Revista de la Facultad de Ciencias
Agrarias. Universidad Nacional de Cuyo. Tomo 57(1). ISSN (en línea) 1853-8665.
Año 2025.
Original article
Preventive
and curative effects of native yeasts on different Botrytis cinerea strains
in “Superior Seedless” (Vitis vinifera L.) table grape cultured in
Argentina
Efectos
preventivos y curativos de levaduras nativas sobre diferentes cepas de Botrytis
cinerea en uva de mesa “Superior Seedless” (Vitis vinifera L.)
cultivada en Argentina
Fabio Vazquez1,
1 Universidad Nacional de San Juan. Facultad de Ingeniería. IBT.
Instituto de Biotecnología. Av. Libertador San Martín 1109 oeste. Capital. CP
5400. San Juan. Argentina.
2 Consejo Nacional de Investigaciones Científicas y Técnicas
(CONICET). Godoy Cruz. Buenos Aires 2290. C1425 FQB. Argentina.
* cristinanally@yahoo.com.ar
Abstract
The fermenting
grape must is a dynamic, stressful, and selective habitat where many yeast species
compete. Specific yeasts isolated from this habitat can play a fundamental role
in table grape biocontrol of fungal diseases. The present study evaluated 225
grapevine yeasts against four Botrytis cinerea strains isolated from
“Superior Seedless” grapes, considering the possible antifungal action
mechanisms. Eighteen enological yeasts (13 Saccharomyces and 5 non- Saccharomyces)
showed preventive antifungal activity against the four native B. cinerea strains,
with disease severity varying between 0 and 49.91%. These 18 strains also
presented curative activity against at least one of the B. cinerea strains
assayed (severity values between 0 and 45.99%). Considering action mechanisms,
thirteen yeast strains inhibited mycelial growth of at least one B. cinerea strain
during dual plating (antibiosis), “killer” activity, and volatile
antifungal assays. Our results showed that 7 yeast strains affected conidial
germination (CG) and germinal tube length (GTL) of at least one B. cinerea isolate.
Two yeast strains occupied the same niche as 4 B. cinerea strains (NOI
values > 0.90). All yeast strains exhibited at least two inhibitory action
mechanisms against gray rot, except for BSc140 with one mechanism. The
possibility of more than one mechanism per yeast strain makes biocontrol an
effective tool to prevent and cure gray rot in table grapes.
Keywords: preventive,
curative, oenological yeasts, Botrytis cinerea, table grape, modes of
action
Resumen
El mosto de uva en
fermentación es un hábitat dinámico, estresante y selectivo donde compiten
diferentes especies de levaduras. Levaduras enológicas pueden desempeñar un
papel fundamental en el biocontrol de las enfermedades fúngicas de la uva de
mesa. El presente estudio evaluó la eficacia de 225 levaduras vitícolas para
controlar cuatro cepas nativas de Botrytis cinerea aisladas de uvas
“Superior Seedless” y los posibles mecanismos de acción antifúngica. Dieciocho
levaduras enológicas (13 Saccharomyces y 5 no Saccharomyces)
mostraron actividad antifúngica preventiva frente a las cuatro cepas de B.
cinerea presentando una severidad de la enfermedad que varía entre 0 y
49,91%. Estas 18 cepas presentaron actividad curativa contra al menos una de
las cepas de B. cinerea ensayadas (valores de severidad entre 0 y
45,99%). Posibles mecanismos de acción: 13 cepas de levadura inhibieron el
crecimiento micelial de al menos una de las cepas de B. cinerea ensayadas
durante los ensayos dual (antibiosis), actividad “killer” y volátiles antifúngicos.
Nuestros resultados mostraron que 7 cepas de levadura afectaron la germinación
de los conidios (CG) y la longitud del tubo germinativo (GTL) de al menos uno
de las cepas patogénicas. Dos cepas de levadura ocuparon el mismo nicho que las
4 cepas de B. cinerea (valores NOI > 0,90). Los presentes hallazgos
indican que todas las cepas de levadura exhibieron al menos dos mecanismos de
acción antifúngico para controlar la pudrición gris, excepto BSc140 (un
mecanismo). La posibilidad de que las cepas de levadura puedan ejercer más de
un mecanismo de acción hace que el biocontrol sea una herramienta más eficaz
para prevenir y curar la pudrición gris en uva de mesa.
