Revista de la Facultad de Ciencias
Agrarias. Universidad Nacional de Cuyo. Tomo 56(2). ISSN (en línea) 1853-8665.
Año 2024.
Original article
Effects
of postharvest treatments based on calcium and silicon in hydro-cooling on the
basic quality attributes of ʹBingʹ sweet cherries (Prunus avium L.)
during storage
Tratamientos
poscosecha a base de calcio y silicio en hidro-enfriamiento sobre atributos
básicos de calidad en cerezas (Prunus avium L.) dulces ʹBingʹ durante
almacenamiento
Irma Ofelia
Maya-Meraz1,
Manuel Francisco
Díaz-Calzadillas1,
María Fernanda
Ruiz-Cisneros1*,
José de Jesús
Ornelas-Paz2,
Claudio Rios-Velasco2,
David I.
Berlanga-Reyes2,
Daniel A.
Pérez-Corral2,
Rodrigo
Alonso-Villegas1
1Universidad Autónoma de Chihuahua. Facultad de Ciencias
Agrotecnológicas. Av. Universidad S/N. Ciudad Universitaria. Chihuahua. C.P.
31110. México.
2Centro de Investigación en Alimentación y Desarrollo A.C. Av.
Río Conchos S/N. Parque Industrial. Cd. Cuauhtémoc. C. P. 31570. México.
*mfruiz@uach.mx
Abstract
Ca2+ and Si2+ treatments confer
resistance to biotic and abiotic stresses in many fruits. In sweet cherries, Ca2+
improves shelf life extension during storage, but only CaCl2
is used. On the other hand, there is scarce information on CaCO3
as a source of Ca2+,
which has shown increased firmness in berries. This study evaluated different treatments
based on Ca2+ (CaCl2 and CaCO3)
+ Si2+ (SiO2)
alone and combined with immersion in hydro-cooling (0°C) on physicochemical
characteristics of ʹBingʹ sweet cherries (Prunus avium L.) during
storage at low temperature (4°C). Results demonstrate that alone or combined
treatments (Ca2+ and Si2+)
with hydro-cooling significantly affected skin and flesh color of sweet
cherries. Chromaticity (C*) was increased in treated fruits, indicating
an intense red color, especially in those cherries treated with CaCl2. Furthermore,
firmness was increased during storage in treatments with Ca2+,
while SiO2 treatment increased total
soluble solids (TSS). Therefore, combined treatments of Ca2+ and Si2+ with hydro-cooling might
be a promising postharvest strategy to maintain desirable physicochemical
characteristics in sweet cherries during low-temperature storage.
Keywords: Prunus avium, fruit firmness, shelf life, non-climacteric fruit,
total soluble solids, skin color
Resumen
Se ha demostrado
que los tratamientos con Ca2+ y Si2+ confieren resistencia al
estrés biótico y abiótico en muchas frutas. En cerezas dulces, el Ca2+
mejora la extensión de la vida útil durante el almacenamiento,
pero solo se ha utilizado CaCl2.
Por otro lado, existe escasa información sobre el CaCO3 como fuente de Ca2+, que ha mostrado
un aumento de la firmeza en bayas. En este estudio, se evaluaron diferentes
tratamientos a base de Ca2+ (CaCl2 y CaCO3)
+ Si2+ (SiO2)
solos y combinados por inmersión en hidro-enfriamiento (0°C) sobre características
fisicoquímicas en cerezas dulces ʹBingʹ (Prunus avium L.) durante el
almacenamiento a baja temperatura (4°C). Los resultados demuestran que los
tratamientos solos o combinados (Ca2+ y Si2+)
en hidro-enfriamiento afectaron significativamente al color de la piel y pulpa
de las cerezas dulces. Se aumentó la cromaticidad (C*) en los frutos
tratados, indicando un color rojo intenso, especialmente en aquellas cerezas
tratadas con CaCl2.
Además, la firmeza aumentó durante el almacenamiento en los tratamientos con Ca2+, mientras que el
tratamiento con SiO2 incrementó la acumulación
de sólidos solubles totales (SST). Por lo tanto, los tratamientos combinados de
Ca2+ y Si2+ con hidro-enfriamiento
podrían ser una estrategia poscosecha prometedora para mantener las
características fisicoquímicas deseables en cerezas dulces durante el
almacenamiento a baja temperatura.
Palabras clave: Prunus avium, firmeza del fruto,
vida útil, fruto no climatérico, sólidos solubles totales,
color de la piel
Originales: Recepción: 09/01/2024 - Aceptación: 02/06/2024
Introduction
Sweet cherry (Prunus
avium L.) is one of the most appreciated fruits worldwide. Attributes such
as sweetness, color, size, and flavor add up to being a rich source of
antioxidants and phytonutrients (14, 39, 40, 66).
In Mexico, the current demand for sweet cherries exceeds the 1,249 tons
imported (17). In this country, cherry
production is 144.45 tons, with only 35.5 ha established in the states of
Chihuahua and Puebla (50). However,
Mexico has regions with high potential for its production (4).
Fruit firmness,
skin and pedicel color, acidity, and sugar content in fresh sweet cherries are
major attributes influencing consumer acceptability (14).
However, these attributes are often lost in between harvest, packaging,
transportation, and storage, especially since sweet cherries are highly
perishable and have a shorter post-harvest shelf life (40, 42, 49). Post-harvest strategies should avoid
water loss, softening, color deterioration, and pedicel browning (14, 30, 53, 66). Nowadays, several technologies
and practices, aimed at preserving post-harvest quality of sweet cherries,
target respiration and senescence, increasing flesh firmness (10, 14, 54, 58, 66). In this regard, pre-harvest
or at-harvest treatments with calcium (Ca2+)
and silicon (Si2+)
on sweet cherries extend storage life and improve flesh firmness by minimizing
respiration and increasing fruit flesh resistance (14,
16, 31, 33, 46, 58, 63, 64).
