Revista de la Facultad de Ciencias
Agrarias. Universidad Nacional de Cuyo. En prensa. ISSN (en línea) 1853-8665.
Original article
Translocation
and experimental adaptation of Distichia muscoides cushions in a wetland
impacted by acid rock drainage, Ancash, Peru
Translocación
y adaptación experimental de cojines de Distichia muscoides en un
bofedal impactado con drenaje ácido de roca, Áncash, Perú
Maria Cristina
Otoya Fernández1*,
Yeidy Montano2,
Marilín
Sánchez-Purihuamán3,
Pedro M. Tapia4,
Junior Caro-Castro5,
Carmen
Carreño-Farfán6
1 Universidad Nacional Pedro Ruiz Gallo. Facultad de Ciencias
Biológicas. Laboratorio de Investigación Biotecnología Microbiana.
Lambayeque-Perú.
2 Instituto Nacional de Investigación en Glaciares y Ecosistemas
de Montaña. Huaraz. Ancash-Perú.
3 Universidad Nacional Pedro Ruiz Gallo. BlyME: BS-CA. Grupo de
Investigación Biodiversidad y Manejo Ecológico del Bosque Seco y Cultivos
Tropicales. Lambayeque-Perú.
4 Universidad Peruana Cayetano Heredia. Facultad de Ciencias e
Ingeniería. Lima-Perú.
5 Universidad Nacional Mayor de San Marcos. Facultad de Ciencias
Biológicas. Laboratorio de Ecología Microbiana. Lima-Perú.
6 Universidad Nacional Pedro Ruiz Gallo. BlyME: BS-CA. Facultad
de Ciencias Biológicas. Laboratorio de Investigación Biotecnología Microbiana.
Lambayeque-Perú.
*crisotofer13@gmail.com
Abstract
The deglaciation of
the Andean mountain range negatively impacts ecosystems and water bodies,
primarily increasing the concentration of heavy metals. However, their
concentration can be reduced by applying bioremediation techniques. The
objective of this study was to evaluate the effect of the translocation and
adaptation of Distichia muscoides cushions in a wetland impacted by acid
rock drainage in a high Andean region. For this purpose, the characteristics of
water, peat, and D. muscoides tissue were compared in two wetlands, and
the behavior of translocated D. muscoides was evaluated based on the
bioaccumulation and translocation factors of metals. The quantification of Al,
Fe, and Mn in peat, root, and aerial tissue of D. muscoides showed
higher concentration values after the translocation of the cushions.
Additionally, the bioaccumulation factor classified the transplanted cushions
as accumulators of Al, Cu, As, Fe, Mn, and Zn, while the translocation factor
classified the cushions as phytoextractors of Al, As, Cr, Fe, Mn, and Zn, and
phytostabilizers of Pb and Cu. It is concluded that translocated and adapted D.
muscoides cushions have potential for the bioremediation of wetlands
contaminated with acid rock drainage.
Keywords: Bioremediation,
wetland, acid rock drainage, Distichia muscoides, heavy metals
Resumen
La desglaciación de la cordillera andina impacta de manera
negativa los ecosistemas y cuerpos de agua, incrementando la concentración de
metales pesados como principal consecuencia; sin embargo, su concentración
puede ser disminuida aplicando técnicas de biorremediación. El objetivo de este
trabajo fue evaluar el efecto de la translocación y adaptación de cojines de Distichia
muscoides en un bofedal impactado con drenaje ácido de roca en una región
altoandina. Para ello, se compararon las características del agua, turba y
tejido de D. muscoides en dos bofedales, evaluando el comportamiento de D.
muscoides traslocados basados en los factores de bioacumulación y
traslocación de metales. La cuantificación de Al, Fe y Mn en turba, tejido
radicular y aéreo de D. muscoides demostró mayores valores de
concentración después de la traslocación de los cojines. Por otro lado, el
factor de bioacumulación calificó los cojines trasplantados como acumuladores
de Al, Cu, As, Fe, Mn, Zn, mientras que el factor de traslocación calificó a
los cojines como fitoextractores de Al, As, Cr, Fe, Mn, Zn, y
fitoestabilizadores de Pb y Cu. Se concluye que los cojines de D. muscoides traslocados
y adaptados tienen potencial para la biorremediación de bofedales contaminados
con drenaje ácido de roca.