Palabras clave: preventivo,
curativo, levaduras enológicas, Botrytis cinerea, uva de mesa, modos de
acción
Originales: Recepción: 14/02/2023 - Aceptación: 09/12/2024
Introduction
San Juan is
Argentina’s main producer of Vitis vinifera L. table grapes. In 2023,
the province produced 41264 tons of grapes for fresh consumption or raisins (6). Indoor and
outdoor grapes suffer from a variety of fungal diseases. Botrytis cinerea,
causing “gray mold”, is one important postharvest decay of fresh fruit and
vegetables (4). Treatments
against fungal diseases can be preventive or curative. In Argentina, chemical
products such as azoxystrobin + difenoconazole, benomyl boscalid + pyraclostrobin,
and carbendazim have a preventive effect against this fungus in grapes. In
Argentina, boscalid is registered as preventive and curative fungicide against B.
cinerea (2). However,
excessive use of synthetic fungicides concerns human health and environmental
well-being. B. cinerea is a polycyclic pathogen that can develop
resistance against chemical fungicides (1).
Grape must is a dynamic habitat with high selection pressure
(stress), resulting from physical (osmolarity, low pH) and chemical conditions
(limited nitrogen availability, high ethanol concentrations) rich in different
competing microbial species (18). Yeasts isolated
from environments subjected to various stresses, such as grape fermentation,
are more likely to be effective antagonists (16). Unfortunately,
information on yeasts with antifungal curative activity as novel biocontrol
technique is limited. Only a few reports mention curative activity (24 h after
pathogen infection) of yeasts like Saccharomyces cerevisiae (8), Candida
stellimalicola (3), Candida inconspicua, Pichia
kluyveri (22), Pichia
kudriavzevii and Rhodotorula glutinis (9) against different
fungal pathogens in various vegetables. No reports consider applying curative
yeasts in white table grapes. Most studies assessed yeast preventive effects
against only one B. cinerea strain (13,
19). Possible modes of action against reported pathogenic fungi
include competition for nutrients and space, reduction in spore germination and
germ tube length, and inhibition of fungal mycelial growth by diffusible and
volatile metabolites (14, 20). Few reports
describe preventive and curative effects against fungi (8,
9). We aimed to assess efficacy of 225 viticultural yeasts against
four native B. cinerea strains, conducting preventive and curative
assays. Possible preventive or curative antifungal mechanisms of selected
yeasts against four B. cinerea isolates were also evaluated in vitro.
Materials
and methods
Yeast
isolates
The present study
assayed two hundred and twenty-five grapevine yeasts belonging to 41 species (13). Seventeen native
yeasts were previously isolated from table grapes, 9 from vineyard soil
(Caucete, San Juan), and 199 from fermenting musts of different varieties from
San Juan, Argentina. These yeasts were previously identified by morphological
and molecular techniques (13).
Botrytis
cinerea strains
Four B. cinerea strains
(B11, B14, B15, B24) were previously isolated from “Superior Seedless” table
grape from Mendoza, Argentina. Previous molecular identification was carried
out using molecular markers based on PCR-RFLP. Amplification of the ribosomal
intergenic spacer (IGS) was performed with PCR, and product restriction was
carried out with CfoI, HaeIII, and HinfI enzymes (12).
Curative
and preventive in vivo assays
Grape
Untreated grapes
with chemical pesticides were washed, superficially disinfected with sodium
hypochlorite 0.2% (v/v) for 3 min, and subsequently rinsed with distilled water
to eliminate sodium hypochlorite. A single wound (3 mm diameter and 3 mm deep)
was made at the equator of each fruit.
Preventive
treatments
First, the
antifungal effect of the 225 yeast strains against 4 B. cinerea strains
was evaluated in preventive bioassays in the “Superior Seedless” table
grape. A 20 μL of a yeast cell suspension (106 cells/mL) was inoculated
in the artificially wounded area and treated 24 h later with 20 μL of a B.
cinerea conidial suspension (104 conidia/mL).
Curative
treatments
Wounded grapes were
initially inoculated with B. cinerea suspension and 24 h later with
yeast suspension with preventive antifungal activity (severity 50% or less).
Microbial concentrations were as mentioned above.
Controls: a- A
wounded grape initially inoculated with B. cinerea suspension (104 conidia/mL), b- A
wounded grape initially inoculated with water, and c- A wounded grape
inoculated with yeast alone.
Treatments were
arranged in a completely random design, with 3 replicates and 9 grapes per
replicate. Assays were performed three times. The fruit was stored for 10 d at
25°C and 90% RH.
After the bioassays, disease severity (%) considered as the
average diameter of gray rot lesions (cm, using a digital caliper) was
calculated as follows:
![]()
In preventive and
curative assays, antagonistic yeasts reduced disease severity by 50% or more.
