Revista de la Facultad de Ciencias
Agrarias. Universidad Nacional de Cuyo. En prensa. ISSN (en línea) 1853-8665.
Original article
Valorization
of regional crude glycerol. Culture media optimization for batch
docosahexaenoic acid (DHA) production with Aurantiochytrium sp.
Valorización
de glicerol crudo regional. Optimización de medios de cultivo para la
producción batch de ácido docosahexaenoico (DHA) con Aurantiochytrium sp.
Debora Laura Manuale1,
Pablo Antonio Torresi1,
Vanina Elizabet Marquez2,
Francisco Collombati3,
Alejandro José Beccaria2,
Juan Carlos Yori1
1 Universidad Nacional del Litoral (UNL). Facultad de Ingeniería
Química (FIQ). Programa de Valorización, Desarrollo y Escalado de Procesos
Agroindustriales (PROVADE). Ruta Nacional 168 Km 0 (S3000). Santa Fe.
Argentina.
2 Universidad Nacional del Litoral (UNL). Facultad de Bioquímica
y Ciencias Biológicas (FBCB). Laboratorio de Fermentaciones. Ruta Nacional 168
Km 0 (S3000). Santa Fe. Argentina.
3 Universidad Nacional del Litoral (UNL). Ruta Nacional 168 Km 0
(S3000). Santa Fe. Argentina.
* esanchez@fiq.unl.edu.ar
Abstract
Docosahexaenoic
acid (DHA) has many benefits for human health. Commercial DHA sources derive
from marine fish but present several production challenges. Aurantiochytrium
sp., an abundant marine microalga, becomes an alternative for DHA
production. Crude glycerol produced by small-scale biodiesel refineries is a
regional, available, and inexpensive waste that can be converted into
value-added compounds. This study investigated crude glycerol as a potential
carbon source for DHA-rich oil production using an aerobically isolated Aurantiochytrium
sp. in batch shake flasks. We also
optimized the culture medium formulation by varying carbon and nitrogen
sources, thereby reducing medium costs while maximizing DHA production. A
larger initial Aurantiochytrium sp. inoculum
improved cell concentration and medium carbon depletion, increasing DHA
productivity (PDHA).
Increasing culture time showed no differences in Aurantiochytrium sp. growth parameters, but reduced DHA production. The absence
of yeast extract in the culture media resulted in faster substrate metabolism
by Aurantiochytrium sp. and increased DHA
production. Crude glycerol yielded the highest PDHA (15.35 mg L-1
h-1)
at 120 h. Crude glycerol can be used as a cheaper carbon source in media
formulation with Aurantiochytrium sp. cultures
for DHA production.
Keywords: crude glycerol, Aurantiochytrium
sp., DHA, inoculum, culture medium
Resumen
El ácido
docosahexaenoico (DHA) posee muchos beneficios para la salud humana. Las
fuentes comerciales de DHA se obtienen de peces marinos, pero presenta
desventajas. Aurantiochytrium sp., una microalga marina abundante, surge
como alternativa para la producción de DHA, solucionando los problemas de
obtener DHA a partir de peces marinos. El glicerol crudo producido por pequeñas
refinerías de biodiesel es un descarte regional, disponible y barato, capaz de
ser transformado en compuestos de valor agregado. El objetivo de este trabajo
fue investigar la potencial utilización de glicerol crudo regional como fuente
de carbono para producir aceite rico en DHA, utilizando la cepa aeróbica
aislada Aurantiochytrium sp. en matraces batch
agitados; y optimizar la formulación de medios de cultivo, variando las fuentes
de carbono y nitrógeno, para reducir costos y maximizar la producción de DHA.
Los tamaños de inóculo iniciales más grandes de Aurantiochytrium sp
mejoran la concentración celular y agotan la fuente de carbono, mejorando la
productividad a DHA (PDHA).
El aumento del tiempo de cultivo no mostró diferencias en los parámetros de
crecimiento de Aurantiochytrium sp., pero disminuyó la producción de
DHA. La ausencia de extracto de levadura en los medios de cultivo produjo una
metabolización más rápida del sustrato por Aurantiochytrium sp.,
mejorando la producción de DHA. Con glicerol crudo se alcanzó la mejor PDHA
(15,35 mg L-1 h-1)
a 120 h. El glicerol crudo se puede utilizar como fuente de carbono barata en
la formulación de medios en cultivos con Aurantiochytrium sp. para producir DHA.
Palabras clave: glicerol crudo, Aurantiochytrium
sp., DHA, inóculo, medio de cultivo
Originales: Recepción: 10/04/2024
- Aceptación: 05/04/2025
Introduction
Polyunsaturated
fatty acids (PUFA), particularly omega-3 (ω-3), play an important role in
physiological functions (13). Docosahexaenoic
acid (DHA, C22:6 ω-3) and eicosapentaenoic acid (EPA, C20:5 ω-3) have
vasodilatory and anti-inflammatory capacity, prevent atherosclerosis and
hypertension, reduce risk factors for thrombosis, arthritis, and Alzheimer’s
disease, and increase development of the central nervous system and retinal
tissue, improving visual acuity and cognitive capacity in children (5,
33). DHA is commonly presented in pharmaceuticals (nutraceutical
and functional products), medicine (Alzheimer’s and cerebrovascular drugs), and
food (soft drinks, dairy, and infant products) (12,
33).
Commercial sources
of ω-3 DHA are obtained from marine fish and shellfish using a well-known
technology that produces 600,000 tonnes annually (21). However, the
process faces overfishing, strong seasonal dependence, low DHA yield (< 50%
w/w), high levels of marine pollutants (dioxins, methylmercury, polychlorinated
biphenyls, metals), fish odor, and low DHA stability (17). Given these
problems and the constant demand for high-quality ω-3 DHA, new strategies like
microalgae cultures become promising alternatives (6).
Microalgae cultures
for DHA production offer high purity and good organoleptic properties, use
renewable waste, are toxins-free, and have low fermentation costs (23). Heterotrophic
strains of the Thraustochytriaceae family (Aurantiochytrium, Schizochytrium,
and Thraustochytrium) have significant growth rates compared to
photoautotrophic microalgal cultivation, producing more than 50% of their dry
cell weight as lipids, with ω-3 DHA reaching 50% of total fatty acids in some
species (8, 20). Aurantiochytrium sp., an
aerobic thraustochytrid abundant in marine environments, has been an
alternative source of ω-3 DHA. This microalga can grow readily on various
carbon sources and has high PDHA,
replacing traditional marine fish oil production (20,
37).
