Revista de la Facultad de Ciencias
Agrarias. Universidad Nacional de Cuyo. Tomo 57(2). ISSN (en línea) 1853-8665.
Año 2025.
Original article
Fungicide
Management of Late Leaf Spot and Peanut Smut
Uso
de fungicidas para el manejo de la viruela tardía y del carbón del maní
Damian Francisco
Giordano1*, 2,
Agostina Del Canto1,
Jessica Gabriela
Erazo1,
Nicolas Alejandro
Pastor1,
Melina Rosso3,
Adriana Mabel
Torres1,
1 Universidad Nacional de Río Cuarto (UNRC). Instituto de
Investigación en Micología y Micotoxicología (IMICO). Consejo Nacional de
Investigaciones Científicas y Técnicas (CONICET). 5800. Ruta Nacional 36 km
601. Río Cuarto. Cordoba. Argentina.
2 Universidad Nacional de Río Cuarto (UNRC). Facultad de
Agronomía y Veterinaria. Departamento de Biología Agrícola.
3 Criadero El Carmen. 5809. Av. Italia 871. General Cabrera.
Córdoba. Argentina.
Abstract
Late leaf spot
(LLS), caused by Nothopassalora personata, is the most devastating
peanut disease in the world. In Argentina, peanut smut (Thecaphora frezii)
has increased significantly in recent decades. LLS is mainly managed through
chemical fungicides, however, peanut smut is not effectively controlled, except
for some resistant peanut genotypes. This study evaluated the effects of widely
used fungicides for LLS control on both diseases and crop yield. Field trials
were conducted over three consecutive years in two locations, with different
fungicide doses and number of applications. Disease intensities were
significantly higher in General Cabrera (GC) than in Vicuña Mackenna (VM)
resulting in higher yields in VM. This could be due to the longer history of
peanut cultivation in GC, where fungicide applications reduced LLS intensity.
Among fungicides, chlorothalonil showed the best performance. However, these
treatments were ineffective against peanut smut, likely due to difficulties
reaching the infection site. Considering fungicides are one major management
tool, further study of different active ingredients against both diseases
should also consider sustainable integrated management.
Keywords: Arachis hypogaea, chemical control,
fungal diseases, Nothopassalora personata, Thecaphora frezii
Resumen
La viruela tardía
(VT) ocasionada por Nothopassalora personata es la enfermedad del maní
más desvastadora a nivel mundial, mientras que el carbón (Thecaphora frezii),
es la enfermedad con mayor incremento en Argentina en las últimas décadas. VT
es principalmente manejada a través de fungicidas químicos, mientras que, para
el carbón del maní, no existen herramientas efectivas, salvo algunos genotipos
resistentes. En este trabajo, se evaluó el efecto de fungicidas ampliamente
utilizados para el control de VT, sobre ambas enfermedades y sobre el
rendimiento del cultivo. Los ensayos de campo fueron realizados en dos localidades
por tres años consecutivos, donde se probaron fungicidas en diferentes dosis y
número de aplicaciones. La intensidad de ambas enfermedades fue más alta en
General Cabrera (GC) que en Vicuña Mackenna (VM), resultando en mayores
rendimientos en VM. Esto se debió posiblemente al mayor historial de producción
de maní en GC, donde la aplicación de fungicidas redujo la intensidad de VT.
Entre los fungicidas, clorotalonil demostró la mejor performance. Sin embargo,
estos tratamientos no fueron efectivos frente al carbón del maní, posiblemente
debido a no alcanzar el sitio de infección. Teniendo en cuenta que los
fungicidas son una de las principales herramientas de manejo, se necesitan más
estudios de diferentes ingredientes activos sobre ambas enfermedades,
considerando un manejo integrado sustentable.
Palabras clave: Arachis hypogaea, control químico,
enfermedades fúngicas, Nothopassalora personata, Thecaphora frezii
Originales: Recepción: 11/02/2024 - Aceptación: 16/10/2024
Introduction
Peanut (Arachis
hypogaea L.) world production exceeds 49 million metric tons in pods. This
oilseed is cultivated in over 100 countries, but approximately 80% of the
production is concentrated in 10 countries, with China leading 18 million
metric tons annually. Argentina is the tenth peanut producer with more than
950.000 metric tons, and the second exporter, with 16% of worldwide production.
The province of Córdoba is the largest producer, accounting for 80% of the
national output (32).
Several diseases
affect peanut production in Argentina and other countries. Early leaf spot
(ELS) caused by Passalora arachidicola (Hori) and late leaf spot (LLS)
caused by Nothopassalora personata (Berk. & Curtis) are the most
important foliar diseases worldwide, being LLS the most frequent in some main
producing regions (14, 24). These diseases
can generate important yield losses, and consequent economic imbalance (2). On the other
hand, peanut smut by Thecaphora frezii (Carranza & Lindquist) has
become the most important soil-borne disease in Argentina due to recent
increasing prevalence and intensity (30), causing
significant yield losses (25). LLS benefits from
rainfall (16), while smut typically thrives under
drought conditions, particularly during grain filling (28).