Evaluation
of possible antifungal mechanisms of the selected preventive and curative
yeasts against four B. cinerea strains (B11, B14, B15, B24) (18)
Dual
culture assays (antibiosis)
Mycelial agar disks (5mm) obtained from the margin of 7-day-old
fungus cultures were placed in the center of the dishes containing Czapeck-Agar
(Sigma-Aldrich ®). Around the fungus, four aliquots (20 μL) of a yeast cell
suspension (106 cells/mL) were
spot-inoculated, 3 cm from the center. Plates were incubated at 25°C for 5 d,
and subsequently, mycelial growth (cm) was measured with a digital caliper.
Results are expressed as % of B. cinerea mycelial growth inhibition
compared to control (100%) (21).
Detection
of killer activity
Plates with
YMB-MB-Phosphate Citrate Buffer- Agar (Britania®) at pH 4.5 were inoculated
with 100 μL of B. cinerea conidia (104 conidia/mL) as lawn.
After the plates solidified, spot inoculation of 20 μL of each yeast (106 cells/mL) was performed
with an automatic pipette. Plates were incubated at 25 °C for 5 d in the dark.
A clear zone around yeast colonies was recorded as positive (+).
Antifungal
activity of volatile organic compounds (VOCs)
B. cinerea mycelial discs (5
mm diameter) were taken from margins of 7-day-old cultures. The mycelial disc
was inoculated on the center in the base plate with Potato Dextrose Agar (PDA)
(Britania ®) medium. Another base plate with YEPD-Agar medium was inoculated
superficially with 100 μL of a yeast suspension (106 cells/mL). The two base
plates were faced and sealed with plastic Parafilm® (13). Controls were
performed by inoculating B. cinerea (without yeast cells) on PDA. The
sealed plates were incubated at 25°C for 5 d. At the end of the assay, mycelial
growth (diameter, cm) was measured with a digital caliper. Results are
expressed as the % of mycelial growth inhibition of B. cinerea compared
with fungal control.
Yeast
effect on conidial germination (CG) and germinal tube length (GTL) of four B.
cinerea strains in low-nutrient medium
A suspension of 100
μL yeast cells (106 cells/mL), 25 μL fungal
conidia (104 conidia/mL), and 100 μL
of 1% diluted (v/v) white grape must (Superior Seedless) were inoculated on
sterile excavated slides (13). Controls
consisted of B. cinerea conidia without yeast. In Petri dishes, the
excavated slides were incubated at 25°C for 24 h in the dark at 80% RH. The CG
results are expressed as percentage of germinated conidia compared with the
control (observation of 100 conidia). Conidia were considered germinated when
the germ tube length was larger or equal to the conidia. GTL (μm) was measured
with an ocular micrometer calibrated in a 40X light microscope objective
(observation of 30 conidia).
Niche
Overlap Index (NOI)
Fungal conidia (20 μL, 104 conidia/mL) and yeast
cells (20 μL, 106 cells/mL) were inoculated
on separate plates containing distilled water agar (2% agar, pH 4.5) with
different nutritional sources (10 mM). Carbon sources assayed are generally
present in table grapes and represent niche size: proline, asparagine,
rhamnose, alanine, melibiose, glycine, malic acid, glutamic acid, tyrosine,
raffinose, arginine, lysine, fructose, methionine, mannitol, glucose,
saccharose, citric acid, galactose and tartaric acid (14). Plates were
incubated at 25°C for 7 d in the dark. NOI values were obtained as follows:
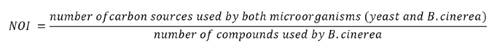
NOI values >0.90
represent occupation of the same niche (competitive exclusion), and values <
0.90 represent occupation of separate niches (coexistence) (23).
Statistical
analysis
All experiments were performed in triplicate and thrice. SPSS® software was used for
statistical analysis. ANOVA assumptions were examined before statistical
analyses, and mean values were compared with Tukey’s test at a p-value = 0.05.
Percentages of wounds infected by B. cinerea and germination conidia
were arcsine-square-root transformed before ANOVA. When ANOVA assumptions were
not met, the non-parametrical Kruskal-Wallis test was used.
Results
Table
grape bioassays (preventive and curative)
In preventive assays with artificially wounded grapes, 18 isolates
of the 225 enological yeasts assayed reduced disease severity by 50% or more in
4 B. cinerea strains: B11, B14, B15, and B24 (table 1).
Table 1. Disease
severity (%) caused by B. cinerea strains (B11, B14, B15, and B24) in white
table grapes treated with yeast strains in preventive and curative assays.
Tabla
1. Severidad de la enfermedad (%)
causada por cepas de B. cinerea (B11, B14, B15 y B24) en uva de mesa
blanca tratadas con cepas de levadura en ensayos preventivos y curativos.

Different lowercase letters
within the same column indicate significant differences among severity means
and control according to Tukey’s test (p < 0.05). The • symbol indicates
non-significant difference between disease severity and control (100%). Gray
highlight indicates 0% disease severity.