Efforts regarding Aurantiochytrium
sp. have focused on optimizing culture conditions
and media (C, N, and micronutrients) to increase DHA production, mainly because
costs highly depend on C sources (26, 28). Carbon
concentration affects the synthesis of organic molecules and energy
availability, while nitrogen concentration affects amino acids and nucleic acid
synthesis (10). A traditional C
source for fermentative DHA synthesis is glucose, but in microalgal
heterotrophic fermentation, glucose represents almost 80% of media cost (24,
37). The use of regional, highly available, and low-cost medium
components with high C and/or N content instead of glucose economizes
fermentation processes and provide energy for cell maintenance and biosynthesis
(32).
In central
Argentina, many companies produce large quantities of agro-industrial waste.
The biodiesel industry produces crude glycerol (10% w/w) as a by-product. World
biodiesel production will reach 40 million tonnes in 2025, producing 6.3
million tonnes of crude glycerol, and the market is estimated to grow to 3,670
million USD by 2030 (1, 16, 34). In biodiesel
refineries, small-scale purification is not economically viable and the
by-product is sold at low cost, depending on its quality level. This calls for
a sustainable and economic process for converting crude glycerol into value-added
compounds. Thus, crude glycerol could constitute a cheaper carbon source in
microalgae cultures for ω-3 DHA production.
This study investigated two main points: i) the use of crude
glycerol, without any purification treatment, as substrate for ω-3 DHA-rich oil
production using a locally isolated oleaginous Aurantiochytrium sp. strain in batch cultures; and ii) the optimization of
culture medium formulation, varying carbon and nitrogen sources, reducing costs
and increasing ω-3 DHA-rich oil yield (PDHA).
Materials
and methods
Materials
Crude glycerol (CG)
was obtained as biodiesel by-product from Bolzán (Argentina). Pure anhydrous
glycerol (AG), monohydrated glucose (MG) and methanol, ethanol, and isopropanol
for extractive techniques were purchased from Cicarelli (Argentina). Peptone and
yeast extract were purchased from Britania (Argentina). Salts for media
preparation, KOH and n-hexane for fatty acid extraction, and Na2SO4 for total fatty acid
determination were purchased from Research AG (Argentina). Fatty acid methyl
ester (FAME) analytical standards were obtained from Merck (Argentina).
Isolation
and identification of microorganisms
Aurantiochytrium sp. was obtained from seawater samples from the Argentinean
coast and isolated in our laboratory. Aurantiochytrium sp. was isolated using the streak plate technique in Petri
dishes (2). The culture medium was a synthetic
AS100 (35) modified with 0.2 M NaCl (31), and supplemented
with peptone (10 g L-1),
yeast extract (10 g L-1),
and monohydrated pure glucose (35 g L-1),
designed as PMG* medium. After adjusting pH to 7.00 and adding 1% agar, the
medium was autoclaved at 115°C for 20 min. Cool but still liquid PMG* agar
medium was distributed in sterile Petri dishes. Approximately 0.1 mL of the
sample to be isolated was placed in these Petri dishes and spread with a
sterilized bacteriological loop. Petri dishes were incubated at 28 ± 2°C for 3
to 4 days. Isolated colonies were then selected and transferred to another
Petri dish until pure microalgae were obtained. Tubes containing 5 mL of liquid
PMG* medium were prepared and autoclaved at 115°C for 20 min. Isolated
microalgae were incubated at 28 ± 2°C for 2 to 3 days obtaining a
higher-density culture for working stocks preparation. At all isolation stages,
possible contamination with other microorganisms was detected by observing the
microalgae under a 40X light microscope. The strain was stored in 25% (v/v)
glycerol at -80 °C and cell viability was checked monthly.
Genomic DNA extraction was performed using the Easy Pure Plant
Genomic DNA Kit (Transgene Biotech, China) identifying the microalgae at a
molecular level. Polymerase chain reaction (PCR) was performed using a
universal primer set (ITS Fo: 5’ TCCGTAGGTGAACCTGCGG 3’, ITS Rev: 5’
TCCTCCGCTTATTGATATGC 3’) to amplify the internal transcriber spacer (ITS)
region at the ribosomal locus, including the 5.8S rRNA gene. The resulting PCR
product was sequenced and the nucleotide sequence was analyzed with NCBI Basic
Local Alignment Search Tool (BLAST). The sequence analysis showed 100% identity
with the 5.8S rRNA gene of Aurantiochytrium sp. strain
CCAP_4062/3 (MF766428.1). It also had a high percentage of identity (~98%) with
Aurantiochytrium limacinum IMB188 (KP899823.1). Considering these
results, the isolated organism would belong to the genus Aurantiochytrium, closely
related to A. limacinum.
Batch
shake flask cultures
Aliquots of 1.5 mL of cells preserved in 25% (v/v) glycerol were
used to inoculate 125 mL shake flasks containing 15 mL PMG* medium and placed
on a heated orbital shaker at 150 rpm and 28 ± 2°C for 48 h. Four fermentation
media were used for screening assays in batch shake flasks. Table 1 shows substrate
carbon composition for media preparation, comparing useful carbon used per L of
culture medium.
Table 1. Substrate
carbon composition used in modified artificial seawater medium for cultures of Aurantiochytrium
sp.
Tabla
1. Composición de carbón de los
sustratos utilizados en el medio agua de mar artificial modificado para
cultivos de Aurantiochytrium sp.
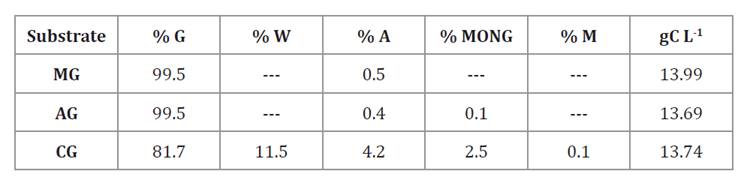
% G: % glucose or glycerol; % W:
% water; % A: % ash; % MONG: % matter organic non-glycerol; % M: % methanol; gC
L-1:
g of useful carbon per L of culture medium.