Even though
different tools aim to control LLS, its management relies mainly on chemical
fungicides (17) like systemic
single-site mode carboxamides, strobilurins and triazoles. Many studies have
shown beneficial effects of using fungicide mixtures from different chemical groups,
mainly with carboxamides (10, 23). Among contact
fungicides with multiple modes of action, the majorly used chlorothalonil
presents consistent results (9). However, other
options must be considered given that some active ingredients (a.i.) may soon
be prohibited or useless against resistant strains.
Different
management strategies have been tested against peanut smut, without successful
effects on intensity. On the other hand, genetics have contributed resistant
varieties (5): EC - 191 RC (AO),
EC - 394 RC (AO) and EC - 420 RC (AO) (2), still grown only
on a reduced area of the country. Meanwhile, biological control agents have
proven useful regarding disease severity and grain weight at field scale (15), although still
mostly preliminary. Regarding chemical control, many fungicide groups have
shown variable results (8, 22). Such variability
in disease control may be due to low fungicide efficacy or the impossibility of
accessing gynophores, the infection site for T. frezii (20). Considering this,
Paredes
et al. (2021) tested 12 different a.i. in vitro, pots and field
trials, using 1.5 times the recommended dose for the LLS control. Fungicides
were applied at night directly to the plant base and pegs in pot trials and
targeting the soil in field trials. These authors observed high disease control
with azoxystrobin (strobilurin) in pots and a 2016 field trial, and with
cyproconazole (triazole) in a 2015 field trial, while chlorothalonil did not
control peanut smut, probably given its limited mobility in the plant compared
to the other a.i. (6). The one product
registered against peanut smut, composed of two triazole fungicides
(triadimenol and myclobutanil), is ineffective against LLS (19).
Currently, chemical
control of fungal pathogens can be achieved by different target site
fungicides, depending on their mode of action. Fungicides with varying modes of
action can be used mixed or in alternating regimes on the same crop. Before
testing new a.i. against a given disease we should evaluate efficacy of
currently registered fungicides. Another key aspect is to evaluate dose and
number of applications with the lowest environmental impact. If some a.i.
registered for LLS could impact smut, a simultaneous control of both diseases
would be highly beneficial. Thus, we evaluated the effect of widely used
fungicides against LLS in peanut crops, simultaneously considering LLS and smut
intensities, and crop production.
Materials
and methods
Field trials were conducted during three consecutive seasons,
2017/18, 2018/19 and 2019/2020, in General Cabrera (GC) and Vicuña Mackenna
(VM), Córdoba, Argentina (table
1).
GC is representative of the historical peanut-producing area, while in VM,
peanut has been recently introduced. They have loam and sandy loam soil
texture, respectively.
Table 1. Coordinates,
sowing and harvest dates and accumulated rainfalls at both locations for three
agricultural seasons.
Tabla 1. Coordenadas,
fechas de siembra y cosecha y precipitaciones acumuladas para ambas localidades
durante las tres campañas agrícolas.
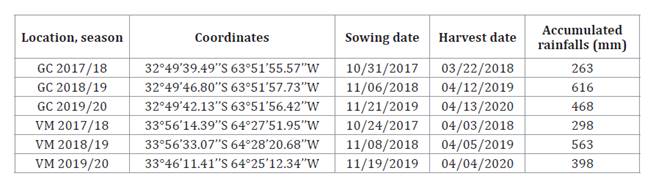
GC: General Cabrera. VM: Vicuña
Mackenna.
All trials followed a randomized complete block design with three
replications, and four furrows 5 m long, spaced 0.70 m. Ten treatments were
composed of different a.i. or mixtures, doses, and number of applications (table 2).
Table 2. Treatments at both
locations during three agricultural seasons.
Tabla 2. Tratamientos en ambas localidades durante tres campañas
agrícolas.
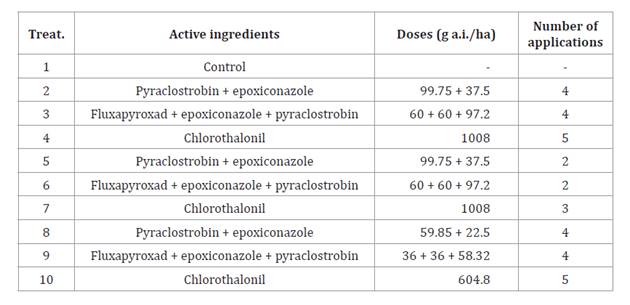
g a.i/ha: grams of active
ingredient per hectare.
Gramos de ingrediente activo por
hectárea.