Diferentes letras minúsculas en
la misma columna indican diferencias significativas entre las medias de
severidad con respecto al control en relación al Test de Tukey (p < 0,05). El
símbolo • representa valores de severidad que no presentan diferencias
significativas en relación con el control (100%). Resaltado con gris indica 0%
de severidad.
Yeast strains belonged to different species, including Saccharomyces
cerevisiae (BSc14, BSc16, BSc27, BSc60, BSc90, BSc96, BSc97, BSc102,
BSc103, BSc112, BSc140, BSc206), Saccharomyces chevalieri (BSch26), Torulaspora
delbrueckii (BTd156, BTd165), Candida sake (BCs54), Hanseniaspora
vineae (BHv86), and Debaryomyces vanrijiae (BDv197). All 18 strains
had been isolated from fermented musts. Antifungal preventive treatments with
these yeasts reduced disease severity between 50.14 and 100%. Notably, S.
cerevisiae BSc103 and S. chevalieri BSch26 inhibited total fungal
growth of B14 and B15, respectively, during preventive assays (table 1 and figure 1).

Figure 1. Grapes
inoculated with S. cerevisiae BSc103- B. cinerea B15 (A) (0%
severity) and inoculated with water- B. cinerea B15 (B) (100% severity),
in preventive assays.
Figura
1. Uvas inoculadas con S.
cerevisiae BSc103- B. cinerea B15 (A) (0% severidad) e inoculadas
con agua- B. cinerea B15 (B) (100% severidad), en ensayos preventivos.
In curative in
vivo experiments, 15 of the 18 preselected yeasts presented curative
activity against two of the four B. cinerea strains assayed (9 S.
cerevisiae, 1 S. chevalieri, 1 C. sake, 1 H. vineae,
and 2 T. delbrueckii), reducing disease severity between 54.01 and 100%.
Additionally, four isolates (3 S. cerevisiae and 1 D. vanrijiae)
significantly reduced the rot halo caused by one B. cinerea strain with
a severity between 0 and 39.88% (table 1).
S. cerevisiae BSc206 and BSc212
inhibited total growth of B11 during curative assays (table 1).
Possible
mechanism of action
Eighteen yeast
isolates that showed antifungal effectivity (table 1) were analyzed.
Dual
culture assay
Five of the 18
yeast isolates did not inhibit any B. cinerea strain (B11, B14, B15,
B24) in dual culture assays (table 2). Two yeasts (BSc27, BCs54) significantly inhibited three B.
cinerea strains, whereas five isolates (BSc14, BSc16, BSc90, BSch26, BHv86)
significantly reduced fungal development of two strains. Five yeast strains
only inhibited one pathogenic strain (BSc96, BSc97, BSc103, BSc112, BSc140,
BTd156). Table
2,
shows the highest inhibition percentages (50% or more) in Saccharomyces strains
BSc27, BSch26, and BSc14 against B11, B15, and B24, respectively.
Table 2. Mycelial
growth inhibition (%) of B. cinerea strains after released dual culture
assays with 18 yeast strains.
Tabla
2. Inhibición del crecimiento micelial
(%) de cepas de B. cinerea luego de realizar ensayos de cultivos duales
con 18 cepas de levaduras.

Different lowercase letters
within the same column indicate significant differences among means and SD of
mycelial growth according to Tukey’s test (p ≤ 0.05). The •symbol represents
values not significantly different from control (0%). Gray highlight indicates
a mycelial growth inhibition percentage of 50% or more.
Diferentes letras minúsculas en
la misma columna indican diferencias significativas entre las medias y el DS
del crecimiento fúngico micelial con respecto al control (p ≤ 0,05). El símbolo
• representa valores que no difieren significativamente con el control (0%). Resaltado
con gris indica porcentajes de inhibición del crecimiento micelial de 50% o
superior.
Yeast
‘Killer’ activity
Thirteen yeast
strains presented ‘killer’ activity against at least one B. cinerea pathogenic
strain. Seven yeasts (5 S. cerevisiae, 1 T. delbrueckii, 1 D.
vanrijiae) showed ‘killer’ activity against B11 strain, four Saccharomyces
yeasts against B14, two yeasts against B15 and none yeast against B24 (table 3).
Table 3. Yeast
“Killer” activity against four B. cinerea isolates (B11, B14,
B15, B24) at 25°C.
Tabla
3. Actividad “killer” de las cepas de
levaduras frente a los cuatro aislamientos de B. cinerea (B11, B14, B15,
B24), a 25°C.