% G: % glucosa o glicerol; % W: %
agua; % A: % cenizas; % MONG: % materia orgánica no glicerol; % M: % metanol;
gC L-1:
g de carbono útil por L de medio de cultivo.
We prepared PMG* medium and three other media identically
formulated to PMG* but without yeast extract. The first was supplemented with
35 g L-1 of pure monohydrate
glucose (PMG medium), the second replaced glucose with 35 g L-1 pure anhydrous glycerol
(PAG medium), and the third replaced glucose with 42.5 g L-1 biorefinery crude
glycerol (BCG medium) achieving identical amounts of available carbon in each
medium. Media pH was adjusted to 7.00 before autoclaving at 115°C for 20 min.
Ten mL of 10% (v/v) inoculum medium were transferred to 250 mL shake flasks
containing 90 mL medium and placed in a heated orbital shaker at 150 rpm and 28
± 2°C for 120 and 240 h assays.
Cell
disruption and extraction of algal lipids
Once cultures
finished, a 40 mL aliquot was transferred to a glass beaker with 10 mL of
distilled water. Cells were homogenized and lysed using an ultrasonic cell
disruptor for 1.2 kW and 20 kHz (Bald Design, Argentina) at 25°C for 30 s.
Then, 50 mL of pure n-hexane was added to the lysed cells, maintaining a
5:1 (v/v) ratio with respect to distilled water. The mixture was vigorously
shaken with a magnetic stirrer for 10 min. Finally, the organic phase was
transferred to a 250 mL balloon and total lipids were extracted by vacuum
distillation at 55°C.
Analytical
procedures
Biomass,
glucose and glycerol determinations
Culture samples (1
mL) were periodically taken and centrifuged at 9000 g and 25°C for 10 min. The
supernatant was stored at -20°C. Biomass concentration was determined by cell
counting using a Neubauer chamber (Boeco, Germany). Glucose and glycerol concentrations
were identified with Glycemia and TG colorimetric kits (Wiener Lab, Argentina),
in a UV spectrophotometer at 505 nm.
Dry cell
weight and total fatty acids
Culture samples (50
mL) were centrifuged at 3000 g and 25°C for 15 min. The supernatant was
discarded and the biomass was washed twice with distilled water. Biomass was
transferred to Petri dishes with filter paper (previously tared), oven-dried at
70°C for 12 h, and weighed until constant weight, obtaining dry cell mass. The
filter paper (with dry biomass) was then removed from the Petri dishes and
placed in a 50 mL Falcon tube for saponifiable lipid determination. The Falcon
tube was filled with 5 mL of 30% w/v KOH and 5 mL of 96% v/v ethanol, and
incubated in a thermostatic bath at 70°C for 16 h. The biomass-KOH-ethanol
mixture was cooled to room temperature. Then 10 mL of n-hexane was
added, the tube was vortexed for 1 min and the contents were centrifuged at
6000 rpm for 5 min, discarding the organic phase. This step was done twice. The
washed aqueous phase was incubated in an ice bath. Concentrated HCl was added
until a pH of 1. Next, 10 mL of n-hexane was added, the tube was stirred
vigorously for 1 min and the contents were centrifuged at 6000 rpm for 5 min to
recover the organic phase. This step was also done twice. The two organic
fractions (10 mL each) were transferred to a glass beaker (previously tared),
oven-dried at 80 °C for 12 h, and weighed to constant weight. Saponifiable
lipids obtained were compared with a calibration curve using crescent
concentrations of standard fatty acid vs. the
saponifiable fraction obtained by the described technique, obtaining total
fatty acid (TFA) mass for each culture.
Fatty acids
composition
Total lipids
extraction employed a mixture of n-hexane:isopropanol
(3:2) and 6% w/v Na2SO4
at room temperature (36). Extracted lipids
were dried under a N2 stream at 40°C. FAME was
prepared following a cold method using n-hexane and KOH 2N in methanol (3). The obtained FAME
fraction was quantified with a gas chromatograph GC-2014 (Shimadzu) equipped
with a capillary column CP-Sil 88, 100 m x 0.25 mm ID (Varian) and a flame
ionization detector (FID), using operating conditions as previously described (22).
Fatty acids were
identified by comparing retention times of each peak and quantified from peak
areas obtained from chromatograms with a FAME standard, using nonadecanoic acid
methyl ester (19:0 ME) as internal standard (4,
11, 22).
DHA quality
The nutritional
quality and sensory properties of DHA-rich oil are highly dependent on the
efficiency of the extraction method used (29). Peroxide value
(PV, AOCS Official Method Cd 8b-90), representing the amount of peroxide (meq
of active oxygen per kg of lipids) in a sample was determined as follows.
Hydroperoxides (dissolved in acetic acid and chloroform) reacted with iodide
ions from KI to form iodine, and PV was determined by titration of the
liberated iodine with a known concentration of Na2S2O3 solution, using starch
as an indicator (27). Acid value (AV,
AOCS Official Method Da 14-48), i.e. free fatty acids (% oleic acid) in a
sample was determined by titration technique determining the mg of KOH (0.1 N,
in ethanolic solution) required to neutralize free FA per g of sample,
dissolved in a mixture of ethyl ether:ethanol,
employing phenolphthalein as an indicator (14). Elemental
analysis for metal identification (Pb, Cd, Hg, As, Ni, Cu, Fe, Cr, and Co) was
performed using an Optima 2100 DV inductively coupled plasma optical emission
spectrometer (ICP-OES) (Perkin Elmer) with a CCD detector. Limit values were
extracted from the results published by the European Union report (9).
Kinetic
and stoichiometric parameters
Specific growth
rate (μ, h-1),
substrate consumption rate (SR,
mg L-1 h-1),
DHA productivity (PDHA,
mg L-1 h-1)
and yield (YX/S,
g g-1) were
calculated from experimental culture data obtained from dry cell weight (DCW, g
L-1), total
fatty acid concentration (CTFA,
g L-1), ω-3 PUFA
concentration (CPUFA,
g L-1) and DHA
concentration (CDHA,
g L-1).
Results
and discussion
Influence
of inoculum size on culture parameters
To understand the influence of the initial inoculum size added
to the media on main culture parameters, tests were developed in 250 mL batch
shake flasks with 100 mL PMG* medium at 150 rpm and 28 ± 2°C for 120 h. Figure 1, shows the initial
inoculum concentration of Aurantiochytrium sp. in
relation to final cell concentration (fDCW),
SR, and PDHA.