Palabras clave: Biorremediación,
bofedales, drenaje ácido de roca, Distichia muscoides, metales pesados
Originales: Recepción: 27/02/2024 - Aceptación: 23/09/2024
Introduction
The Huascaran
Biosphere Reserve is a natural heritage site located in Ancash, Peru,
encompassing an area of 1,155,800 hectares. This
includes the core area, Huascaran National Park (HNP), which contains 95% of
the Cordillera Blanca (11). Between 1962 and
1970, the Cordillera Blanca covered 723 km² and 658 km², respectively (6). However, by 2016,
the glacial area had decreased to 448.81 km², representing a 38.2% reduction
and a loss of 277.45 km² (38). Additionally, in
the Santa River, which is fed by western glaciers, heavy metals have been
detected at levels exceeding the Maximum Permissible Limits (MPL) of the
Environmental Quality Standards (EQS) for Water. This contamination results
from glacial erosion, rock weathering, and anthropogenic activity (18,
39).
The retreat of
Andean glaciers exposes rock material that typically contains sulfide minerals
(pyrite), which, when oxidized and leached, generates acid rock drainage (ARD)
with an acidic pH and high metal concentrations, impacting water bodies and
ecosystems (17). The ARD formation
process begins when sulfide minerals, exposed to atmospheric oxygen, become
unstable and oxidize (34). The oxidation of
pyrite, the main mineral responsible for ARD generation, requires oxygen and
water and can be accelerated by microbial action. Metals in ARD originate from
the oxidation of sulfides and the dissolution of acid-consuming minerals. ARD
has an acidic pH, a high concentration of sulfates, and primarily dissolved
metals such as iron (Fe) and aluminum (Al). However, trace metals like lead
(Pb), zinc (Zn), copper (Cu), cadmium (Cd), manganese (Mn), cobalt (Co), and
nickel (Ni) can reach high concentrations on certain occasions (38). Heavy metals are
persistent pollutants because they bioaccumulate, are not biodegradable, and
are highly toxic even at low concentrations, affecting plants, animals, and
humans (27).
Wetlands are
characteristic of the tropical and subtropical Andes (5,
36), where cushion plants such as Distichia muscoides and Oxychloe
andina are found. These are primary species of the Andean Altiplano with
the capacity to store water and elevate it above the groundwater level (31). These cushion
plants regulate water release, act as sinks for organic carbon, and promote
wildlife (7, 20). However, they are threatened by
glacial retreat (20), overgrazing,
anthropogenic extraction, and heavy metal contamination from ARD (10,
31, 36). Previous studies conducted in the Cordillera Blanca, Ancash,
Peru, have demonstrated the effectiveness of transplanting species like D.
muscoides to accelerate the bioremediation of environments impacted by ARD (17,
38).
Based on previous
evidence, bioremediation is proposed as a solution, involving the application
of microorganisms, plants, or derived enzymes for environmental restoration.
This approach relies on the biological entities’ ability to reduce or eliminate
contaminants (12). Phytoremediation,
the use of plants for bioremediation, allows them to absorb, mobilize, and
accumulate heavy metals and other contaminants through strategies like phytoextraction,
phytostabilization, and phytovolatilization (16,
28). In phytoextraction, metals are absorbed by roots, transported,
and accumulated in stems and leaves. Phytostabilization reduces contaminant
mobility and prevents migration to groundwater (24,
30). Phytovolatilization involves the absorption of metals by
roots, transport via the xylem, and release from the aerial parts of plants (23).
The response of
plants to heavy metals classifies them as excluders, indicators or accumulators.
Excluders have lower metal accumulation in their above-ground parts compared to
the soil concentration. Indicators maintain a direct relationship between metal
concentration in their above-ground parts and the soil. Accumulators have
higher metal concentrations in their above-ground parts compared to the soil.
To implement phytoremediation through phytostabilization, excluder plants are
used, which accumulate metals in their roots, while for phytoextraction,
accumulator plants are necessary to transport metals to their above-ground
parts (1, 16, 24).
Plants like D. muscoides are efficient for the
stabilization and extraction of heavy metals (17);
however, it is necessary to analyze the technical, ecological, and economic
feasibility before their use, considering the conditions of the damaged
ecosystem and the possibilities of application (12).
Therefore, the objective of this study was to evaluate the effect of translocating
and establishing D. muscoides cushions in a wetland impacted by ARD
located in the Ancash region, Peru, aiming to remediate ARD-affected wetlands.