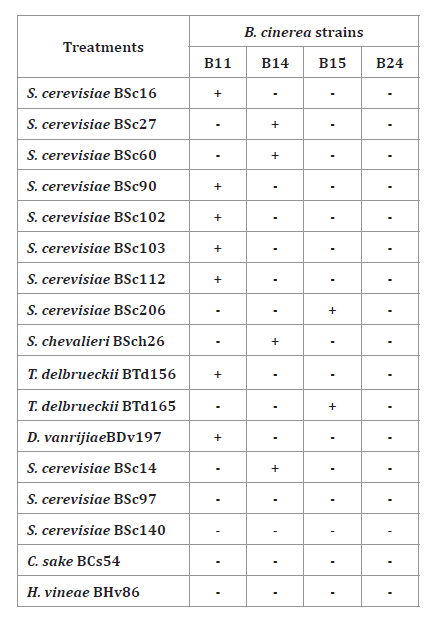
Positive signs (+) indicate
killer activity (presence of a clear zone around the yeast colony), and
negative signs (-) indicate no “killer” activity.
Signos positivos (+) indican la
presencia de zona transparente alrededor de las colonias de levadura, y los
signos negativos (-) indican ausencia de actividad “killer”.
Antifungal
activity of volatile organic compounds (VOCs)
From the 18
isolates, 13 yeast strains produced volatile compounds that significantly
inhibited mycelial growth of at least one Botrytis strain (table 4). The most
susceptible B. cinerea strain was B. cinerea B24 (7/18), followed
by B11, B15 (6/18) and B14 (3/18).
Table 4. Effect
of volatile compounds produced by 18 yeast isolates on mycelial growth of four B.
cinerea strains (%).
Tabla
4. Efecto de los compuestos volátiles
producidos por 18 levaduras sobre la inhibición del crecimiento micelial de las
cuatro cepas de B. cinerea ensayadas (%).
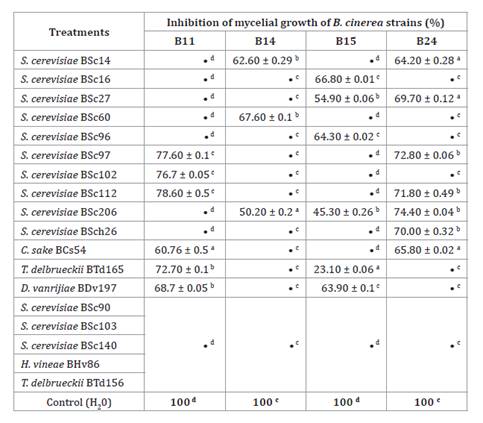
Different lowercase letters
within the same column indicate significant differences between means of
mycelial growth according to Tukey's test (p ≤ 0.05). The •symbol represents
mycelial growth not significantly different from the control (100%).
Letras distintas en de la misma
columna indican diferencias significativas entre las medias para el crecimiento
fúngico según la prueba de Tukey (p ≤ 0,05). El símbolo • representa valores de
crecimiento micelial que no difieren significativamente con el del control
(100%).
Volatile compounds produced by S. cerevisiae BSc206
significantly inhibited mycelial growth of three B. cinerea strains,
B14, B15, and B24, between 25.6 and 54.7% (table 4). Seven strains (4
Saccharomyces and 3 non- Saccharomyces) produced antifungal
volatile compounds against 2 B. cinerea strains and 5 Saccharomyces
yeasts against one B. cinerea strain. These 12 yeast strains inhibited mycelial
growth between 21.4 and 76.9% (table 4).
Yeast
effect on conidial germination (CG) and germinal tube length (GTL) of B.
cinerea in low-nutrient medium (diluted grape must)
In this assay, seven yeast strains (6 Saccharomyces and 1
non-Saccharomyces) significantly affected conidial germination (CG) and
germinal tube length (GTL) of at least one B. cinerea strain. B24 was
the most susceptible strain, inhibited by five yeasts (5/18), followed by B15
inhibited by 3 yeasts (3/18), B14 (2/18), and B11 inhibited by 1 yeast (1/18).
Five Saccharomyces yeasts (S. cerevisiae BSc27, BSc102, BSc112,
BSc206, and S. chevalieri BSch26) significantly inhibited conidial
germination and reduced the germ tube length of one B. cinerea strain. S.
cerevisiae Bsc60 significantly reduced two B. cinerea strains: B24
and B15 (table
5).
Table 5. Evaluation
of conidial germination (CG; %) and germ tube length (GTL; μm) of B. cinerea
strains in co-cultures with yeasts (excavated slides).
Tabla
5. Evaluación de la germinación de
conidios (CG; %), y longitud del tubo germinal (GTL; μm) de cepas de B.
cinerea en co-cultivos con levaduras (portaobjetos excavados).