Calcium is considered a critical, quality-defining nutrient in
sweet cherries (63), mainly promoting
firmness by acting in association with pectin molecules at cell-wall level (8, 38). CaCl2 is the most widely used
source of calcium in sweet cherries, both pre and post-harvest, preserving
fruit quality and reducing physiological disorders like cracking (12, 14, 16, 27, 64). CaCO3 is another less-known
source of calcium for agriculture, shown to increase firmness of ‘Shiraz’
grapes after pre-harvest foliar application (32).
On the other hand, silicon (Si2+),
although not considered an essential element for plant nutrition (7), has been suggested against various biotic and
abiotic stresses in sweet cherry cultivation (2, 7,
28, 46). Si2+ improves strength and stiffness
of plant tissues and increases wall extensibility (2,
23, 28). In addition, available literature demonstrates the safe use
of physical treatments like hydro-cooling on vegetables and fruits to extend
postharvest quality, especially by delaying firmness loss, reducing respiration
rate and preserving fruit flavor (58, 60).
Therefore, chemical
strategies like Ca2+ and Si2+ applications and physical
treatments like hydro-cooling on freshly harvested sweet cherries might
maintain storage quality (58, 59).
However, studies considering a combination of Ca2+ and Si2+ with hydro-cooling and
cool storage on post-harvest quality and shelf life of sweet cherries, are
scarce (29, 53, 58).
Considering the
aforementioned, the study aimed to evaluate the effect of post-harvest
treatments with Ca2+ and Si2+ combined with
hydro-cooling on physicochemical quality of ‘Bing’ sweet cherries during
low-temperature storage.
Materials
and methods
Fruits
and chemical inputs
Sweet cherries
ʹBingʹ (12 kg) were harvested from the commercial orchard “El Fulano”
(28°26’46” N; 106°45’1.6” W and 2013 m above sea level) located in the “Tres
Lagunas” ejido, in Cuauhtemoc, Chihuahua, Mex. Fruits were randomly collected
from several trees on east-facing branches and from the center of the canopy.
For the treatments of Ca2+ and Si2+;
food-grade CaCl2,
CaCO3, and SiO2
were purchased from Food Technologies Trading S.A. de C.V.
Mexico.
Immersion
of fruits
Before starting
treatments, cherries were disinfected by immersion in a 1% (v/v) sodium
hypochlorite for 5 min, washed twice with sterile distilled water, and left to
dry at room temperature while packaged in commercial polyethylene boxes. Six
treatments (solutions) simulated hydro-cooling, using distilled water and
enough ice to keep the solutions at 0°C (58).
Sweet cherries were immersed for 5 min in the evaluated solutions, all of them
at 0.5% according to previous studies (58).
The evaluated solutions were T1 (CaCl2),
T2 (CaCO3), T3 (SiO2), T4 (CaCl2
+ SiO2),
T5 (CaCO3 + SiO2)
and a control treatment T6 (distilled water at 0°C). Thirty-two selected fruits
were used in each treatment considering post-harvest evaluation dates 0, 7, 14
and 21 days after treatment. After the treatments, fruits were drained, placed
on brown paper to dry at room temperature, packed in commercial polyethylene
boxes (500 g) and immediately stored at 4°C with relative humidity of ~85%.
Basic
physicochemical properties
Physicochemical
changes were measured by monitoring weight, firmness, color, total soluble
solids (TSS; °Brix), and titratable acidity (TA). Measurements were expressed
as the average of 32 fruits. The standard error (SE) was estimated at each
evaluation time. Fruit weight was determined with an electronic balance, 0.01g
precision, Precisa BJ 610C (Precisa Gravimetrics AG/Switzerland). Fruit
firmness was evaluated as fruit resistance to a deformation of 15% of fruit
diameter using a plunger of Ø=6 mm on a stationary steel plate, attached to a
Universal Texture Analyzer TA-XT2i (Texture Technologies Corp. USA) according
to previous studies (6). Data were
expressed in Newtons (N) using the Texture Exponent Lite program. Skin color
(CIELab parameters L*, C* and h*) was measured at opposite
sites of each fruit with a colorimeter CR-300, Minolta, (Japan). Total soluble
solids content (TSS= °Brix) was determined in fruit juice with a digital
refractometer PAL-1 pocket (Atago, Japan). Finally, titratable acidity (TA
expressed as g 100 g−1 of fresh weight ‘FW’) was
measured by diluting 1 g of flesh in 9 mL of distilled water, followed by 3
drops of phenolphthalein and titrated with 0.1 N NaOH until pH 8.2 (6). The maturity index was expressed as the ratio
of TSS: TA (34).
Experimental
design and statistical analysis
Results were statistically evaluated according to a split-plot
in-time design. ANOVA and LSD mean tests were used to detect significant
differences among treatments at p≤0.05 using SAS System for Windows 9.0
(SAS Institute. Inc. Cary, N.C., USA, 2002) after testing assumptions. All
experiments were conducted using four replicates.
