiuza, L. M. 2017.
Genetically modified soybean expressing insecticidal protein (Cry1Ac):
Management risk and perspectives. Facets. 2: 496-512.
https://doi.org/10.1139/facets-2017-0006
26.
Montezano, D. G.; Specht, A.; Sosa-Gómez, D. R.; Roque-Specht, V. F.; de
Barros, N. M. 2014a. Immature stages of Spodoptera eridania (Lepidoptera:
Noctuidae): developmental parameters and host plants. Journal of Insect
Science. 14: 1-11. https://doi.org/10.1093/jisesa/ieu100
27.
Montezano, D. G.; Specht, A.; Sosa-Gómez, D. R.; Roque-Specht, V. F.; Bortolin,
T. M.; Fronza, E.; Pezzi, P. P.; Luz, P. C.; Barros, N. M. 2014b. Biotic
potential, fertility and life table of Spodoptera albula (Walker)
(Lepidoptera: Noctuidae), under controlled conditions. Anais da Academia
Brasileira de Ciências. 86(2): 723-732. http://dx.doi.org/10.1590/0001-
3765201402812
28.
Murúa, M. G.; Vera, M. T.; Michel, A.; Casmuz, A. S.; Fatoretto, J.;
Gastaminza, G. 2019. Performance of field-collected Spodoptera frugiperda (Lepidoptera:
Noctuidae) strains exposed to different transgenic and refuge maize hybrids in
Argentina. Journal of Insect Science. 19 (6) 21: 1-7.
https://doi.org/10.1093/jisesa/iez110
29.
Paez Jerez, P. G.; Hill, J. G.; Pereira, E. J.; Medina Pereyra, P.; Vera, M. T.
2022. The role of genetically engineered soybean and Amaranthus weeds on
biological and reproductive parameters of Spodoptera cosmioides (Lepidoptera:
Noctuidae). Pest Management Science. 78(6): 2502-2511. https://doi.org/10.1002/ps.6882
30.
Páez Jerez, G. P.; Hill, J. G.; Pereira, E. J.; Alzogaray, R. A.; Vera, M. T.
2023. Ten years of Cry1Ac Bt soybean use in Argentina: Historical shifts in the
community of target and non-target pest insects. Crop Protection. 170: 106265. https://doi.org/10.1016/j.cropro.2023.106265
31.
Parra, J. R. P.; Coelho, A.; Cuervo-Rugno, J. B.; Garcia, A. G.; de Andrade
Moral, R.; Specht, A.; Neto, D. D. 2022. Important pest species of the Spodoptera
complex: Biology, thermal requirements and ecological zoning. Journal of
Pest Science. 95(1): 169-186. https://doi.org/10.1007/ s10340-021-01365-4
32.
Perotti, E.; Boero, L.; Gamundi, J. 2016. Manejo del complejo de plagas de
soja: MIP versus Control Preventivo. En: Para Mejor la Producción 54.
INTA-EEA Oliveros. Santa Fe. Argentina. 169-176.
33. Poitout, S.; Bues, R. 1974. Elevage de chenilles de
veingt-huit espeses de lépidopterés Noctuidae. Annales de Zoologie Ecologie
Animale. 6: 341-411.
34.
Rabelo, M. M.; Matos, J. M. L.; Santos-Amaya, O. F.; França, J. C.; Gonçalves,
J.; Paula-Moraes, S. V.; Guedes, R. N. C.; Pereira, E. J. G. 2020. Bt-toxin
susceptibility and hormesis-like response in the invasive southern armyworm (Spodoptera
eridania). Crop Protection. 132: 105129. https://doi.org/10.1016/j.cropro.2020.105129
35.
Rahman, K.; Abdullah, M. A. F.; Ambati, S.; Taylor, M. D.; Adang, M. J. 2012.
Differential protection of Cry1Fa toxin against Spodoptera frugiperda larval
gut proteases by cadherin orthologs correlates with increased synergism. Applied
and Environmental Microbiology. 78: 354- 362.
https://doi.org/10.1128/AEM.06212-11
36.
Ramírez, M.; Gómez, V. 2010. Biología de Spodoptera eridania (Cramer,
1782) (Lepidoptera; Noctuidae) en dieta natural y artificial, en condiciones de
laboratorio. Investigación Agraria. 12(1): 17-21.
37.