Dashed lines show
linear adjustment for each parameter. Tests were conducted in triplicate.
Las
líneas discontinuas muestran el ajuste lineal para cada parámetro. Los ensayos
fueron realizados por triplicado.
Figure
1. Final dry cell concentration (fDCW), SR, and PDHA vs. initial dry cell concentration (iDCW) for Aurantiochytrium
sp. in 250 mL batch shake flasks with 100 mL PMG*
medium, tested at 150 rpm and 28 ± 2 °C for 120 h.
Figura
1. Concentración celular final en base
seca (fDCW), SR y
PDHA vs.
Concentración celular inicial en base seca (iDCW) para Aurantiochytrium sp.
en matraces batch agitados de 250 mL con 100 mL de
medio PMG*, testeados a 150 rpm y 28 ± 2 °C durante 120 h.
Increasing the initial inoculum size lowered final Aurantiochytrium
sp. concentration only for the first inoculum size
(0.5% v/v), but did not significantly increase the last three inoculum sizes
(2.5, 5.0 and 10.0% v/v), which showed similar DCW levels. As Aurantiochytrium
sp. metabolism produces a maximum cell
concentration after 5 culture days, this was not much affected when the
inoculum size added was between 2.5 and 10.0% v/v of fresh culture medium.
Larger inoculum sizes could be tested but increasing inoculum sizes would not
be economically viable in a scale-up process. As expected, SR slightly increased with
increasing inoculum size given a greater number of Aurantiochytrium sp. cells growing in the same volume of initial culture medium.
Only the two largest initial inoculum sizes (5.0 and 10.0% v/v) practically
exhausted the medium substrate. The PDHA markedly
increased with increasing inoculum size, due to higher SR obtained by a greater
amount of Aurantiochytrium sp. cells available
in culture. PDHA was 2.85 and 6.84 mg L-1
h-1 at inoculum size of 0.5
and 10.0% v/v fresh culture medium, respectively.
Previous results
with A. limacinum SR21 demonstrate that inoculum preparation is
fundamental for DHA production, with inoculum size having a significant effect
on biomass production. High lipid accumulation was obtained with 10.0% v/v inoculum
size, with higher PDHA and DHA production (11 g
L-1) in a
shorter time (30). In addition, the
greatest increase occurred when inoculum size changed from 0.5 to 2.5% v/v of
fresh culture medium, obtaining 60% more PDHA.
When inoculum size was doubled, from 2.5 to 5.0% v/v, and then from 5.0 to
10.0% v/v, 25% increase in PDHA was
achieved in both cases. Increasing initial inoculum size to 10.0% v/v had a
direct effect on PDHA and SR,
as a greater number of Aurantiochytrium sp. cells
metabolize high amounts of substrate at the same medium volume and culture
time.
Influence
of yeast extract and test time on culture parameters
The development of Aurantiochytrium sp. cultures in 250 mL batch shake flasks at two final culture
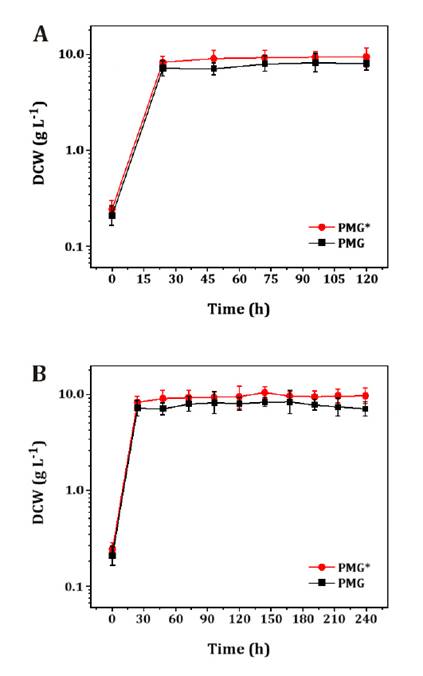
times was analyzed using PMG* and PMG media. Figure 2, shows cell
concentration curves at 120 h (figure 2a) were slightly higher for PMG* than for PMG medium, but showed
similar behavior. An increase in culture time until 240 h (figure 2b) displayed the
same behavior as for 120 h, with no major difference in DCW, and remaining
constant during the stationary phase.
Tests were
conducted in duplicate.
Los
ensayos fueron realizados por duplicado.
Figure
2. Cell concentration curves of Aurantiochytrium sp.
cultures in 250 mL batch shake flasks with 100 mL PMG*
and PMG, tested at 150 rpm and 28 ± 2 °C for: a) 120 h and b) 240 h culture
times.
Figura
2. Curvas de concentración celular de
cultivos Aurantiochytrium sp. en matraces batch
agitados de 250 mL con 100 mL de medios PMG* y PMG, ensayados a 150 rpm y 28 ±
2 °C durante: a) 120 h y b) 240 h de cultivo.
Table 2, exhibits the main
parameters obtained during fermentation of Aurantiochytrium sp. with 100 mL medium in batch shake flasks at 120 to 240 h
culture times.
Table 2. Culture
parameters obtained during Aurantiochytrium sp. tests
in 250 mL batch shake flasks with 100 mL PMG* and PMG, tested at 150 rpm and 28
± 2 °C for two culture times.
Tabla
2. Parámetros de cultivo obtenidos
durante los ensayos con Aurantiochytrium sp. en
matraces batch agitados de 250 mL con 100 mL de medios PMG* y PMG, testeados a
150 rpm y 28 ± 2 °C a dos tiempos de cultivo.