Results
and discussion
In hydro-cooling, calcium and silicon treatments (alone or
combined) significantly influenced some quality parameters and shelf life in
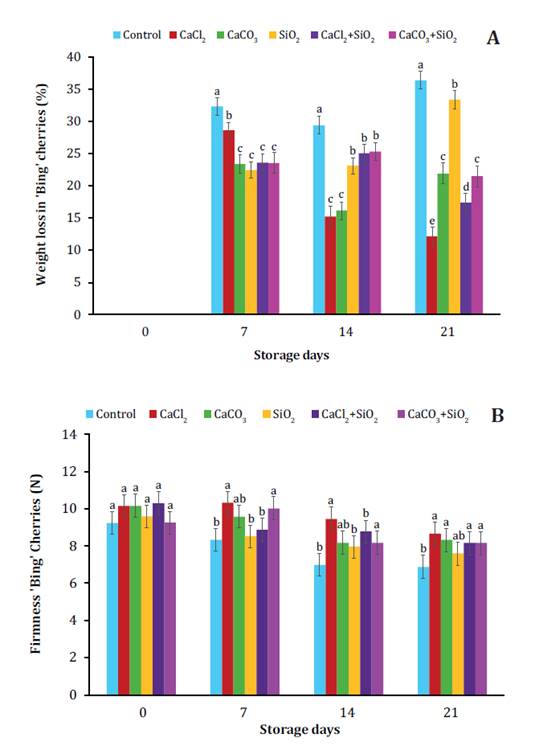
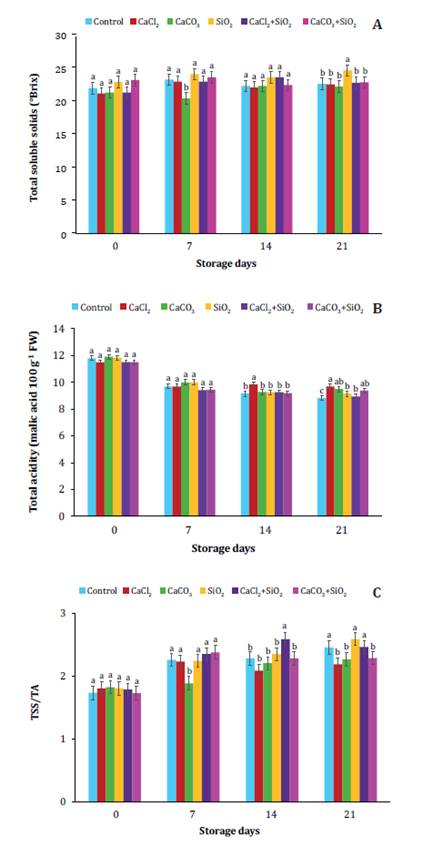
sweet cherries during low-temperature storage (figure 1, figure 2 and figure 3).
Different
letters indicate significant differences (p≤0.05) between treatments for
each storage date.
Las
letras diferentes indican diferencias significativas (p≤0,05) entre
tratamientos para cada fecha de almacenamiento.
Figure
1. Effect of post-harvest treatments based on calcium
(Ca2+)
and silicon (Si2+)
sources, alone and combined with hydro-cooling on weight loss (A) and firmness
(B) in ʹBingʹ sweet cherries during storage at low temperature.
Figura
1. Efecto de los tratamientos
poscosecha basados en fuentes de calcio (Ca2+) y silicio (Si2+) solas y combinadas con
hidro-enfriamiento sobre la pérdida del peso (A) y la firmeza (B) en cerezas
dulces ʹBingʹ durante el almacenamiento a baja temperatura.
Different
letters indicate significant differences (p≤0.05) between treatments for
each storage date.
Las
letras diferentes indican diferencias significativas (p≤0,05) entre
tratamientos para cada fecha de almacenamiento.
Figure
2. Effect of post-harvest treatments of calcium (Ca2+)
and silicon (Si2+)
sources alone and/or combined with hydro-cooling on skin color (L* C* h°)
in ʹBingʹ sweet cherries during low-temperature storage.
Figura
2. Efecto de los tratamientos
poscosecha de fuentes de calcio (Ca2+) y silicio (Si2+) solas y/o combinadas con
hidro-enfriamiento sobre el color de la piel (L* C* h°) en cerezas
ʹBingʹ dulces durante el almacenamiento a baja temperatura.
Different
letters indicate significant differences (p≤0.05) between treatments for
each storage date.
Las
letras diferentes, indican diferencias significativas (p≤0,05) entre
tratamientos para cada fecha de almacenamiento.
Figure
3. Effect of post-harvest treatments of calcium (Ca2+)
and silicon (Si2+)
sources alone and/or combined with hydro-cooling on total soluble solids (TSS;
A), titratable acidity (TA; B) and maturity index (TSS/ TA; C) in ʹBingʹ sweet
cherries during low-temperature storage.
Figura
3. Efecto de los tratamientos
poscosecha de fuentes de calcio (Ca2+) y silicio (Si2+) solas y/o combinadas con
hidro-enfriamiento sobre los sólidos solubles totales (SST; A), la acidez
titulable (AT; B) y el índice de madurez (SST/AT; C) en cerezas dulces ʹBingʹ
durante el almacenamiento a baja temperatura.
Various studies have extensively documented that Ca2+
applications in fruits favor storage conservation. In sweet
cherries, it has been documented that Ca2+ delays deterioration,
favorably influencing physicochemical attributes like weight, color, firmness,
TSS, TA, pH, respiration rate, and anthocyanin content, especially during
storage (14, 31, 57, 58, 59, 60). Shelf
life extension in sweet cherries could be attributed to Ca2+ increase in the cell
walls, favored by rapid absorption of Ca2+ by the fruit flesh under
hydro-cooling immersion (19, 27, 59, 61).
Weight loss is the
most important parameter for horticultural crops and fruit quality and shelf
life. All treatments based on Ca2+ and Si2+ sources, alone and
combined with hydro-cooling, affected weight loss of sweet cherries during
storage (figure 1). According to previous studies (51, 66), weight loss in stored fruits mainly
depends on transpiration and respiration. Interestingly, cherries treated with
Ca2+ lost less weight during
storage compared to untreated cherries (figure 1),
suggesting that Ca2+ ions increased cell wall
stability. Other studies mention increased cell wall stability after Ca2+
ions bind non-esterified pectins and stabilize cell membranes,
preventing electrolyte leakage and consequently preventing fruit moisture and
weight loss (1, 38, 41). The observed
weight values in fruits treated with Ca2+ could have been
influenced by the amount of this element absorbed through the skin (through the
lenticels and peduncle pores) during the 5-minutes exposure (44). Similarly, previous studies (15) documented that combined Ca-Glu (calcium
gluconate) treatment, limited weight loss in sweet cherries.
Sweet cherries
treated with SiO2 showed rapid weight loss
on day 21 of storage, however less evident than for control fruits (figure 1A). Similarly, other studies (3) have documented that SiO2 was less effective in preventing
weight loss in post-harvest fruits of Citrus × sinensis, while Rombolà et al. (2023) found that foliar sprays
with sodium silicate (Na2SiO3) decreased cherry weight at
harvest.
Firmness is a major
attribute in fruits (43). Broadly, our
study showed a gradual loss of firmness concerning storage time indicating
senescence, with significant differences among monitoring dates and treatments.