All fungicides are registered in Argentina for LLS control (7). Applications were
performed with a carbon dioxide pressurized backpack sprayer equipped with six
hollow cone spray nozzles spaced 0.35 m apart, calibrated to 180 L/ha.
Applications began upon the first symptoms of LLS. Seeds of cv. Granoleico
(INASE Reg. N° 7907) were treated with 2.5 g (a.i.) of ipconazole + 2 g of
metalaxyl and 30 g of carboxin + 30 g of thiram per 100 kg of seeds, preventing
other soil pathogens and those carried by seeds.
Treatments 2, 3 and
4 implied using fungicides in the registered doses and number of applications,
considering residual periods. Treatments 5, 6 and 7 maintained doses but
reduced applications. Finally, treatments 8, 9 and 10, reduced a.i. dose to 60%
of the recommended. Treatments 5, 6, 7, 8, 9 and 10 tested whether a different
dose and application number was as efficient as the registered, representing a
more interesting option for peanut producers. However, risks of generating
resistance must be considered at reduced doses that should not be massively
adopted (as in treatments 8 to 10) (1). For all cases,
the Environmental Impact Quotient (EIQ) was calculated according to Kovach et
al. (1992), determining the environmental impact for each a.i. based on
physicochemical and toxicological information. This widely used indicator
evaluates pesticide risks and is useful for selecting less harmful molecules (13).
Before harvest, two
cotyledonary branches per plot were collected (one branch per central furrow)
and LLS intensity was calculated through incidence and severity. The first
represents the percentage of diseased leaflets and the last considers the
percentage of affected tissue. Incidence was calculated as the number of
leaflets with LLS spots over the total number of leaflets. Severity (S) was
calculated through the equation proposed by Plaut and Berger (1980): S = [(1-D)
* Sx] + D, considering defoliation (D), and average severity (Sx) (calculated
by a diagrammatic scale) (31).
At harvest maturity (150 days after planting), all plants in 1 m2
per plot were collected. Pods were separated and allowed to dry
until constant weight, in a dry and ventilated place. Once weighted and
shelled, yield was estimated via total and confectionery quality weights,
considering confectionary quality as those grains greater than 7,5 mm sieve
size. Simultaneously, peanut smut incidence (percentage of affected pods) and
severity (degree of symptoms in pods, through the disease severity index (DSI))
(27) were evaluated.
The DSI involves a five levels scale: 0 = healthy pod, 1 = normal pod with a
small sorus in a single seed, 2 = deformed or normal pod with one seed half
affected, 3 = deformed pod and a completely smutted seed, 4 = deformed pod,
both seeds completely smutted. The DSI was calculated using the following equation:
where:
n = number of pods
corresponding to each level (0-4)
N = total number.
For all parameters, ANOVA was performed, and means were compared
using Tukey’s test (p ≤ 0.05) with InfoStat software (12).
Results
and Discussion
According to a.i.,
concentration, dose and number of applications, EIQ values per treatment were:
1=0, 2=17.16, 3=21.80, 4=202.5, 5=8.58, 6=10.9, 7=121.23, 8=10.29, 9=13.08, and
10=121.5.
During the first
year, LLS was not observed given environmental conditions, mainly precipitation
(21). For the other
campaigns, the disease appeared in both locations with higher intensity values
in GC. In the GC 2018/19 trial (figure 1A), the lowest incidence levels were observed with chlorothalonil
in five applications (treatments 4 and 10), while severity was higher only in
the control (treatment 1). This agrees with Culbreath et al. (2018), who found
chlorothalonil more efficient than almost all evaluated triazoles. Concerning
the 2019/20 trial, treatment 4 had, once more, the best performance.
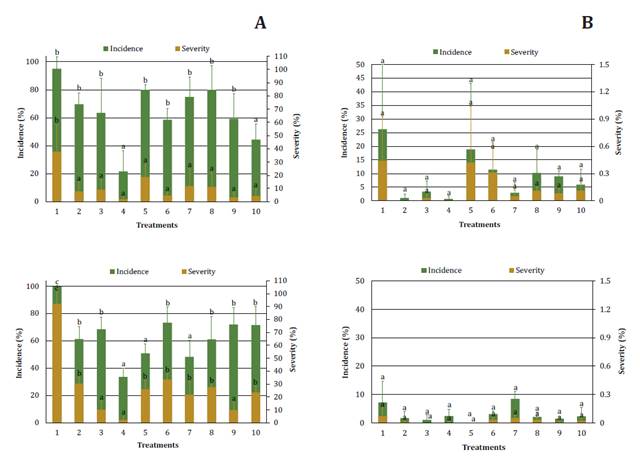
Tratamientos:
1) control sin fungicida; 2) pyraclostrobin + epoxiconazole 4 aplicaciones; 3)
fluxapyroxad + epoxiconazole + pyraclostrobin 4 aplicaciones; 4) clorotalonil 5
aplicaciones; 5) pyraclostrobin + epoxiconazole 2 aplicaciones; 6) fluxapyroxad
+ epoxiconazole + pyraclostrobin 2 aplicaciones; 7) clorotalonil 3
aplicaciones; 8) pyraclostrobin + epoxiconazole 4 aplicaciones, dosis reducida;
9) fluxapyroxad + epoxiconazole + pyraclostrobin 4 aplicaciones, dosis
reducida; y 10) clorotalonil 5 aplicaciones, dosis reducida. Letras diferentes
indican diferencias significativas (p<0,05).