The specific objectives were: a) to compare the characteristics of wetlands
unaffected and affected by ARD, b) to monitor the adaptation of translocated D.
muscoides cushions by comparing the concentration of heavy metals, and c)
to determine the behavior of translocated cushions in an ARD-impacted wetland.
Materials
and methods
Comparison
of wetlands affected and unaffected by ARD
Study area
The study was
conducted within HNP, in the district of Catac, province of Recuay, Ancash
region. Two wetlands were selected, designated as wetland 1 and 2, both
predominantly populated with D. muscoides cushions. Three zones (A, B,
and C) were established within each wetland (figure 1), based on pH
conditions (3.95-7.27), temperature (8.7- 14.7 °C), altitude (4506-4818 meters
above sea level), and the condition of the D. muscoides cushions. For
sampling in each zone of both wetlands, nine sampling points were considered
(A1, A2, A3, B1, B2, B3, C1, C2, and C3) with a 50 cm separation between them.
Each wetland involved nine samples of root tissue, nine of aerial tissue, three
water samples, and three peat samples.
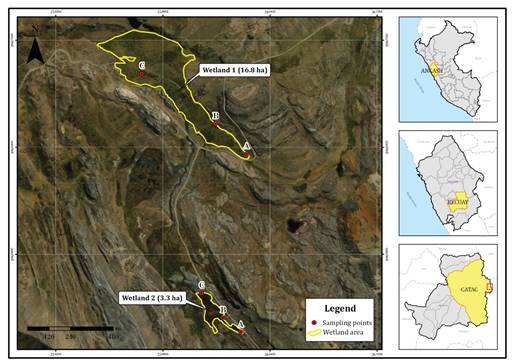
Figure 1. Location
of the sampling zones in wetlands 1 and 2.
Figura
1. Ubicación de las zonas de muestreo
en los bofedales 1 y 2.
Sampling of
water from the wetlands
Water quality was
assessed according to the National Protocol for the Quality of Natural Bodies
of Surface Water (3). In each wetland,
three water samples were collected in October 2021. Five-hundred mL of water
were collected using sterile polyethylene bottles placed at the imaginary
triangle center formed by the three sampling points. The samples were taken
against the water flow direction at a depth of 20-30 cm, with the addition of 1
mL of nitric acid as a chemical preservative. Physicochemical parameters such
as pH and temperature were measured in each wetland water sample using a
handheld meter (HANNA HI8424 model).
Sampling of
peat, root and foliage of D. muscoides
Sampling of organic peat was conducted following the established
technique in the Peruvian Soil Sampling Guide (21).
Nine cushions of D. muscoides were selected in each wetland, one per
sampling point, at a depth of 30 cm. From each cushion, a rectangle of 20x15 cm
consisting of peat, roots, and aerial tissue was extracted. In the laboratory,
peat samples from each zone (three sampling points) were mixed, and a 300 g
sample of the most decomposed peat was selected, totaling three samples per
wetland. Root tissue was separated from aerial tissue, and 250 g from each
rectangle was weighed, resulting in nine samples per wetland.
The concentration of heavy metals in all samples was determined
using Inductively Coupled Plasma Atomic Emission Spectrometry (ICP-AES) for
water according to method EMW 200.7 (19).
For peat, root tissue, and aerial tissue, method 3050-B (2)
was employed. Metal concentration values in water were compared with EQS fow
water, Category 4, Subcategory 1 (22).
Presence of sulfate-reducing bacteria
in the sediment of water bodies
For sampling, three
zones were considered in each wetland, with two sampling points in each zone
(A1, A2, B1, B2, C1, and C2). Samples of 1000 g of sediment from the water
bodies were collected using an auger that extracts perforated solid material (37) at a depth of 70
cm. The samples were immediately deposited into transparent glass jars and
transported in a thermal box (10±1°C) to the laboratory, where enrichment of
sulfate-reducing bacteria (SRB) was conducted in twelve Winogradsky columns (25).
Translocation
and adaptation of D. muscoides and
comparison of heavy metal concentrations
Translocation
and adaptation of D. muscoides
The phase of translocation or transplantation and adaptation of D.
muscoides took place from December 2021 to May 2022 in zone B, the
experimental area of wetland 2, previously delineated (total area: 7.7 ha).