According to previous studies (14),
decreases in this parameter are more noticeable during storage. Softening of
sweet cherries is attributed to enzymatic degradation of pectic compounds in
the middle lamella of the cell walls by polygalacturonases, pectin methyl
esterases, cellulases, and β-galactosidases (62).
All sweet cherries treated with Ca2+ and Si2+ were firmer than control
fruits (figure 1B). Studies have suggested that pre- and
post-harvest treatments with Ca2+ and Si2+ favor greater firmness in
fruits at harvest time and during storage (27, 55).
Sweet cherries containing insufficient Ca2+ are softer, and,
therefore, more susceptible to quality losses during storage (10). Fruits treated with CaCl2 were the firmest compared
to control fruits after 21 days of storage (figure 1B). It
has been evidenced that CaCl2 applied before and/or
after cherry harvest increases firmness values up to 0.6 N (14, 63, 64). Our study is consistent with
previous studies (10, 14, 15, 27, 55),
reporting increased fruit firmness in treatments with Ca2+ before harvest and/or in
recently harvested cherries. The treatments (CaCO3 and CaCO3+SiO2) also favored
greater firmness of sweet cherries but to a lesser extent than CaCl2
(figure 1B). Similarly, other studies (32) documented firmer ‘Shiraz’ grapes after
pre-harvest foliar treatment with CaCO3.
In our study, the treatment with SiO2 alone was the least
effective, although slightly superior to the control.
The greater
firmness of sweet cherries treated with Ca2+ is attributed to the
ability of this element to maintain cell wall mechanical properties and
integrity during storage, which consequently delays softening (14, 44, 47). According to previous studies (38), Ca2+ acts in association with
pectin molecules in fruit cell walls. It has also been suggested that Ca2+
maintains fruit firmness by reducing water loss and stabilizing
the membrane, given this ion is responsible for binding phosphate and
carboxylate groups of membrane phospholipids and proteins (62, 65).
Surface color of cherries is determined by factors such as
radiation at the end of fruit development, and temperatures near harvest (13). Recently, it has been documented that color
of sweet cherries is influenced by post-harvest treatments based on Ca2+
and Si2+ (14, 46). On the other hand, according to other
studies (21, 36), the chromatic functions
L*, C* and h° are closely correlated with color change and
anthocyanin accumulation in sweet cherries during ripening. Interestingly,
after 21 days of storage, sweet cherries treated with Ca2+ or Si2+ showed increased
chromaticity (figure 2), redder and intensity (C*),
especially in cherries treated with CaCl2.
This effect could be due to the inhibition of skin color development by Ca2+
or Si2+.
The delayed skin color darkening may be related to senescence inhibition (58, 59). Control fruits showed a darker red color
attributed to chlorophyll degradation and accumulation of anthocyanins during
storage (5, 18). Coincidentally, other
studies (21) reported that the higher the
anthocyanin content in sweet cherries, the lower the values of L* and h°.
The L* value in sweet
cherries decreased during storage in all treatments, not showing significant
differences among treatments (figure 2). Sweet cherries
treated with CaCO3+SiO2 and CaCl2+SiO2 showed a
higher h° angle (figure 2), indicating reduced red
tones (h°) than control fruits and suggesting lower skin anthocyanin
content (21, 37). In contrast, Rombolà et al. (2023) documented that Si2+
reduced hue (h°), brightness (C), and saturation of cherry
skin/flesh, while, Karagiannis et al. (2021)
documented that foliar sprays with Si2+ induced skin color development in
apples by stimulating anthocyanin accumulation. In this experiment, sweet
cherries treated with CaCO3+SiO2 and CaCO3 showed higher L* and h° values (figure 2) compared with control fruits, probably given to
suppression of respiratory processes by CaCO3, as previously established in cherries
treated with Ca2+
at harvest (14). The positive effect of
CaCO3 on skin
and flesh color in sweet cherries is given by Ca2+ activation of ABA biosynthesis, which
influences anthocyanin biosynthesis in non-climacteric fruits such as cherries (20, 32).
The TSS
concentration in sweet cherries significantly increased according to storage
time in all treatments (figure 3A). Increasing TSS
concentrations during storage is only frequent in climacteric fruits (22, 35). Therefore, the highest TSS
concentrations in non-climacteric sweet cherries might be favored by a
pronounced weight/moisture loss in SiO2 treated and control
fruits (figure 1A). The SiO2 and CaCl2+SiO2
treatments significantly increased TSS in sweet cherries (figure 3A), like previously documented by Rombolà et al. (2023), who suggested that Si2+
forms a protective film covering fruit surface and preventing
transpiration, slowing down phloem translocation, and subsequent sugar
accumulation. The high concentration of TSS (figure 3A) in
SiO2 -treated
fruits might also be due to sugar concentration after greater weight loss (figure 1A) (11),
something not observed in CaCl2-,
treated ones.
On the contrary,
lower TSS values were observed in sweet cherries treated with CaCl2
compared with control fruits. This coincides with other studies (9, 15), documenting low TSS contents in Ca2+-treated
cherries. Both studies attributed these results to lower respiration rates in
treated cherries, leading to cell wall and membrane stabilization. This could
also be attributed to delayed moisture and weight loss (figure
1A) after pectin stabilization and consequent effects on cell wall and
membrane structure (32).
TA in sweet
cherries also decreased over time during storage for control, Ca2+ and Si2+ treatments evidencing
significant differences (figure 3B). Similar results were
documented in ‘Sweetheart’ and ‘Lapins’ sweet cherries during storage (58). Low acidity mainly depends on ripeness state
(45); however, during storage, organic
acids might be used as carbon source during respiration (15, 25, 26, 60). After 21 days of storage, sweet
cherries treated with Ca2+ and Si2+ maintained TA above
values recorded for control cherries. However, the highest TA values were
measured in CaCl2-treated
fruits (figure 3B). Sweet cherries treated with CaCO3
and CaCO3+SiO2
also showed high TA values. Coincidentally, treatments with Ca2+
(such as CaCl2 and Ca-Glu/calcium
gluconate) in pre-harvest and/or before storage of sweet cherries, also
preserved or retarded TA loss during storage, compared to control fruits (14, 15, 48, 55, 58).