Figure
1. Incidence and severity of late leaf spot in 2018/19
and 2019/20 on General Cabrera (A) and Vicuña Mackenna (B) field trials.
Figura
1. Incidencia y severidad de viruela
tardía en 2018/19 y 2019/20 en los ensayos de campo de General Cabrera (A) y
Vicuña Mackenna (B).
However, other
treatments, like treatment 4, achieved lower incidence (5: pyraclostrobin +
epoxiconazole in 2 moments, and 7: chlorothalonil in 3 moments), and severity
(3 and 9: fluxapyroxad + epoxiconazole + pyraclostrobin in 4 moments) levels.
These last results show effective disease management with mixes including
carboxamides, and better behavior with shorter periods between applications, as
previously found (10, 23). Treatments 8 to
10, with a.i. in reduced doses, achieved effective LLS control. However, it
should not constitute a strategy to be applied solely due to the possibility of
creating fungal resistance (1). Nevertheless,
treatments with fewer applications and thus, lower EIQ, represent a good option
considering environmental risk (13). Another
interesting fact is that treatments 4 and 10, with chlorothalonil, led to
better control than other a.i., but with higher EIQ values. However, this a.i.
has significantly low selection pressure (Fungicide Resistance Action
Committee, Code M5). On the other hand, no differences were evidenced among VM
treatments (figure
1B),
probably because of the low disease intensity registered in that location.
However, the highest LLS incidence and severity were observed without
fungicides (treatment 1).
Thecaphora frezii field inoculum was
quantified before planting according to Marinelli et al. (2008), estimating 10000
and less than 2000 teliospores per gram of soil in GC and VM, respectively.
Given this disease is less dependent on weather conditions than LLS, we could
evaluate smut intensity during three seasons in both locations (27). Intensity was
high in GC and moderate in VM (figure 2A and 2B), incidence reached 72.08% and
severity 2.34 in GC, while in VM, maximum values were 22.66% and 0.55,
respectively. These results may depend on GC long history of peanut cultivation
and processing, and thus, high inoculum (27).
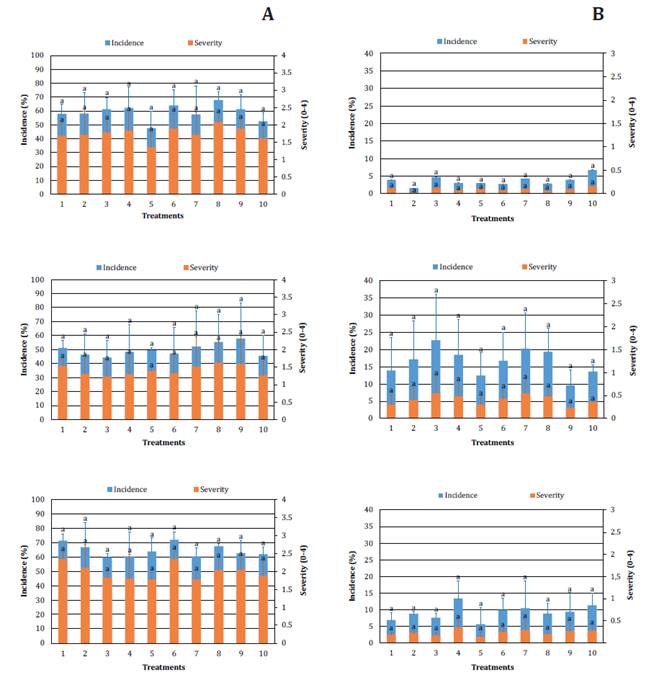
Tratamientos:
1) control sin fungicida; 2) pyraclostrobin + epoxiconazole 4 aplicaciones; 3)
fluxapyroxad + epoxiconazole + pyraclostrobin 4 aplicaciones; 4) clorotalonil 5
aplicaciones; 5) pyraclostrobin + epoxiconazole 2 aplicaciones; 6) fluxapyroxad
+ epoxiconazole + pyraclostrobin 2 aplicaciones; 7) clorotalonil 3
aplicaciones; 8) pyraclostrobin + epoxiconazole 4 aplicaciones, dosis reducida;
9) fluxapyroxad + epoxiconazole + pyraclostrobin 4 aplicaciones, dosis
reducida; y 10) clorotalonil 5 aplicaciones, dosis reducida. Letras diferentes
indican diferencias significativas (p<0,05).