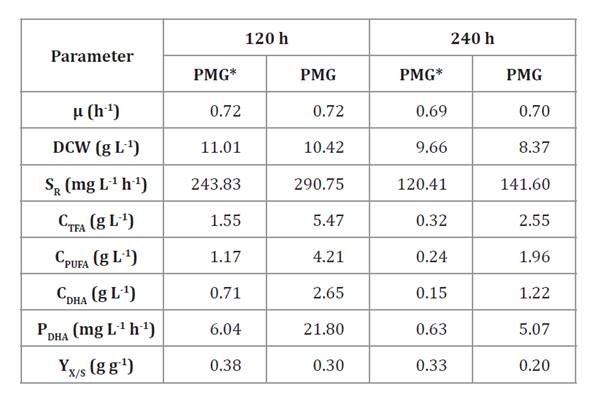
The μ parameter was practically similar for all tests, but DCW
decreased by 12.3 and 18.1% in PMG* and PMG media respectively, between 120 and
240 h culture time. Cultures with yeast extract did not deplete the supplied glucose,
consuming 82.5 and 83.5% of added glucose at 120 and 240 h, respectively. PMG
cultures also showed higher SR than PMG* cultures. With
PMG medium, consumed glucose reached 98.1% of the total added substrate at 120
h, and was practically consumed (99.7%) at 240 h. A preliminary screening
revealed that adding 3.0% w/v glucose concentration from 2 to 10% w/v showed
optimal culture parameters for Aurantiochytrium sp. cultures
(18). In our tests,
adding 35 g L-1 glucose (3.5% w/v) as
initial substrate was appropriate.
Eliminating yeast
extract (an expensive ingredient) in culture media causes Aurantiochytrium sp.
to metabolize glucose 1.2 times faster. These results
agree with a previous study where optimal culture conditions were achieved only
with peptone as N source in culture media, and cell concentration and DHA yield
were significantly increased (7). Another work
replaced yeast extract with discarded orange peel extract and nitrates (NaNO3, NH4Cl,
urea) for a more economical culture formulation in microalgae DHA production (25). Finally, when
culture time was increased to 240 h, SR was reduced by half for
both evaluated cultures. Interestingly, absent yeast extract increased CDHA
by 3.6 times at 120 h and 8.1 times at 240 h. However, at longer
culture time, CPUFA and CDHA were less than half than
at 120 h for both cultures tested.
Decreased PDHA
was also observed when culture time increased from 120 to 240 h,
resulting in 6.04 and 0.63 mg L-1 h-1 for PMG* medium, and
21.80 and 5.07 mg L-1 h-1 for PMG medium. This
suggests that increasing culture time to 240 h was not appropriate, in
agreement with previous results (7). Although DCW
remains at the same level in all cultures, Aurantiochytrium sp. metabolism changes by decreasing lipid synthesis while some
reserve substances are used for cell maintenance. Therefore, no yeast extract
was added to media for the next tests.
Influence
of glycerol quality and assay time on culture parameters
Pure and crude glycerol was used as a carbon source in Aurantiochytrium
sp. cultures to evaluate the differences between
glycerol qualities, analyzing crude glycerol as an economical and accessible
substrate from regional small-scale biodiesel refineries. Figure 3, shows cell
concentration curves of Aurantiochytrium sp. cultures
at 120 and 240 h in 250 mL batch shake flasks with 100 mL PAG and BCG media,
without yeast extract addition. The best performance was observed with BCG
medium compared to PAG medium at 120 h (figure 3a), becoming more evident
at 240 h (figure
3b).

Tests were
conducted in duplicate.
Los
ensayos fueron realizados por duplicado.
Figure
3. Cell concentration curves of Aurantiochytrium sp.
cultures in 250 mL batch shake flasks with 100 mL PAG
and BCG media, tested at 150 rpm and 28 ± 2 °C for: a) 120 h and b) 240 h
culture times.
Figura
3. Curvas de concentración celular de
cultivos Aurantiochytrium sp. en matraces batch
agitados de 250 mL con 100 mL de medios PAG y BCG, testeados a 150 rpm y 28 ± 2
°C durante: a) 120 h y b) 240 h de cultivo.
Cell concentration curves obtained with glycerol cultures and
PMG medium showed similar behaviors but were slightly higher with PMG. Table 3 shows the main
parameters obtained in Aurantiochytrium sp. cultures
at 120 and 240 h with PAG and BCG media in batch shake flasks.
Table 3. Culture
parameters obtained during Aurantiochytrium sp. tests
in 250 mL batch shake flasks with 100 mL PAG and BCG, tested at 150 rpm and 28
± 2 °C for two culture times.
Tabla
3. Parámetros de cultivo obtenidos
durante los ensayos con Aurantiochytrium sp. en
matraces batch agitados de 250 mL con 100 mL de medios PAG y BCG, testeados a
150 rpm y 28 ± 2 °C a dos tiempos de cultivo.
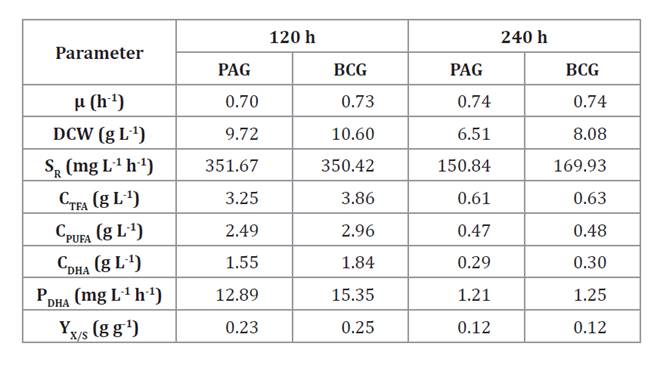
The μ parameters are practically similar for all tests, with
values close to those reported with PMG medium, demonstrating that glycerol was
an adequate substrate for Aurantiochytrium sp. Concerning DCW, BCG
showed the best performance at both culture times, and lower values were
observed at 240 h culture, with a decrease of 10 and 24% for PAG and BCG,
respectively.
PAG and BCG cultures
have a high SR but decreased by 43% for
PAG and 54% for BCG at 240 h. The SR values in cultures with
glycerol are higher than with glucose. Adequate CTFA and CPUFA were obtained at 120 h
but strongly decreased when the culture was extended to 240 h. Higher CDHA
(1.84 g L-1)
was reached with BCG medium at 120 h, whereas with PAG CDHA was 1.55 g L-1, a value 16%
lower than with BCG medium. The best PDHA (15.35 mg L-1
h-1)
was produced with BCG medium at 120 h, slightly lower than the value obtained
with PMG (21.80 mg L-1 h-1)
at 120 h. The use of glycerol or monosaccharides (fructose, glucose, mannose)
in culture media resulted in higher concentrations of DCW and DHA than with
disaccharides (maltose, lactose, sucrose) and polysaccharides (starch) (7). At 240 h, CDHA
decreased between 5 and 6 times with respect to 120 h. This
result suggests that longer culture times cause Aurantiochytrium sp. to consume lipid reserves given lower carbon availability in
batch culture. Lipids are accumulated as carbon and energy sources in the first
culture stage, maybe after the faster cell growth (i.e., culture time
higher than 15 h). Some authors reported that glucose promotes rapid cell
growth and lipid synthesis in early fermentation stages and glycerol produces
late DHA accumulation with A. limacinum SR21 (15). Times over 240 h
were not tested, as no clear improvement in culture parameters could be
demonstrated with Aurantiochytrium sp.