Delayed loss of TA
during storage of sweet cherries treated with Ca2+ sources could be due to
the suppressive effect on fruit metabolic activity, especially respiration (15, 35, 56).
The maturity index TSS/TA indicates commercial and organoleptic
maturity of fruits (34, 45). High
contents of both TSS and TA are associated with good flavor in sweet cherries (52, 53). The TSS/TA ratios in ʹBingʹ sweet
cherries treated with Ca2+ and Si2+ were statistically
different (figure 3C), however increasing over time in all
treatments and indicating a higher acid vs. sugar
content ratio. TSS/TA ratio in sweet cherries treated with CaCO3+SiO2, CaCO3,
and CaCl2 remained lower than
control after 21 days of storage, indicating diminished respiration rates.
While TSS/TA ratios in SiO2 treatments remained above
control values.
Conclusions
Immersion of
freshly harvested ʹBingʹ sweet cherries with hydro-cooled solutions of Ca2+
(CaCl2 and CaCO3)
and Si2+ (SiO2)
alone and combined markedly improved quality properties and extended storage
capacity at low temperatures. All treatments based on Ca2+ and Si2+ alone reduced weight loss
while maintaining firmness, and acidity in sweet cherries. Skin color of sweet
cherries treated with Ca2+ and Si2+ was more intense than
control fruits. Sweet cherries treated with CaCl2 were the firmest and had
the highest TA values. SiO2 increased TSS
concentration in sweet cherries, while CaCl2 decreased it.
1. Angeletti, P.;
Castagnasso, H.; Miceli, E.; Terminiello, L.; Concellon, A.; Chaves, A.;
Vicente, A. R. 2010. Effect of preharvest calcium applications on postharvest
quality, softening, and cell wall degradation of two blueberry (Vaccinium
corymbosum) varieties. Postharvest Biology and Technology. 58(2): 98-103.
https://doi.org/10.1016/j.postharvbio.2010.05.015
2. Bat-Erdene, O.;
Szegő, A.; Gyöngyik, M.; Mirmazloum, I.; Papp, I. 2021. Effects of silicon in
plants with particular reference to horticultural crops - Review article.
International Journal of Horticultural Science. 27: 95-105.
https://doi.org/10.31421/ijhs/27/2021/9096
3. Beltrán, R.;
Otesinova, L.; Cebrián, N.; Zornoza, C.; Breijo, F.; Reig, J.; Garmendia, A;
Merle, H. 2021. Effect of chitosan and silicon oxide treatments on postharvest
Valencia Late (Citrus × sinensis) fruits. Journal of Plant Science and
Phytopathology. 5: 065-071. https://doi. org/10.29328/journal.jpsp.1001063
4.
Chávez-Gutiérrez, N. A.; López de Santana-Pimienta, J. A.; Juárez-Méndez, J.
2023. Zonificación agroecológica del cerezo (Prunus avium L.) en la
región manzanera del estado de Chihuahua. Ciencia Latina Revista Científica
Multidisciplinar. 7(2): 8683-8709. https:// doi.org/10.37811/cl_rcm.v7i2.5983
5. Cogo, S. F.;
Chaves, F.; Schirmer, M.; Zambiazi, R.; Nora, L.; Silva, J.; Rombaldi, C. 2011.
Low soil water content during growth contributes to preservation of green
colour and bioactive compounds of cold-stored broccoli (Brassica oleraceae L.)
florets. Postharvest Biology and Technology. 60(2): 158-163.
https://doi.org/10.1016/j.postharvbio.2010.12.008
6. Correia, S.;
Queirós, F.; Ribeiro, C.; Vilela, A.; Aires, A.; Barros, A. I.; Schouten, R.;
Silva, A. P.; Gonçalves, B. 2019. Effects of calcium and growth regulators on
sweet cherry (Prunus avium L.) quality and sensory attributes at harvest.
Scientia Horticulturae. 248: 231-240. https://
doi.org/10.1016/j.scienta.2019.01.024
7. Coskun, D.;
Deshmukh, R.; Sonah, H.; Menzies, J. G.; Reynolds, O.; Ma, J. F.; Kronzucker,
H. J.; Bélanger, R. R. 2019. The controversies of silicon’s role in plant
biology. New Phytologist. 221: 67-85. https://doi.org/10.1111/nph.15343
8. Daher, F. B.;
Braybrook, S. A. 2015. How to let go: Pectin and plant cell adhesion. Frontiers
in Plant Sciences. 6: 1-8. https://doi.org/10.3389/fpls.2015.00523
9. Díaz-Mula, H. M.;
Valero, D.; Guillén, F.; Valverde, J. M.; Zapata, P.J.; Serrano, M. 2017.
Postharvest treatment with calcium delayed ripening and enhanced bioactive
compounds and antioxidant activity of ‘Cristalina’ sweet cherry. Acta
Horticulturae. 1161: 511-514. https://doi.org/10.17660/ActaHortic.2017.1161.81
10. Dong, Y.; Zhi,
H.; Wang, Y. 2019. Cooperative effects of pre-harvest calcium and gibberellic
acid on tissue calcium content, quality attributes, and in relation to
postharvest disorders of late maturing sweet cherry. Scientia Horticulturae.
246: 123-128. https://doi.org/10.1016/j.
scienta.2018.10.067
11. Dutra de
Vargas, A.; de Oliveira, F. L.; Quintão Teixeira, L. J.; Oliveira Cabral, M.;
dos Santos Gomes Oliveira, L.; Ferreira Pedrosa, J. L. 2022. Physical and
chemical characterization of yacon (Smallanthus sonchifolius) roots
cultivated with different doses of potassium fertilization. Revista de la
Facultad de Ciencias Agrarias. Universidad Nacional de Cuyo. Mendoza.
Argentina. 54(2): 22-31. DOI: https://doi.org/10.48162/rev.39.079
12. Ekinci, N.;
Özdüven, F.; Gür, E. 2016. Effects of preharvest foliar calcium applications on
the storage quality of ‘0900 Ziraat’ sweet cherry cultivar. Erwerbs-Obstbau.
58: 227-231.