Figure
2. Incidence and severity of peanut smut in 2017/18,
2018/19 and 2019/20 in General Cabrera (A) and Vicuña Mackenna (B) field
trials.
Figura
2. Incidencia y severidad de carbón
del maní en 2017/18, 2018/19 y 2019/20 en los ensayos de campo de General
Cabrera (A) y Vicuña Mackenna (B).
We did not observe
fungicide effect on disease intensity when compared to the untreated control
throughout all trials. Some authors (3, 4, 33) cite the action of
chlorothalonil, triazoles, strubilurins and carboxamides for controlling soil
pathogens. However, for peanut smut, effects are variable probably because of
low efficacy or inability to reach gynophores through spraying (8,
20, 25). We evaluated fungicides with different mobility in plants: a
non-penetrating a.i. (chlorothalonil) that cannot translocate through tissues
and penetrant and mobile a.i. (epoxiconazole, pyraclostrobin and fluxapyroxad)
transported through the xylem (6). These mobility differences
could help these a.i reach gynophores and stop infections. Paredes
et al. (2021) observed lower severity with azoxystrobin when compared to
other fungicides and control pots. On the other hand, smut intensity in
untreated control did not differ from treatments with chlorothalonil and
pyraclostrobin. These outcomes align with our findings. Finally, in field
trials, cyproconazole and azoxystrobin showed the best control efficiency among
all treatments.
Since peanut smut intensity is directly related to crop
production losses (25), and no
significant effect of fungicides was observed on the former, we expected no
differences in yield (table
3).
Table 3. Peanut
yield parameters (kg/ha) recorded in General Cabrera (GC) and Vicuña Mackenna
(VM) for the three agricultural seasons.
Tabla
3. Parámetros de rendimiento de maní (kg/ha) medidos en
General Cabera (GC) y Vicuña Mackenna (VM) para las tres campañas agrícolas.
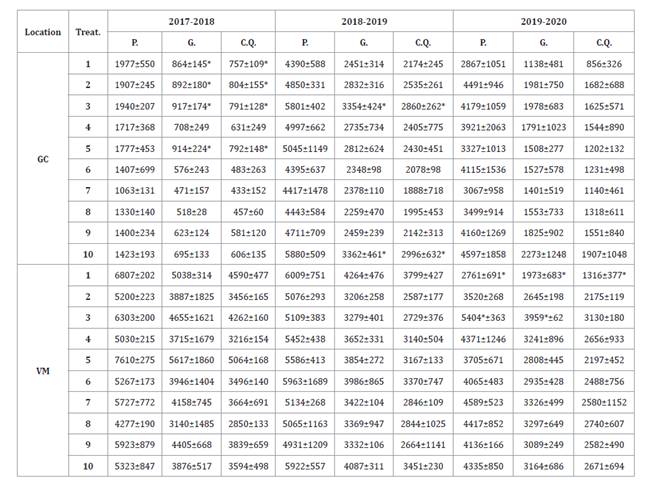
Pod (P.), grain (G.) and
confectionery quality grain (C.Q.) yields (kg/ha). Means ± standard deviation.
Treatments: 1) untreated control; 2) pyraclostrobin + epoxiconazole four times;
3) fluxapyroxad + epoxiconazole + pyraclostrobin four times; 4) chlorothalonil
five times; 5) pyraclostrobin + epoxiconazole twice; 6) fluxapyroxad +
epoxiconazole + pyraclostrobin twice; 7) chlorothalonil three times; 8)
pyraclostrobin + epoxiconazole four times, reduced dose; 9) fluxapyroxad +
epoxiconazole + pyraclostrobin four times, reduced dose; and 10) chlorothalonil
five times, reduced dose. Significant differences (p<0.05) per column
are represented by *.
Rendimientos (kg/ha) de vainas,
granos y granos calidad confitería. Medias ± error estándar. Tratamientos: 1)
control sin fungicida; 2) pyraclostrobin + epoxiconazole 4 aplicaciones; 3)
fluxapyroxad + epoxiconazole + pyraclostrobin 4 aplicaciones; 4) clorotalonil 5
aplicaciones; 5) pyraclostrobin + epoxiconazole 2 aplicaciones; 6) fluxapyroxad
+ epoxiconazole + pyraclostrobin 2 aplicaciones; 7) clorotalonil 3
aplicaciones; 8) pyraclostrobin + epoxiconazole 4 aplicaciones, dosis reducida;
9) fluxapyroxad + epoxiconazole + pyraclostrobin 4 aplicaciones, dosis
reducida; y 10) clorotalonil 5 aplicaciones, dosis reducida. Las diferencias
significativas (p<0,05) dentro de la misma columna, están
representados con *.