Different lowercase letters
within the same column indicate significant differences between means of
germinated conidia (CG) expressed in % and germinal tube length (GTL) in μm,
according to Tukey’s test (p ≤ 0.05). Gray highligh indicates values
significantly differing from the control (p ≤ 0.05).
Letras distintas en la misma
columna indican diferencias significativas entre los valores de conidios
germinados (CG) expresados en %, y longitud del tubo germinal (GTL) en μm, en
relación al Test de Tukey (p ≤ 0,05). Resaltado con color gris indica valores
que difieren significativamente del control (p ≤ 0,05).
H. vineae BHv86 was the only
isolate significantly inhibiting conidial germination and germinal tube length
of all five B. cinerea B11, B14, B15, and B24. This yeast strain reduced
conidial germination between 28.57 and 32.62%, and germ tube length between
12.57 and 50.96% (table
5).
Niche
Overlap Index (NOI)
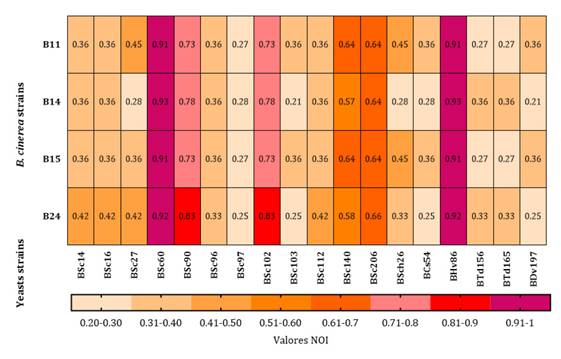
S. cerevisiae BSc60 and H. vineae BHv86 occupied the same niche as the
4 B. cinerea isolates. Both yeast strains competed with B11, B14, B15
and B24 for the nutritional sources. Four S. cerevisiae/four H.
vineae-B. cinerea interactions presented
NOI values between 0.91 and 0.93 (table 6). The remaining interacting pairs (64) presented NOI values
between 0.21 and 0.83, indicating ecological coexistence (separate niches) (table 6).
Table 6. Niche
overlap index (NOI) between 18 yeast and 4 B. cinerea strains.
Tabla
6. Índice de superposición de nichos
(NOI) entre 18 cepas de levaduras y 4 cepas de B. cinerea.
Discussion
Yeast antifungal
activity is strain-dependent, and therefore, screening numerous microorganisms
becomes necessary for finding strains with broad inhibitory spectrums. Few
reports have mentioned the potential use of yeasts of different species and
genera with preventive and curative activity to control fungi on fruit tissues (5,
22). Our study first reports yeast species isolated from grape
fermentation, like S. cerevisiae, S. chevalieri, T.
delbrueckii, D. vanrijiae, H. vineae and C. sake, with
preventive and curative antifungal activity against B. cinerea in table
grapes and postharvest conditions. These enological yeasts demonstrated greater
antifungal activity in preventive than in curative assays in table grape wounds
(table
1,
page XXX). Pesce
et al. (2018) determined that oenological yeasts have stronger antifungal
activity than yeasts isolated from another habitat (olives), presenting
competitive advantages against other microorganisms.
Commercially
developing a product based on native microorganisms involves several steps,
starting with isolation and selection of potential biocontrol strains
exhibiting desirable characteristics, like different antifungal mechanisms of
action (5). Our study examined 18 viticultural
yeast strains promoting biocontrol of gray mold under preventive and curative
conditions in white table grapes (table 2, table
3,
table
4,
table
5 and
table
6).
We assessed biocontrol activity and modes of action of native yeasts against a
pool of four native B. cinerea strains previously isolated from
different vineyards, constituting a representative way to measure biocontrol
activity. To the best of our knowledge, this is the first report on antifungal
modes of action of Saccharomyces, Torulaspora, Debaryomyces, and Candida with
preventive and curative effects against native B. cinerea strains
isolated from Superior Seedless table grapes (table 1). The dual culture
assay revealed that 72.2% (13/18) of the biocontrol yeasts inhibited mycelial
growth of at least one B. cinerea strain in the PDA medium (table 2). Strains belonged to S.
cerevisiae, S. chevalieri, C. sake, H. vineae, and T.
delbrueckii species. Korres et al. (2011) state that yeasts
synthesized and secreted suppressive antifungal substances like diffusible and
volatile metabolites, and observed mycelial inhibition on plates in dual
cultures. “Killer” activity is a widespread characteristic among yeast
species of different genera, including Saccharomyces, Hansenula, Kluyveromyces,
and Pichia, conferring an ecological advantage over competitors (10). In this study, 13
yeasts belonging to Saccharomyces, Torulaspora, and Debaryomyces
species showed positive ‘killer’ activity against at least one B.