13. Ellena, D.
2012. Formación y sistemas de conducción del cerezo dulce. Temuco: Boletín INIA
- Instituto de Investigaciones Agropecuarias. N° 247.
https://hdl.handle.net/20.500.14001/7500 (Accessed: 22 February 2023).
14. Erbaş, D.;
Koyuncu, M. A. 2022. Effect of preharvest calcium chloride treatment on some
quality characteristics and bioactive compounds of sweet cherry cultivars.
Journal of Agricultural Sciences (Tarim Bilimleri Dergisi). 28(3): 481-489.
http://doi.org/10.15832/ ankutbd.874567
15. Erbaş, D.; Koyuncu, M. A. 2023. The effect of pre- and
postharvest calcium gluconate treatments on physicochemical characteristics and
bioactive compounds of sweet cherry during cold storage. Food Science and
Technology International. 29(4): 299-309. https://doi.
org/10.1177/10820132221077515
16. Erogul, D.
2014. Effect of preharvest calcium treatments on sweet cherry fruit quality.
Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 42(1): 150-153.
https://doi.org/10.15835/ nbha4219369
17. FAOSTAT. 2023.
Producción mundial de cereza 2021. https://www.fao.org/faostat/es/#data/
QCL/visualize (Accessed 13 April 2023).
18. Ferrer, A.;
Remón, S.; Negueruela, A.; Oria, R. 2005. Changes during ripening of the very
late season Spanish peach cultivar Calanda: Feasibility of using CIELAB
coordinates as maturity indices. Scientia Horticulturae. 105(4): 435-446.
https://doi.org/10.1016/j.scienta.2005.02.002
19. Figueroa, C.;
Opazo, M. C.; Vera, P.; Arraigada, O.; Díaz, M.; Moya-León, M. 2012. Effect of
postharvest treatment of calcium and auxin on cell wall composition and
expression of cell wallmo difying genes in the Chilean strawberry (Fragaria
chiloensis) fruit. Food Chemistry. 132(4): 2014-2022.
https://doi.org/10.1016/j.foodchem.2011.12.041
20. Gao, Q.; Xiong,
T.; Li, X.; Chen, W.; Zhu, X. 2019. Calcium and calcium sensors in fruit
development and ripening. Scientia Horticulturae. 253: 412-421. https://doi.org/10.1016/j. scienta.2019.04.069
21. Gonçalves, B.;
Silva, A. P.; Moutinho-Pereira, J.; Bacelar, E.; Rosa, E.; Meyer, A. S. 2007.
Effect of ripeness and postharvest storage on the evolution of colour and
anthocyanins in cherries (Prunus avium L.). Food Chemistry. 103:
976-984. https://doi.org/10.1016/j.
foodchem.2006.08.039
22.
Hernández-Muñoz, P.; Almenar, E.; Ocio, M.; Gavara, R. 2006. Effect of calcium
dips and chitosan coatings on postharvest life of strawberries (Fragaria x
ananassa). Postharvest Biology and Technology. 39(3): 247–253.
https://doi.org/10.1016/j.postharvbio.2005.11.006
23. Hossain, M. T.;
Mori, R.; Soga, K.; Wakabayashi, K.; Kamisaka, S.; Fujii, S.; Yamamoto, R.;
Hoson, T. 2002. Growth promotion and an increase in cell wall extensibility by
silicon in rice and some other Poaceae seedlings. Journal of Plant Research.
115: 0023–0027. https://doi. org/10.1007/s102650200004
24. Karagiannis,
E.; Michailidis, M.; Skodra, C.; Molassiotis, A.; Tanou, G. 2021. Silicon
influenced ripening metabolism and improved fruit quality traits in apples.
Plant Physiology and Biochemistry. 166: 270-277.
https://doi.org/10.1016/j.plaphy.2021.05.037
25. Kays, S. J.;
Paull, R. E. 2004. Postharvest biology. Athens, GA: Exon Press.
http://hdl.handle. net/10125/65829
26. Lanchero, O.;
Velandia, G.; Fischer, G.; Varela, N.; García, H. 2007. Comportamiento de la
uchuva (Physalis peruviana L.) en poscosecha bajo condiciones de
atmósfera modificada activa. Ciencia y Tecnología Agropecuaria. 8(1): 61-68.
https://doi.org/10.21930/rcta.vol8_ num1_art:84
27. Lidster, P. D.;
Porritt, S. W.; Tung, M. A. 1978. Texture modification of ‘Van’Sweet cherries
by postharvest calcium treatments. Journal of the American Society for
Horticultural Science. 103(4): 527-530.
https://doi.org/10.21273/JASHS.103.4.527
28. Ma, J. F.;
Yamaji, N. 2006. Silicon uptake and accumulation in higher plants. Trends in
Plant Science. 11: 392-397. https://doi.org/10.1016/j.tplants.2006.06.007
29. Manganaris, G.
A.; Ilias, I. F.; Vasilakasis, M.; Mignani, I. 2007. The effect of hydrocooling
on ripening related quality attributes and cell wall physicochemical properties
of sweet cherry fruit (Prunus avium L.). International Journal of
Refrigeration. 30: 1386-1392. https://doi. org/10.1016/j.ijrefrig.2007.04.001
30.
Martínez-Romero, D.; Alburquerque, N.; Valverde, J. M.; Guillén, F.; Castillo,
S.; Valero, D.; Serrano, M. 2006. Postharvest sweet cherry quality and safety
maintenance by Aloe vera treatment: a new edible coating. Postharvest
Biology and Technology. 39: 93-100. https://doi.
org/10.1016/j.postharvbio.2005.09.006
31. Matteo, M.;
Zoffoli, J. P.; Ayala, M. 2022. Calcium sprays and crop load reduction increase
fruit quality and postharvest storage in sweet cherry (Prunus avium L.).