Silva, G. V. 2013. Efeito de plantas Bt de soja e milho sobre pragas não-alvo e
seus inimigos naturais. Dissertação. Mestrado em Ciências Biológicas.
Universidade Federal do Paraná. Curitiba.
38.
Silva, G.; de Freitas Bueno, A.; Bortolotto, O. C.; dos Santos, A. C.;
Pomari-Fernandes, P. 2016. Biological characteristics of black armyworm Spodoptera
cosmioides on genetically modified soybean and corn crops that express
insecticide Cry proteins. Revista Brasileira de Entomologia. 60: 255-259.
https://doi.org/10.1016/j.rbe.2016.04.005
39.
Silva, A.; Baronio, C. A.; Galzer, E. C. W.; Garcia, M. S.; Botton, M. 2019.
Development and reproduction of Spodoptera eridania on natural hosts and
artificial diet. Brazilian Journal of Biology. 79(1): 80-86.
https://doi.org/10.1590/1519-6984.177219
40.
Snow, A. A.; Andow, D. A.; Gepts, P.; Hallerman, E. M.; Power, A.; Tiedje, J.
M.; Wolfenbarger, L. 2005. Genetically engineered organisms and the
environment: current status and recommendations. Ecological Application. 15:
377-404. https://doi. org/10.1890/04- 0539
41.
Sosa, C.; Gómez, V.; Ramírez, M.; Gaona, E.; Gamarra, M. 2020. Toxicity of the Bt
Protein Cry1Ac expressed in leaves of the event of transgenic soybean
released in Paraguay against Spodoptera cosmioides. Journal of
Agricultural Science. 12(12): 107. https://doi. org/10.5539/jas.v12n12p107
42.
Specht, A.; Roque-Specht, V. F. 2019. Biotic potential and reproductive parameters
of Spodoptera cosmioides (Walker) (Lepidoptera: Noctuidae) in the
laboratory. Brazilian Journal of Biology. 79(3): 488-494.
https://doi.org/10.1590/1519-6984.184595
43.
Teodoro, A. V.; Procopio, S. D. O.; Bueno, A. D. F.; Negrisoli Junior, A. S.;
de Carvalho, H. W. L.; Negrisoli, C. D. C.; Brito, L. F.; Guzzo, E. C. 2013. Spodoptera
cosmioides (Walker) e Spodoptera eridania (Cramer) (Lepidoptera:
Noctuidae): novas pragas de cultivos da Região Nordeste. Circular Técnica 131.
EMBRAPA. Brasília. DF.
44.
Vera, M. A.; Casmuz, A. S.; Murúa, M. G.; Fadda, L.; Gastaminza, G. A. 2019.
Plagas blanco de la soja Bt: Complejo de especies defoliadoras Lepidoptera:
Noctuidae. Avance Agroindustrial; 40(3): 28-29.
https://www.eeaoc.gob.ar/wp-content/uploads/2020/01/403-08Ficha-tecnica.pdf
45.
Yu, H.; Li, Y.; Li, X.; Romeis, J.; Wu, K. 2013. Expression of Cry1Ac in
transgenic Bt soybean lines and their efficiency in controlling lepidopteran
pests. Pest Management Science. 69(12): 1326-1333. https://doi.org/10.1002/ps.3508
46.
Zenker, M. M.; Specht, A.; Corseuil, E. 2007. Estágios imaturos de Spodoptera
cosmioides (Walker) (Lepidoptera, Noctuidae). Revista Brasileira de
Zoologia. 24: 99-107. https://doi. org/10.1590/S0101-81752007000100013
47.
Zhao, J. H.; Ho, P.; Azadi, H. 2011. Benefits of Bt cotton counterbalanced by
secondary pests? Perceptions of ecological change in China. Environmental
Monitoring and Assessment, 173: 985-994.
https://doi.org/10.1007/s10661-010-1439-y
48.
Zurbrügg, C.; Hönemann, L.; Meissle, M.; Romeis, J.; Nentwig, W. 2010.
Decomposition dynamics and structural plant components of genetically modified
Bt maize leaves do not differ from leaves of conventional hybrids. Transgenic
Research. 19: 257-267. https://doi. org/10.1007/s11248-009-9304-x
Funding
Financial support for this study was provided by the Universidad
Nacional del Litoral (Argentina), through the Curso de Acción para la
Investigación y Desarrollo (CAI + D) Program (50120150100131LI).