cinerea strain at pH 4.5 (table 3). Fungal growth inhibition by volatile compounds avoids adverse
environmental or toxicological effects (11). Parafati
et al. (2015) found that the biocontrol activity of S. cerevisiae strains
against B. cinerea on table grape berries was attributed to volatile
organic compounds (VOCs) in vitro and in vivo. In our study, Saccharomyces,
Candida, Torulaspora, and Debaryomyces isolates
significantly inhibited mycelial diameter of different B. cinerea isolates
through volatile compounds in vitro (table 4). Primarily, biocontrol yeasts
compete for nutrients and space (20). Co-cultures of Saccharomyces
(BSc27, BSc60, BSc102, BSc112, BSc206, BSch26) and Hanseniaspora (BHv86)
with B. cinerea conidia in liquid medium with low nutrients (excavated
slides) showed that these yeasts significantly inhibited conidia germination
and reduced germ tube length. In our study, Hanseniaspora yeast
significantly reduced conidial germination (CG) and germinal tube length (GTL)
of four B. cinerea strains (table 5). Qin
et al. (2015) reported similar results in a co-culture of H. uvarum and
B. cinerea, while Wilson and Lindow (1994) previously
suggested that nutritional resources might mediate microorganism coexistence
and competitive exclusion. To the best of our knowledge, our study first
reports two yeast isolates, S. cerevisiae (BSc60) and H. vineae (BHv86)
with NOI values of 0.91 and 0.93 when co-cultured with four B. cinerea strains
(table
6).
S. cerevisiae (BSc27, BSc60,
BSc112) and S. chevalieri (BSch26) presented the highest amount of
possible antifungal mechanisms (four). Our results confirm multiple possible
modes of action against pathogens like antibiosis (dual culture), ‘killer’ activity,
antifungal activity by volatile compounds, inhibition of conidial germination,
reduction of germinal tube length (low nutrient medium) and competitive
exclusion (NOI).
Conclusions
Under preventive and curative conditions, yeasts isolated from
wine fermentation are key biocontrol agents against B. cinerea in white
table grapes. Understanding yeast strategies against B. cinerea strains
in preventive and curative assays is essential for selection and effective
application. Our study highlights the importance of testing diverse mechanisms
that native biocontrol yeasts apply against different B. cinerea isolates.
In addition, considering that different isolates can exert more than one
mechanism of action makes biocontrol an effective tool to prevent gray rot in
white table grapes. Further studies should establish bio-antagonism effects on
quality attributes of table grapes, the application of biofungicide yeast
consortium and the effect of nonviable yeast cells against B. cinerea.
1.
Abbey, J. A.; Percival, D.; Abbey, L.; Asiedu, S. K.; Prithiviraj, B.;
Schilder, A. 2018. Biofungicides as an alternative to synthetic fungicide
control of grey mould (Botrytis cinerea) - prospects and challenges.
Biocontrol Science and Technology. 29: 3.
2.
CASAFE (Cámara de Sanidad Agropecuaria y Fertilizantes). 2024. Guía
fitosanitaria. Argentina. https://guiaonline.casafe.org/
3.
Da Cunha, T.; Ferraz, L. P.; Wehr, P. P.; Kupper, K. C. 2018. Antifungal
activity and action mechanisms of yeasts isolated from citrus against Penicillium
italicum. International Journal of Food Microbiology. 276: 20-27.
4.
Elad, Y.; Vivier, M.; Fillinger, S. 2015. Botrytis: the good, the bad, and the
ugly. In: Fillinger S.; Elad Y.; Vivier M. (Eds.), Botrytis-the Fungus,
the pathogen and its management in agricultural systems. Springer, Heidelberg,
Germany. 1-15.
5.
Ferraz, L. P.; da Cunha, T.; da Silva, A. C.; Kupper, K. C. 2016. Biocontrol
ability and putative mode of action of yeasts against Geotrichum
citri-aurantii in citrus fruit. Microbiol. Res. 188: 72-79.
6.
INV (Instituto Nacional de Vitivinicultura). 2022. Exportaciones. Información:
estadísticas informes. Argentina.
https://www.argentina.gob.ar/inv/vinos/estadisticas
7.
Korres, A. M. N.; Buss, D. S.; Ventura, J. A.; Fernández, P. M. B. 2011. Candida
krusei and Kloeckera apis inhibit the causal agent of pineapple
fusariosis, Fusarium guttiforme. Fungal Biol. 115: 1251-1258.
8.