In GC, differences in grain and confectionery quality grain
yields were observed during the 2017-2018 season, where the untreated control
presented higher production than other treatments. These results could be given
by decreased yield when fungicides are applied to stressed plants (11). Treatments 1
(untreated control), 2, 3 and 5 had the highest total grain yield (864, 892,
917 and 914 kg/ha, respectively), and confectionery quality grain yield (757,
804, 791 and 792 kg/ha, respectively).
On the other hand,
during the 2018-2019 season, treatments 3 and 10 showed the highest grain yield
(3354 and 3362 kg/ha, respectively) and confectionery quality yield (2860 and
2996 kg/ha, respectively). Both treatments achieved lower LLS intensity than control,
as previously found (9, 23). Finally, during
the 2019-2020 season, no statistical differences were found for productivity
parameters in GC. For VM trials, statistical differences for crop yield were
only found in the 2019-2020 season. Treatment 1 had 22-49%, 25-50% and 40-58%
lower values than the rest of the treatments for pod, grain and confectionery
quality grain yields, respectively. In contrast, treatment 3 had 18-53% and
19-50% higher pod and grain yields than the other treatments. Finally, the
difference between treatments 1 and 3 was approximately 100%, as found by Culbreath
et al. (2018).
Considering each season, results were statistically different
between locations. Table
4
shows markedly higher LLS and peanut smut incidence and severity in GC than in
VM.
Table 4. LLS
intensity, peanut smut intensity and crop yield, across trials.
Tabla 4. Intensidad
de VT, carbón del maní y rendimiento del cultivo a lo largo de los años.
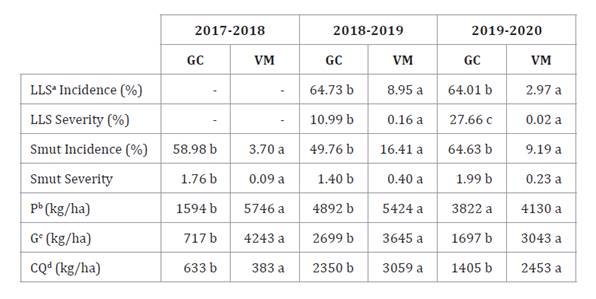
a Late leaf spot. b Pod
yield. c Grain yield. d Confectionery quality grains yield. Comparison
between locations partitioned by year. Different letters represent significant
differences (p<0.05).
a Viruela tardía. b Rendimiento
en vainas. c Rendimiento en granos. d Rendimiento
en granos calidad confitería. Comparación entre localidades, particionadas por
año. Letras diferentes representan diferencias significativas (p<0,05).
Although Paredes et al. (2024) report that peanut
smut benefits from drought, we did not observe any correlation between the
highest intensities and lowest rainfall, except for GC when comparing the first
and second seasons. However, this behavior is not linear and depends on whether
soil moisture falls below 30% of soil water-holding capacity and on which
growth stage (28). Regarding yield,
all values were superior in VM for all years. Disease intensity and crop yield
are possibly explained by cultivation history in each area.
Fungicide
application leads peanut LLS management. Additionally, considering peanut smut
is hard to control, having a fungicide against both diseases simultaneously
would be significantly useful. Considering two different sites and three
cropping seasons, this study showed how some majorly used fungicides for peanut
crops in Argentina could control LLS even at lower doses and application frames
than usual. However, these treatments proved no effects against peanut smut.
Further testing should consider different a.i., their combinations, doses and
application frames against LLS and peanut smut. Additionally, considering
genetic resistance and biocontrol strategies with microorganisms is key for
integrated management strategies.
Conclusions
Disease intensities of late leaf spot (LLS) and peanut smut are
closely linked to the agricultural history of locations and weather conditions
in a certain season. Chemical control of LLS has been effective, and certain
options exhibit a lower environmental impact, particularly important for
integrated management strategies. Conversely, fungicides demonstrated
inefficacy against peanut smut in these field trials. We also demonstrated the
importance of quantifying inoculum density given its direct relationship with
disease levels to avoid certain locations or choose resistant varieties.
Further studies on the biology of T. frezii and management of peanut
smut should contribute to genetic resistance development.
1. Amaradasa, B.
S.; Everhart, S. E. 2016. Effects of sublethal fungicides on mutation rates and
genomic variation in fungal plant pathogen, Sclerotinia sclerotiorum.
PLoS One. 11(12): e0168079. https://doi.org/10.1371/journal.pone.0168079
2. Anco, D. J.;
Thomas, J. S.; Jordan, D. L.; Shew, B. B.; Monfort, W. S.; Mehl, H. L.; Small,
I. M.; Wright, D. L.; Tillman, B. L.; Dufault, N. S.; Hagan, A. K.; Campbell,
H. L. 2020. Peanut yield loss in the presence of defoliation caused by late or
early leaf spot. Plant Disease. 104: 1390-1399.
https://doi.org/10.1094/PDIS-11-19-2286-RE
3. Augusto, J.;
Brenneman, T. B. 2011. Implications of fungicide application timing and
post-spray irrigation on disease control and peanut yield. Peanut Science.