These results are
key since no prior (and more expensive) purification step, and no conditioning
treatment was necessary before using crude glycerol as substrate in culture
media. The good performance reached with BCG medium was explained by glucose
being a C-6 compound, and glycerol a C-3 compound, easier to incorporate and
assimilate by Aurantiochytrium sp. Increasing CTFA and CDHA induced by glycerol in Aurantiochytrium
sp. cultures could be explained by the
up-regulation of two important metabolites, oxalic acid (intermediate in citric
acid cycle) and myo-inositol (growth promoter) (18). The cumulative
effect of these metabolites produces an up-regulation of the citric acid cycle
directly affecting cell metabolism, together with the pentose phosphate
pathway, which generates reducing power, causing dynamic changes at a molecular
level in enzymatic activities (18, 19).
Lipid
composition and quality
Fatty acid production by Aurantiochytrium sp. cultures was analyzed with different substrates in a
modified artificial seawater medium, prepared from a mixture of salts and
nutrients in water. Figure
4
shows these strategies concerning total fatty acid production expressed as DHA,
DPA (docosapentaenoic acid), EPA, and other fatty acid concentrations.

Numbers on bars
represent PDHA (mg
L-1 h-1)
for each culture strategy. Assays were run in duplicate.
Los
números sobre las barras representan la PDHA (mg L-1 h-1) para cada estrategia de cultivo. Los
ensayos fueron realizados por duplicado.
Figure
4. CTFA, as concentration of DHA, DPA, EPA, and
other fatty acids, obtained in Aurantiochytrium sp. cultures
in 250 mL batch shake flasks with 100 mL media, tested at 150 rpm and 28 ± 2 °C
for 120 h culture time.
Figura
4. Concentración de ácidos grasos
totales (CTFA),
expresada como concentración de DHA, DPA, EPA y otros ácidos grasos, obtenidos
en cultivos Aurantiochytrium sp. en matraces
batch agitados de 250 mL con 100 mL de medio de cultivo, testeados a 150 rpm y
28 ± 2 °C durante 120 h de cultivo.
The most efficient culture for CDHA was PMG medium, with a
maximum CDHA of 2.65 g L-1
and CTFA of 5.47 g L-1
(table
2).
CDHA increased 3.7 times in
PMG compared to PMG* medium, while PDHA is about 3.5 times
higher, indicating that absent yeast extract greatly favors cellular machinery
for lipid production, allowing CTFA to increase. It should be
noted that the DPA concentration (healthy ω-3 PUFA) also increases in the PMG
culture and represents almost half of the total amount of DHA obtained. The
highest CDHA for glycerol cultures was
obtained with BCG, reaching 1.84 g L-1,
and CTFA of 3.86 g L-1. In this medium,
DPA concentration was more than half of the total amount of DHA obtained, and
the PDHA reached a maximum of
15.35 g L-1 h-1,
superior to PAG medium. In all tests, EPA concentration (another healthy ω-3
PUFA) is negligible, reaching a maximum value of 0.10 g L-1 with PMG medium.
The obtained
DHA-rich oil constitutes a safe product for human and animal food formulation.
However, it requires certain quality parameters to be a stable product. After
extraction from the obtained TFA, DHA quality parameters were obtained by
titrimetric assays and metal identification. For PV and AV assays, DHA products
comply with the limit (< 1.20 meq O2 kg-1,
and < 0.10 meq KOH g-1,
respectively) set by the European Union standards for algal oils (9). Since the main
difference between ω-3 DHA obtained from microalgae and marine fish was the
presence of heavy metals, ICP-OES analysis of DHA products obtained from Aurantiochytrium
sp. cultures showed that metal contents (Pb <
0.001, Cd < 0.020, Hg < 0.015, As < 0.030, Ni < 0.135, Cu <
0.090, Fe < 0.505, Cr < 0.060, Co < 0.135, expressed in ppm) were
below the maximum levels established by the European Union legislation (9). This ω-3 DHA-rich
oil obtained from microalgae was safer and had greater competitiveness and
commercial advantage than marine fish DHA.
Our results
demonstrated that crude glycerol is an adequate, cheap, and available substrate
resource for producing ω-3 DHA-rich oil employing the microalgae Aurantiochytrium
sp. in batch cultures. Applying simple culture
strategies allowed increasing CTFA,
CPUFA, and CDHA production in batch shake
flasks, reducing culture costs by eliminating useless components and employing
a cheaper and widely available regional waste, presenting identical substrate
consumption and product yield rates as those using purified sugars. Our next
challenge is to develop future assays for ω-3 DHA-rich oil production in
bioreactor systems at 10X or 100X scale factor. These experiences will allow
the implementation an integrated process also able to evaluate other valuable
wastes from regional agro-industries minimizing effluent production in a
circular economy system.
Conclusions
Crude glycerol has great potential as substrate for ω-3 DHA-rich
oil production using a locally isolated strain of Aurantiochytrium sp. in batch cultures. The largest initial inoculum (10.0% v/v)
of Aurantiochytrium sp. improved DCW,
practically exhausted glucose substrate, and enhanced PDHA.
Absent yeast extract in culture media enabled faster glucose metabolism in Aurantiochytrium
sp. favoring lipid production and improving CTFA
and CDHA.
Longer culture times showed no differences in μ and DCW but decreased SR
and CDHA due to Aurantiochytrium
sp. consumed lipid reserves for cell maintenance.
Better DCW performance was observed with BCG medium, with μ values similar to
those reported with PMG medium. For glycerol cultures, the best CDHA
and PDHA (1.84 g L-1 and 15.35 mg L-1
h-1,
respectively) were reached with BCG at 120 h, and SR was higher than in
glucose cultures. Quality assays showed ω-3 DHA-rich oil product is safer for
human and animal food formulation. This study confirms that crude glycerol,
without prior and expensive purification steps or conditioning treatment,
constitutes a cheap and highly available carbon source for media formulation in
Aurantiochytrium sp. cultures for ω-3 DHA-rich
oil production. The next experiences for ω-3 DHA-rich oil production will focus
on evaluating other wastes from regional agro-industries and developing
scale-up assays in bioreactor systems.