Lopes, M. R.; Klein, M. N.; Ferraz, L. P.; Silva, A. C.; Kupper, K. C. 2015. Saccharomyces
cerevisiae: a novel and efficient biological control agent for Colletotrichum
acutatum during pre-harvest. Microbiol. Res. 175: 93-99.
9.
Madbouly, A. K.; Kamal, A. M.; Ismail, M. I. 2020. Biocontrol of Monilinia
fructigena, the causal agent of brown rot of apple fruit, by using
endophytic yeasts. Biological Control. 144: 104239.
10.
Magliani, W.; Conti, S.; Travassos, L. R.; Polonelli, L. 2008. From yeast
killer toxins to antibiobodies and beyond. FEMS Microbiol Lett. 288: 1-8.
11.
Mari, M.; Bautista-Baños, S.; Sivakumar, D. 2016. Decay control in the
postharvest system: role of microbial and plant volatile organic compounds.
Postharvest Biol. Technol. 122: 70-81.
12.
Muñoz, C.; Gomez, S.; Oriolani, E.; Combina, M. 2010. Genetic characterization
of grape vine-infecting Botrytis cinerea isolates from Argentina.
Revista Iberoamericana de Micología. 27: 66-70.
13.
Nally, M. C.; Pesce, V. M.; Maturano, Y. P.; Muñoz, C. J.; Combina, M.; Toro,
M. E.; Castellanos de Figueroa, L. I.; Vazquez F. 2012. Biocontrol of Botrytis
cinerea in table grapes by non-pathogenic indigenous Saccharomyces
cerevisiae yeast isolated from viticultural environments in Argentina.
Post. Biology and Technology. 64: 40-48.
14.
Nally, M. C.; Pesce, V. M.; Maturano, Y. P.; Rodriguez Assaf, L. A.; Toro, M.
E.; Castellanos de Figueroa, L. I.; Vazquez, F. 2015. Antifungal modes of
action of Saccharomyces and other biocontrol yeasts against fungi
isolated from sour and grey rots. Int J Food Microbiol. 204: 91-100.
15.
Parafati, L.; Vitale, A.; Restuccia, C.; Cirvilleri, G. 2015. Biocontrol
ability and action mechanism of food-isolated yeast strains against Botrytis
cinerea causing table g. Food Microbiol. 47: 85-92.
16.
Pesce, V. M.; Nally, M. C.; Carrizo, G.; Rojo, C.; Pérez, B.; Toro, M. E.;
Castellanos de Figueroa, L.; Vazquez, F. 2018. Antifungal activity of native
yeasts from different microenvironments against Colletotrichum
gloeosporioides on ripe olive fruits, Biological Control. 120: 43-51.
17.
Qin, X. J.; Xiao, H. M.; Xue, C. H.; Yu, Z. F.; Yang, R.; Cai, Z. K. 2015.
Biocontrol of gray mold in grapes with the yeast Hanseniaspora uvarum alone
and in combination with salicylic acid or sodium bicarbonate. Postharvest Biol.
Tec. 100: 160-167.
18.
Querol, A.; Fernandez-Espinar, M. T.; Olmo, M. L.; del Barrio, E. 2003.
Adaptive evolution of wine yeast. Int. J. Food Microbiol. 86: 3-10.
19.
Sepúlveda, X.; Vargas, M.; Vero, S.; Zapata, N. 2023. Indigenous Yeasts for the
Biocontrol of Botrytis cinerea on Table Grapes in Chile. J. Fungi. 9:
557.
20.
Spadaro, D.; Droby, S. 2016. Development of biocontrol products for postharvest
diseases of fruit: the importance of elucidating the mechanisms of action of
yeast. Trends in Food Science and Technology. 47: 39-49.
21.
Varela Pardo, R. A.; López Lastra, C. C.; Manfrino, R. G.; Balcazar, D.;
Mónaco, C.; Wright, E. R. 2024. Selection of fungal isolates from Buenos Aires,
Argentina, as biological control agents of Botrytis cinerea and Sclerotinia
sclerotiorum. Revista de la Facultad de Ciencias Agrarias. Universidad
Nacional de Cuyo. Mendoza. Argentina. 56(2): 72-86. DOI: https://doi.
org/10.48162/rev.39.138.
22.
Vilaplana, R.; Cifuentes, C.; Vaca, L.; Cevallos-Cevallos, J. M.;
Valencia-Chamorro, S. 2020. The curative activity of possible biocontrol agents
in the postharvest of yellow pitahaya and organic banana. Postharvest Biology
and Technology. 159-111030.
23.
Wilson, M.; Lindow, S. 1994. Ecological similarity and coexistence of epiphytic
ice-nucleating Pseudomonas syringae strains and non-ice-nucleating biological
control agent. Applied and Environmental Microbiology. 60: 3128-3137.