Agronomy. 12: 829. https://doi.org/10.3390/agronomy12040829
32. Maya-Meraz, I.
O.; Ornelas-Paz, J. J.; Pérez-Martínez, J. D.; Gardea-Béjar, A. A.; Rios-Velasco,
C.; Ruiz- Cruz, S.; Pérez-Leal, R.; Virgen-Ortiz, J. J. 2023. Foliar
application of CaCO3-rich industrial residues on ‘Shiraz’ vines improves the
composition of phenolic compounds in grapes and aged wine. Foods. 12: 1566.
https://doi.org/10.3390/foods12081566
33. Mitre, V.;
Erzsébet, B. U.; Lukacs, L.; Mitre, I.; Teodorescu, R.; Dorel, H. O.; Sestraș,
A. F.; Stănică, F. 2018. Management of apple scab and powdery mildew using
bicarbonate salts and other alternative organic products with fungicide effect in
apple cultivars. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 46(1):
115-121. https://doi.org/10.15835/ nbha46110783
34. Monte Andrade,
A. D.; Moura, E. A.; Mendonça, V.; Mendes Oliveira, L.; Souza Ferreira, E.;
Ferreira Melo, B. E.; Andrade Figueiredo, F. R.; Ferreira Melo, M.; Freitas
Medeiros Mendonça, L. 2022. Production and physicochemical characterization of
genotypes of Eugenia uniflora L. Revista de la Facultad de Ciencias
Agrarias. Universidad Nacional de Cuyo. Mendoza. Argentina. 54(2): 1-11. DOI:
https://doi.org/10.48162/rev.39.077
35. Moradinezhad, F.; Ghesmati, M.; Khayyat, M. 2019.
Postharvest calcium salt treatment of fresh jujube fruit and its effects on
biochemical characteristics and quality after cold storage. Journal of Horticultural
Research. 27(2): 39-46. https://doi.org/10.2478/johr-2019-0009
36. Mozetič, B.;
Simčič, M.; Trebše, P. 2006. Anthocyanins and hydroxycinnamic acids of Lambert
Compact cherries (Prunus avium L.) after cold storage and
1-methylcyclopropene treatment. Food Chemistry. 97(2): 302-309.
https://doi.org/10.1016/j.foodchem.2005.04.018
37. Opiyo, A. M.;
Ying, T. J. 2005. The effects of 1-methylcyclopropene treatment on the shelf
life and quality of cherry tomato (Lycopersicon esculentum var. cerasiforme)
fruit. International Journal of Food Science & Technology. 40: 665-673.
https://doi.org/10.1111/j.1365- 2621.2005.00977.x
38. Ornelas-Paz, J.
J.; Quintana-Gallegos, B. M.; Escalante-Minakata, P.; Reyes-Hernández, J.;
Pérez- Martínez, J. D.; Ríos-Velasco, C.; Ruiz-Cruz, S. 2018. Relationship
between the firmness of Golden delicious apples and the physicochemical
characteristics of the fruits and their pectin during development and ripening.
Journal of Food Science and Technology. 55: 33- 41. https://doi.org/10.1007/s13197-017-2758-6
39. Ozturk, B.;
Aglar, E.; Karakaya, O.; Saracoglu, O.; Gun, S. 2019. Effects of preharvest GA3, CaCl2 and modified
atmosphere packaging treatments on specific phenolic compounds of sweet cherry.
Turkish Journal of Food and Agriculture Sciences. 1(2): 44-56. https://doi.
org/10.14744/turkjfas.2019.009
40. Parsa, Z.;
Roozbehi, S.; Hosseinifarahi, M.; Radi, M.; Amiri, S. 2021. Integration of
pomegranate peel extract (PPE) with calcium sulphate (CaSO4):
A friendly treatment for extending shelf-life and maintaining postharvest
quality of sweet cherry fruit. Journal of Food Processing and Preservation.
45:e15089. https://doi.org/10.1111/jfpp.15089
41. Pérez, A. R.;
Quintero, E. M. 2015. Funciones del calcio en la calidad poscosecha de frutas y
hortalizas: Una revisión. Alimentos hoy. 23(34): 13-25.
42. Petriccione,
M.; De Sanctis, F.; Pasquariello, M. S.; Mastrobuoni, F.; Rega, P.;
Scortichini, M.; Mencarelli, F. 2015. The effect of chitosan coating on the
quality and nutraceutical traits of sweet cherry during postharvest life. Food
and Bioprocess Technology. 8: 394-408.
https://doi.org/10.1007/s11947-014-1411-x
43. Pugliese, M.
B.; Guzmán, Y.; Pacheco, D.; Bottini, R.; Travaglia, C.; Avenant, J. H.;
Avenant, E.; Berli, F. 2022. Indole-3-butyric acid, an alternative to GA3 for
bunch quality enhancing of table grape Vitis vinifera L. cv. Superior
Seedless. Revista de la Facultad de Ciencias Agrarias. Universidad Nacional de
Cuyo. Mendoza. Argentina. 54(1): 163-174. DOI: https://doi. org/10.48162/rev.39.075
44. Reyes-Medina,
A. J.; Pinzon, E. H.; Alvarez-Herrera, J. G. 2017. Effect of calcium chloride
and refrigeration on the quality and organoleptic characteristics of cape
gooseberry (Physalis peruviana L.). Acta Agronómica. 66(1): 15-20. https://doi.org/10.15446/acag.
v66n1.50610
45. Rodrigues
Gomes, F.; Morais Silveira, C.; Marques Rodrigues, C. D.; Alves Ferreira, B.;
Lopes Barros, Â.; Hurtado Salazar, A.; Pereira da
Silva, D. F.; Nunes da Silveira Neto, A. 2023. Correlations between physical
and chemical characteristics of Cortibel guava (Psidium guajava L.)
fruits grown in the Brazilian Cerrado. Revista de la Facultad de Ciencias
Agrarias. Universidad Nacional de Cuyo. Mendoza. Argentina. 55(1): 10-16. DOI:
https://doi.org/10.48162/ rev.39.091
46. Rombolà, A. D.;
Quartieri, M.; Rodríguez-Declet, A.; Minnocci, A.; Sebastiani, L.; Sorrenti, G.
2023. Canopy-applied silicon is an effective strategy for reducing sweet cherry
cracking. Horticulture, Environment, and Biotechnology. 64: 371-378.
https://doi.org/10.1007/ s13580-022-00486-8
47. Saba, M. K.;
Sogvar, O.B. 2016. Combination of carboxymethyl cellulose-based coatings with
calcium and ascorbic acid impacts in browning and quality of fresh-cut apples.