38(1): 48-56. https://doi. org/10.3146/PS10-11.1
4. Augusto, J.;
Brenneman, T. B.; Culbreath, A. K.; Sumner, P. 2010. Night spraying peanut
fungicides. I. Extended fungicide residual and integrated disease management.
Plant Disease. 94(6): 676-682. https://doi.org/10.1094/PDIS-94-6-0676
5. Bressano, M.;
Massa, A.; Arias, R.; De Blas, F.; Oddino, C.; Faustinelli, P.; Soave, J.;
Soave, S.; Perez, A.; Sololev, V.; Marshall, C.; Balzarini, M.; Buteler, M.;
Seijo, G. 2019. Introgression of peanut smut resistance from landraces to elite
peanut cultivars (Arachis hypogaea L.). PLoS ONE. 14(2): e0211920.
https://doi.org/10.1371/journal.pone.0211920
6. Carmona, M.;
Sautua, F.; Pérez-Hérnandez, O.; Reis, E. M. 2020. Role of fungicide
applications on the integrated management of wheat stripe rust. Frontiers in
Plant Science. 11: 733. https:// doi.org/10.3389/fpls.2020.00733
7. CASAFE. 2023.
Cámara de Sanidad Agropecuaria y Fertilizantes. Guía online de productos
fitosanitarios. https://guiaonline.casafe.org/ (Accessed on: Sep. 6 2023).
8. Cazón, I.;
Bisonard, E. M.; Conforto, C.; March, G.; Rago, A. 2013. Estrategias para el
manejo del carbón del maní. Actas de resúmenes XXVIII Jornada Nacional del
Maní. General Cabrera, Córdoba. Argentina. p: 28-30.
9. Culbreath, A.
K.; Gevens, A. J.; Stevenson, K. L. 2018. Relative effects of
demethylation-inhibiting fungicides on late leaf spot of peanut. Plant Health
Progress. 19(1): 23-26. https://doi. org/10.1094/PHP-09-17-0053-RS
10. Culbreath, A.
K.; Brenneman, T. B.; Kemerait, R. C.; Stevenson, K. L.; Henn, A. 2020. Effect
of DMI and QoI fungicides mixed with the SDHI fungicide penthiopyrad on late
leaf spot of peanut. Crop Protection. 137: 105298.
https://doi.org/10.1016/j.cropro.2020.105298
11. Dias, M. A.
2012. Phytotoxicity: An overview of the physiological responses of plants
exposed to fungicides. Journal of Botany. Article ID 135479.
https://doi.org/10.1155/2012/135479
12. Di Rienzo, J.
A.; Casanoves, F.; Balzarini, M. G.; Gonzales, L.; Tablada, M.; Robledo, C. W.
InfoStat versión 2020. Centro de Transferencia InfoStat, FCA, Universidad
Nacional de Córdoba, Argentina. http://www.infostat.com.ar
13. Dugan, S. T.;
Muhammetoglu, A.; Uslu, A. 2023. A combined approach for the estimation of
groundwater leaching potential and environmental impacts of pesticides for
agricultural lands. Science of The Total Environment. 901: 165892. https://doi.org/10.1016/j. scitotenv.2023.165892
14. Fulmer, A. M.
2017. Differentiation, prediction and management of early and late leaf spot of
peanut in the southeastern United States and Haiti. Ph.D. thesis. University of
Georgia, Athens, GA.
15. Ganuza, M.;
Pastor, N.; Erazo, J.; Andrés, J.; Reynoso, M.; Rovera, M.; Torres, A. 2018.
Efficacy of the biocontrol agent Trichoderma harzianum ITEM 3636 against
peanut smut, an emergent disease caused by Thecaphora frezii. European
Journal of Plant Pathology. 151(1): 257-262.
https://doi.org/10.1007/s10658-017-1360-0
16. Giordano, D.F.;
Pastor, N.; Palacios, S.; Oddino, C.; Torres, A. 2021. Peanut leaf spot caused
by Nothopassalora personata. Tropical plant pathology. 46: 139-151.
https://doi. org/10.1007/s40858-020-00411-3
17. Jordan, B. S.;
Culbreath, A. K.; Brenneman, T. B.; Kemerait, R. C.; Branch, W. D. 2017. Late
leaf spot severity and yield of new peanut breeding lines and cultivars grown
without fungicides. Plant Disease. 101(11): 1843-1850.
https://doi.org/10.1094/PDIS-02-17-0165-RE
18. Kovach, J.;
Petzoldt, C.; Degni, J.; Tette, J. 1992. A method to measure the environmental
impact of pesticides. New York’s Food and Life Sciences Bulletin. 139: 1-8.
19. Laboratorios
NOVA. 2023. IRIDIUM.
https://laboratorios-nova.com/fungicidas-fungicidas-insecticidas/iridium/
(Accessed on Sep. 12 2024).