Acknowledgements
The authors thank CONICET (Consejo Nacional de Investigaciones
Científicas y Técnicas) and FIQ-UNL (Facultad Ingeniería Química, Universidad
Nacional del Litoral) for financial support.
1. Attarbachi, T.;
Kingsley, M. D.; Spallina, V. 2023. New trends on crude glycerol purification:
A review. Fuel 340: 127485. DOI: 10.1016/j.fuel.2023.127485
2. Band Schmidt, C.
J. 2007. Aislamiento, purificación y mantenimiento de cepas de microalgas. In:
Arredondo-Vega, B. O.; Voltolina, D. (Eds.), Métodos y herramientas analíticas
en la evaluación de la biomasa microalgal. La Paz (Mexico), Centro de Investigaciones
Biológicas del Noroeste. p. 1-11.
3. Bannon, C. D.;
Breen, G. J.; Craske, J. D.; Hai, N. G.; Harper, N. L.; O’Rourke, K. L. 1982.
Analysis of fatty acid methyl esters with high accuracy and reliability. III.
Literature review of and investigations into the development of rapid
procedures for the methoxide-catalysed methanolysis of fats and oils, J.
Chromatogr. 247(1): 71-89. DOI: 10.1016/S0021- 9673(00)84857-8
4.
Bautista-Martínez, Y.; Granados-Rivera, L. D.; Jimenez-Ocampo, R.;
Maldonado-Jáquez, J. A. 2024. Morphostructural composition and meat quality in
local goat kids from the northeastern region of Mexico. Revista de la Facultad
de Ciencias Agrarias. Universidad Nacional de Cuyo. Mendoza. Argentina. 56(1):
127-137. DOI: https://doi.org/10.48162/rev.39.129
5. Campoy, C.;
Escolano-Margarit, M.; Anjos, T.; Szajewska, H.; Uauy, R. 2012. Omega-3 fatty
acids on child growth, visual acuity neurodevelopment. Br. J. Nutr. 107:
S85-106. DOI: 10.1017/ S0007114512001493
6. Chauton, M. S.;
Reitan, K. I.; Norsker, N. H.; Tveteras, R.; Kleivdal, H. T. 2015. A
techno-economic analysis of industrial production of marine microalgae as a
source of EPA and DHA-rich raw material for aquafeed: research challenges and
possibilities. Aquaculture. 436: 95-103. DOI: 10.1016/j.aquaculture.2014.10.038
7. Chen, X.; Sen,
B.; Zhang, S.; Bai, M.; He, Y.; Wang, G. 2021. Chemical and physical culture
conditions significantly influence the cell mass and docosahexaenoic acid
content of Aurantiochytrium limacinum strain PKU#SW8. Mar. Drugs.
19(12): 671. DOI: 10.3390/ md19120671
8. Du, F.; Wang, Y.
Z.; Xu, Y. S.; Shi, T. Q.; Liu, W. Z.; Sun, X. M.; Huang, H. 2021.
Biotechnological production of lipid from thraustochytrids. Biotechnol. Adv.
48(8): 107725. DOI: 10.1016/j. biotechadv.2021.107725
9. EFSA Panel on
Nutrition. Novel Foods and Food Allergens. 2020. Safety of Schizochytrium
sp. oil as a novel food pursuant to regulation EU
2015/2283. EFSA J 18(10): e06242. DOI: 10.2903/j. efsa.2020.6242
10. Furlan, V.; Maus, V.; Batista, I.; Bandarra, N. 2017.
Production of docosahexaenoic acid by Aurantiochytrium sp. ATCC PRA-276.
Braz. J. Microbiol. 48(2): 359-365. DOI: 10.1016/j. bjm.2017.01.001
11.
González-Martinez, A.; Angón, E.; González, M. A.; Rodríguez Tobar, J. M.;
Barba Capote, C.; García Martínez, A. R. 2021. Effect of rearing system and sex
on the composition and fatty acid profile of Andinoacara rivulatus meat
from Ecuador. Revista de la Facultad de Ciencias Agrarias. Universidad Nacional
de Cuyo. Mendoza. Argentina. 53(2): 232-242. DOI:
https://doi.org/10.48162/rev.39.056
12. Hermida, L. G.;
Gallardo, G. 2015. Food applications of microencapsulated omega-3 oils. In:
Sagis L.M.C. (Ed.), Microencapsulation and microspheres for food applications.
Academic Press. p. 271-299. DOI: 10.1016/B978-0-12-800350-3.00018-2
13. Janssen, C. I.;
Kiliaan, A. J. 2014. Long-chain polyunsaturated fatty acids (LC-PUFA) from
genesis to senescence: the influence of LC-PUFA on neural development, aging,
and neurodegeneration, Prog. Lipid Res. 53: 1-17. DOI:
10.1016/j.plipres.2013.10.002
14. Kamal Eldin, A.
2010. Methods to determine the extent of lipid oxidation in foods. In: Decker,
E.; Elias, R.; McClements, D. (Eds.). Oxidation in foods and beverages and
antioxidant applications. Understanding mechanisms of oxidation and antioxidant
activity. Sawston (UK), Woodhead Publishing. p. 181-195.
15. Li, J.; Liu,
R.; Chang, G.; Li, X.; Chang, M.; Liu, Y.; Jin, Q.; Wang, X. 2015. A strategy
for the highly efficient production of docosahexaenoic acid by Aurantiochytrium
limacinum SR21 using glucose and glycerol as the mixed carbon sources.