This area is traversed by water from Pastoruri Creek, covering an area of 5x5
m² (georeferenced points E: 260088 and N: 8905452, at an altitude of 4804
meters above sea level). Eight cushions were selected from zones A and B of
wetland 1, located at the edge in contact with the water, from which a rectangle
(30 x 15 cm) consisting of roots, aerial tissue, and root peat was extracted to
a depth of 30 cm. Among the eight rectangles extracted from the cushions, five
were designated for translocation and three for the initial analysis of heavy
metal concentration in peat, root tissue, and aerial tissue before
transplantation. Monitoring of the adaptation of transplanted cushion rectangles
was conducted over 5 months, during what is known as the rainy season due to
the significant influence of wetland water levels (7).
Monitoring occurred every 10 days during the first two months and subsequently
every 15 days from the third to the fifth month. Vigor and persistence of green
color were evaluated in each cushion rectangle.
After 5 months of
transplantation, a random sampling of three cushions was conducted from among
the five that were transplanted. The presence of few, moderate, or abundant
roots allowed for assessing the adaptation of the cushions to the
transplantation. In contrast, the absence of roots indicated inadequate
adaptation of the cushions to the transplantation.
Comparison
of heavy metal concentrations
The concentration
of heavy metals in the peat, root tissue, and aerial tissue of three D.
muscoides cushions before translocation was compared with the concentration
determined in three cushions 5 months after translocation. Additionally, the
concentration of heavy metals in the peat, root tissue, and foliage of three
translocated cushions was compared with that in three native cushions (not
transplanted) from the wetland impacted by ARD where the translocation
occurred.
Behavior
of translocated cushions in the wetland impacted by ARD
The behavior of transplanted D. muscoides was evaluated
using the bioaccumulation factor (BAF) and translocation factor (TF) (30)
of the root tissue and foliage of both translocated and non-translocated D.
muscoides.

Statistical
analysis
The concentrations
of heavy metals (mg/kg) in peat, root tissue, and foliage before and after the
translocation of D. muscoides were analyzed using the paired Student’s
t-test for normally distributed samples and the Wilcoxon test for samples with
a non-normal distribution. Additionally, the concentrations of heavy metals
(mg/kg) in translocated and non-translocated cushions were analyzed using the
one-sample Student’s t-test. A significance level of 0.05 was applied to all
statistical tests. The analyses were conducted using RStudio 2021.09.
Results
and discussion
Characteristics
of the studied wetlands
Wetland 2 was
classified as impacted by ARD based on the acidic pH of the water, higher
concentrations of metals in both the water and tissues of D. muscoides,
as well as the presence of sulfate-reducing bacteria (table 1).
Table 1. Concentration
of heavy metals (mg/kg) in D. muscoides from the evaluated wetlands.
Tabla
1. Concentración de metales pesados
(mg/kg) en D. muscoides procedentes de los bofedales evaluados.
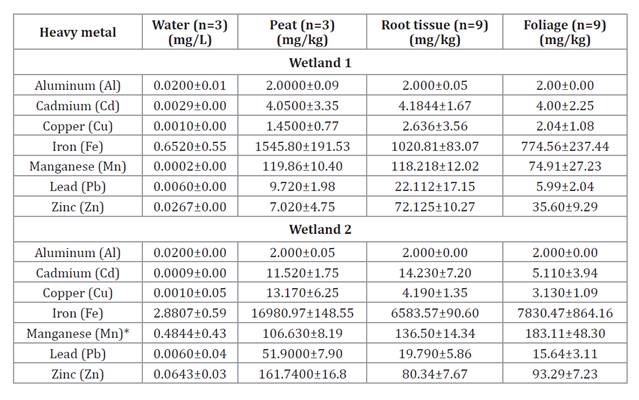
*Peruvian EQS: Category 4,
subcategory 1: 0.2 mg/L.
*ECA peruano: Categoría 4,
subcategoría 1: 0,2 mg/L.
The pH range of
water in wetland 2, impacted by ARD (3.95 - 6.35), was more acidic compared to
wetland 1, which showed no apparent impact from ARD (water: 7.13 - 7.30). In
ARD-impacted environments, a permanent acidity (3.54-4.47) has been reported (39), or significantly
lower pH (3.60-4.42) compared to non-ARD water (6.95-7.13) (15). The acidic pH is
a consequence of ARD, resulting from glacier retreat and subsequent oxidation
of exposed mineralized rocks. The acidic pH increases the availability of
dissolved metal ions in the water, thereby increasing toxicity to living
organisms (38).