20. Marinelli, A.;
March, G.; Oddino, C. 2008. Aspectos biológicos y epidemiológicos del carbón
del maní (Arachis hypogaea L.) causado por Thecaphora frezii Carranza
& Lindquist. AgriScientia. 25(1): 1-5.
21. Marinelli, A.;
Oddino, C.; March, G. 2017. 2ª ed. Enfermedades fúngicas del maní. En:
Fernández, E.; Giayetto, O. (Ed.). El cultivo de maní en Argentina. Río Cuarto,
Córdoba. Ediciones UNRC. p: 285-311.
22. Oddino, C.; Mortigliengo, S.; Moresi, A.; Soave, J.;
Giuggia, J.; Ferrari, S.; Cassano, C.; Martinez, F.; Molineri, A.; Moran, F.;
Soave, S.; Torre, D.; Butteler, M.; Bianco, C.; Bressano, M.; De Blas, F. 2017.
Efecto de fungicidas foliares sobre la intensidad de viruela y carbón en
diferentes cultivares de maní. Ciencia y tecnología de Cultivos Industriales.
6(9): 99-105.
23. Oddino, C.;
Giordano, F.; Paredes, J.; Cazón, L.; Giuggia, J.; Rago, A. 2018. Efecto de
nuevos fungicidas en el control de viruela del maní y el rendimiento del
cultivo. Ab Intus. 1(1): 9-17.
24. Oddino, C.;
Rosso, M.; Soave, J.; Soave, S.; Mendoza, M.; Giordano, D. F.; Bressano, M.; De
Blas, F.; Mortigliengo, S.; Butteler, M. 2023. Comportamiento de variedades de
maní resistentes a carbón a través de los años. Actas de resúmenes XXXVIII
Jornada Nacional del Maní. General Cabrera, Córdoba. Argentina.
25. Paredes, J.
2017. Importancia regional del carbón del maní (Thecaphora frezii) y
efecto de ingredientes activos de fungicidas sobre la intensidad de la
enfermedad. Master thesis. Universidad Nacional de Río Cuarto, Córdoba.
26. Paredes, J. A.;
Cazón, L. I.; Oddino, C.; Monguillot, J. H.; Rago, A. M.; Edwards Molina, J. P.
2021. Efficacy of fungicidal management of peanut smut. Crop Protection. 140:
105403. https:// doi.org/10.1016/j.cropro.2020.105403
27. Paredes, J. A.;
Edwards Molina, J. P.; Cazón, L. I.; Asinari, F.; Monguillot, J. H.;
Morichetti, S. A.; Rago, A. M.; Torres, A. M. 2022. Relationship between incidence
and severity of peanut smut and its regional distribution in the main growing
region of Argentina. Tropical Plant Pathology. 47: 233-244.
https://doi.org/10.1007/s40858-021-00473-x
28. Paredes, J. A.;
Guzzo, M. C.; Monguillot, J. H.; Asinari, F.; Posada, G. A.; Oddino, C. M.;
Giordano, D. F.; Morichetti, S. A.; Torres, A. M.; Rago, A. M.; Monteoliva, M.
I. 2024. Low water availability increases susceptibility to peanut smut (Thecaphora
frezzii) in peanut crop. Plant Pathology. 73(2): 316-325. https://doi.org/10.1111/ppa.13810
29. Plaut, J. L.;
Berger, R. D. 1980. Development of Cercosporidium personatum in three
peanut canopy layers. Peanut Science. 7(1): 46-49.
https://doi.org/10.3146/i0095-3679-7-1-11
30. Rago, A.;
Cazón, I.; Paredes, J.; Edwards Molina, J.; Bisonard, M.; Oddino, C. 2017.
Peanut Smut: From an emerging disease to an actual threat to Argentine peanut
production. Plant Disease. 101(3): 400-408.
http://dx.doi.org/10.1094/PDIS-09-16-1248-FE
31. Shokes, F. M.;
Berger, R. D.; Smith, D. H.; Rasp, J. M. 1987. Reliabity of disease assessment
procedures. A case study with late leafspot of peanut. Oléagineux. 42: 245-251.
32. USDA. 2023.
United States Department of Agriculture. Peanut explorer. https:// ipad.fas.
usda.gov/cropexplorer/cropview/commodityView.aspx?cropid=2221000&sel_
year=2022&rankby=Production (Accessed on Sep. 12 2023).
33. Woodward, J. E.; Brenneman, T. B.; Kemerait, R. C.; Smith,
N. B.; Culbreath, A. K.; Stevenson, K. L. 2008. Use of resistant cultivars and
reduced fungicide programs to manage peanut diseases in irrigated and
non-irrigated field. Plant Disease. 92(6): 896-902. http://dx.doi.
org/10.1094/PDIS-92-6-0896