Biores. Technol. 177: 51-57. DOI: 10.1016/j.biortech.2014.11.046
16. Liu, Y.; Zhong,
B.; Lawal, A. 2022. Recovery and utilization of crude glycerol, a biodiesel
byproduct. RSC Adv. 12: 27997-28008. DOI: 10.1039/D2RA05090K
17. Lu, Q.; Li, H.;
Xiao, Y.; Liu, H. 2021. A state-of-the-art review on the synthetic mechanisms,
production technologies, and practical application of polyunsaturated fatty
acids from microalgae. Algal Res. 55: 102281. DOI: 10.1016/j.algal.2021.102281
18. Mariam, I.;
Kareya, M. S.; Nesamma, A. A.; Jutur, P. P. 2021. Delineating metabolomic
changes in native isolate Aurantiochytrium for production of
docosahexaenoic acid in presence of varying carbon substrates. Algal Res. 55:
102285. DOI: 10.1016/j.algal.2021.102285
19. Martínez-Reyes,
I.; Chandel, N. S. 2020. Mitochondrial TCA cycle metabolites control physiology
and disease. Nat. Commun. 11(1): 102. DOI: 10.1038/s41467-019-13668-3
20. Martins, D.;
Custódio, L.; Barreira, L.; Pereira, H.; Ben-Hamadou, R.; Varela, J.;
Abu-Salah, K. 2013. Alternative sources of n-3 long-chain polyunsaturated fatty
acids in marine microalgae. Marine Drugs. 11(7): 2259-2281. DOI:
10.3390/md11072259
21. Nazir, Y.;
Halima, H.; Al-Shorgani, N. K. N.; Manikan, V.; Hamid, A. A.; Song, Y. 2020.
Efficient conversion of extracts from low-cost, rejected fruits for high-valued
docosahexaenoic acid production by Aurantiochytrium sp. SW1. Algal Res.
50(5528): 101977. DOI: 10.1016/j. algal.2020.101977
22. Negro, E.;
González, M. A.; Bernal, C. A.; Williner, M. R. 2016. Saturated and trans fatty
acids content in unpackaged traditional bakery products in Santa Fe city,
Argentina: nutrition labeling relevance. Int. J. Food Sciences Nutr. 68(5):
546-552. DOI: 10.1080/09637486.2016.1268100
23. Ochsenreitheri,
K.; Gluck, C.; Stressler, T.; Fischer, L.; Syldatk, C. 2016. Production
strategies and applications of microbial single cell oils. Front. Microbiol. 7:
1539. DOI: 10.3389/ fmicb.2016.01539
24. Oliver, L.;
Dietrich, T.; Marañón, I.; Villarán, M.; Barrio, R. 2020. Producing Omega-3
polyunsaturated fatty acids: A review of sustainable sources and future trends
for the EPA and DHA market. Resources 9(12): 148. DOI: 10.3390/resources9120148
25. Park, W. K.;
Moon, M.; Shin, S. E.; Cho, J. M.; Suh, W. I.; Chang, Y. K.; Lee, B. 2018.
Economical DHA (Docosahexaenoic acid) production from Aurantiochytrium sp.
KRS101 using orange peel extract and low cost nitrogen sources. Algal Res. 29:
71-79. DOI: 10.1016/j. algal.2017.11.017
26. Patel, A.;
Rova, U.; Christakopoulos, P.; Matsakas, L. 2020. Mining of squalene as a
value-added byproduct from DHA producing marine thraustochytrid cultivated on
food waste hydrolysate, Sci. Total Environ. 736: 139691. DOI:
10.1016/j.scitotenv.2020.139691
27. Patterson, H.
B. W. 2011. Quality and control. In: List, G.R.; King, J.W. (Eds.).
Hydrogenation of fats and oils. Theory and practice. AOCS Press. p 329-350.
28. Pleissner, D.;
Lam, W. C.; Sun, Z.; Lin, C. S. 2018. Food waste as nutrient source in
heterotrophic microalgae cultivation. Bioresour. Technol. 137: 139-146. DOI:
10.1016/j. biortech.2013.03.088
29. Plua Montiel,
J.; Neira Mosquera, J. A.; Sanchez Llaguno, S. N.; Aldas Morejon, J. P.;
Revilla Escobar, K. Y.; Caicedo-Álvarez, E. (en prensa). omparison
of fatty acid profiles of sacha inchi oil (Plukenetia huayllabambana),
sesame oil (Sesamum indicum), and peanut oil (Arachis hypogaea)
using two extraction methods for food purposes. Revista de la Facultad de
Ciencias Agrarias. Universidad Nacional de Cuyo. Mendoza. Argentina.
30. Rosa, S.;
Soria, M.; Vélez, C.; Galvano, M. 2010. Improvement of a two-stage fermentation
process for docosahexaenoic acid production by Aurantiochytrium limacinum SR21
apply statistical experimental designs and data analysis, Biores. Technol.
101(7): 2367-2374. DOI: 10.1016/j.biortech.2009.11.056
31. Santos Jesus, S.; Maciel Filho, R. 2010. Modeling growth of
microalgae Dunaliella salina under different nutritional conditions. Am.
J. Biochem. Biotechnol. 6(4): 279-283. DOI: 10.3844/ ajbbsp.2010.279.283
32. Sartori, M.;
García, D.; Grossi Vanacore, M. F.; Fessia, A.; Nesci, A. (en prensa).
Biofungicide formulation based on Bacillus velezensis EM-A8 for control
of maize foliar diseases. Revista de la Facultad de Ciencias Agrarias.
Universidad Nacional de Cuyo. Mendoza. Argentina.
33. Siriwardhana,
N.; Kalupahana, N. S.; Moustaid-Moussa, N. 2012. Health benefits of n-3
polyunsaturated fatty acids: eicosapentaenoic acid and docosahexaenoic acid.
Adv. Food Nutr. Res. 65: 211-222. DOI: 10.1016/B978-0-12-416003-3.00013-5
34. Verified Market
Research. 2023. Global glycerin market size. Report ID 17400. 202 p. www.
verifiedmarketresearch.com/product/glycerin-market. Accessed December 2023.
35. Vonshak, A.
1986. Laboratory techniques for the cultivation of microalgae. In: Richmond, A.
(Ed.), Handbook of microalgal mass culture. CRC Press. p. 117-145.
36. Wolff, R. L.
1995. Content and distribution of trans-18:1 acids in ruminant milk and meat
fats. Their importance in European diets and their effect on human milk. J. Am.
Oil Chem. Soc. 72(1): 259-272. DOI: 10.1007/BF02541081
37. Yu, X. J.; Liu, J. H.; Sun, J.; Zheng, J. Y.; Zhang, Y. J.;
Wang, Z. 2016. Docosahexaenoic acid production from the acidic hydrolysate of
Jerusalem artichoke by an efficient sugar-utilizing Aurantiochytrium sp. YLH70.
Ind. Crops Prod. 83: 372-378. DOI: 10.1016/j. indcrop.2016.01.013