LWT-Food Science and Technology. 66: 165-171.
https://doi.org/10.1016/j.lwt.2015.10.022
48. Safa M.;
Hajilou J.; Nagshiband-Hasani R.; Ganbari-Najar M. 2015. Effect of postharvest
oxalic acid and calcium chloride on quality attributes of sweet cherry (Prunus
avium L.). Journal of Horticulture Science. 29(2): 196-206.
https://doi.org/10.22067/JHORTS4.V0I0.29791
49. Sharma, M.;
Jacob, J. K.; Subramanian, J.; Paliyath, G. 2010. Hexanal and 1-MCP treatments
for enhancing the shelf life and quality of sweet cherry (Prunus avium L.).
Scientia Horticulturae. 125(3): 239-247.
https://doi.org/10.1016/j.scienta.2010.03.020
50. SIAP. Sistema
de Información Agrícola y Pesquera. 2023.
https://nube.siap.gob.mx/cierreagricola/ (Accesed August 2023).
51. Sohail, M.;
Ayub, M.; Khalil, S. A.; Zeb, A.; Ullah, F.; Afridi, S. R.; Ullah, R. 2015.
Effect of calcium chloride treatment on postharvest quality of peach fruit
during cold storage. International Food Research Journal. 22(6): 2225-2229.
52. Toivonen, P. M.
A. 2014. Relationship of typical core temperatures after hydrocooling on
retention of different quality components in sweet cherry. HortTechnology. 24:
457-462. https:// doi.org/10.21273/HORTTECH.24.4.457
53. Toivonen, P. M.
A; Manganaris, G. A. 2020. Chapter 15.2 - Stone fruits: Sweet cherries (Prunus
avium L.), Editor(s): Maria Isabel Gil, Randolph Beaudry. Controlled and
modified atmospheres for fresh and fresh-cut produce. Academic Press. 323-328.
https://doi.org/10.1016/ B978-0-12-804599-2.00018-1
54. Tsaniklidis, G.; Kafkaletou, M.; Delis, C.; Tsantili, E.
2017. The effect of postharvest storage temperature on sweet cherry (Prunus
avium L.) phenolic metabolism and colour development. Scientia
Horticulturae. 225: 751-756. https://doi.org/10.1016/j.scienta.2017.08.017
55. Tsantili, E.;
Rouskas, D.; Christopoulos, M. V.; Stanidis, V.; Akrivos, J.; Papanikolaou, D.
2007. Effects of two pre-harvest calcium treatments on physiological and
quality parameters in ‘Vogue’ cherries during storage. The Journal of
Horticultural Science and Biotechnology. 82(4): 657-663.
https://doi.org/10.1080/14620316.2007.11512287
56. Valero, D.;
Pérez-Vicente A.; Martínez-Romero D.; Castillo S.; Guillén F.; Serrano M. 2002.
Plum storabil-ity improved after calcium and heat postharvest treatments: Role
of polyamines. Journal of Food Science. 67(7): 2571-2575.
https://doi.org/10.1111/j.1365-2621.2002. tb08778.x
57. Vangdal, E.;
Hovland, K. L.; Børve, J.; Sekse, L.; Slimestad, R. 2006. Foliar application of
calcium reduces postharvest decay in sweet cherry fruit by various mechanisms.
Acta Horticulturae. 768: 143-148.
https://doi.org/10.17660/ActaHortic.2008.768.16
58. Wang, Y.; Xie,
X.; Long. L. E. 2014. The effect of postharvest calcium application in
hydro-cooling water on tissue calcium content, biochemical changes, and quality
attributes of sweet cherry fruit. Food Chemistry. 160: 22-30.
https://doi.org/10.1016/j.foodchem.2014.03.073
59. Wang, Y.; Long,
L. E. 2015a. Physiological and biochemical changes relating to postharvest
splitting of sweet cherries affected by calcium application in hydrocooling
water. Food Chemistry. 181: 241-247. https://doi.org/10.1016/j.foodchem.2015.02.100
60. Wang, Y.; Bai,
J.; Long, L. E. 2015b. Quality and physiological responses of two late-season
sweet cherry cultivars ‘Lapins’ and ‘Skeena’ to modified atmosphere packaging
(MAP) during simulated long distance ocean shipping. Postharvest Biology and
Technology. 110: 1-8. https://doi.org/10.1016/j.postharvbio.2015.07.009
61. Wani, A. A.;
Singh, P.; Gul, K.; Wani, M. H.; Langowski, H. C. 2014. Sweet cherry (Prunus
avium): Critical factors affecting the composition and shelf life. Food
Packaging Shelf. 1: 86-99. https://doi.org/10.1016/j.fpsl.2014.01.005
62. Wei, J.; Qi,
X.; Guan, J.; Zhu, X. 2011. Effect of cold storage and 1-MCP treatment on
postharvest changes of fruit quality and cell wall metabolism in sweet cherry.
Journal of Food Agriculture and Environment. 9: 118-122.
https://doi.org/10.1234/4.2011.2235
63. Winkler, A.;
Knoche, M. 2019. Calcium and the physiology of sweet cherries: A review.
Scientia Horticulturae. 245: 107-115.
https://doi.org/10.1016/j.scienta.2018.10.012
64. Winkler, A.;
Knoche, M. 2021. Calcium uptake through skins of sweet cherry fruit: effects of
different calcium salts and surfactants. Scientia Horticulturae. 276: 109761.
https://doi. org/10.1016/j.scienta.2020.109761
65. Winkler, A.;
Fiedler, B.; Knoche, M. 2020. Calcium physiology of sweet cherry fruits. Trees.
34: 1157-1167. https://doi.org/10.1007/s00468-020-01986-9
66. Zhao, H.; Wang, B.; Cui, K.; Cao, J.; Jiang, W. 2019.
Improving postharvest quality and antioxidant capacity of sweet cherry fruit by
storage at near-freezing temperature. Scientia Horticulturae. 246: 68-78.
https://doi.org/10.1016/j.scienta.2018.10.054