Neutralization
reactions also influence ARD. While most carbonate minerals dissolve rapidly,
hydrolysis of Fe or Mn, following the dissolution of their respective
carbonates and subsequent precipitation, can generate acidity (34). The concentration
of metals in the water of wetland 2 (Fe, Mn, Zn) was higher than in wetland 1.
0.4844 mg/L of Mn was quantified in wetland 2, contrasting with wetland 1
(0.0002 mg/L), a value that exceeds the EQS for water, Category 4 (22).
Regarding water temperature, the ranges were similar in wetlands
1 and 2 (10.5 - 12.3 ˚C and 8.7 - 14.7 ˚C, respectively). Similarly, in the
Quillcay basin, Ancash region, it was determined that water temperature
remained constant throughout the year at different sampling points, thus not
contributing to differences in water quality found in potentially ARD-affected
and unaffected areas (15).
The concentration
range of metals in the peat (Cd, Cu, Fe, Pb, Zn), root tissue (Cu, Fe, Zn, Cd,
Mn), and foliage (Cd, Fe, Mn, Zn) in wetland 2 was higher than that in wetland
1 (table
1).
Fe and Al are the main metals dissolved in ARD, as previously reported by Zimmer et
al. (2018). However, the high concentration of heavy metals in the peat
and tissues of D. muscoides suggests that these plants are adapted to
decontamination efforts (10). Al, Fe, and Mn
are the heavy metals quantified in higher concentrations in D. muscoides exposed
to ARD in an artificial wetland, with other metals such as Cu, Cd, Fe, Ni, and
Zn also present (17).
Regarding the
Winogradsky columns from sediment in water bodies, several characteristics were
observed related to coloration, gas production, and turbidity, verifying the
presence of sulfate-reducing bacteria (BSR), similar to Winogradsky columns
processed with residual sludge from wastewater treatment (25). The black
coloration of the sulfate-reducing zone results from sulfide precipitation
with reduced metals such as iron, which deposits at the bottom of the columns (29). BSR produce
approximately 2 moles of alkalinity per mole of reduced sulfate, thereby
neutralizing the pH of acidic waters. Additionally, the generated bicarbonate
ions consume protons and raise the pH of acidic water (8,
33). In Wetland 2, the presence of BSR was confirmed in 66.7% of
the Winogradsky columns, compared to 16.67% in columns prepared from wetland 1
samples. This difference may be attributed to the greater impact of ARD in
wetland 2. As a consequence of increased oxidation of sulfide minerals, sulfate
concentrations rise, which are used as electron acceptors by BSR, while organic
matter in lower layers of the peat serves as a carbon source (38).
Translocation
and adaptation of D. muscoides and comparison of heavy metal
concentrations
From days 20 to 80, the vigor and green color persisted in all
cushions. By day 100, there was no discernible difference in vigor and color
due to flooding. From days 120 to 160, a blackish coloration appeared on the
edges and middle tissue of all translocated rectangles. At day 160, it was
determined that the green coverage ranged from 34.3% to 97.9%, and the black
coverage from 2.1% to 65.7% in the translocated cushions, compared to 100%
green coverage in the controls. The major radius of the translocated cushions
ranged from 26 to 38 cm, and the minor radius from 21 to 44 cm, compared to
30-32 cm and 20-21 cm in the non-translocated controls. Additionally, a regular
to abundant number of roots was observed in the translocated cushions compared
to the abundant root presence in the controls.
The growth of D.
muscoides is very slow, requiring more than 5 months for translocated
cushions to develop morphology similar to non-translocated ones. A growth rate
of 1-2 cm per year has been reported, with an increase in height of more than 1
cm in summer and less than 1 cm in winter over 6 months (7). In contrast, the
root production of D. muscoides was 2000-2800 g m-2 year-1,
a range that exceeds other cushion species such as Plantago rigida,
which produces 1000-1080 g m-2 year-1 (32). In areas with
shallow and stable water tables, D. muscoides is dominant and exhibits a
high capacity for peat accumulation, owing to its abundant underground biomass
that can facilitate the adaptation of translocated plants (26). The establishment
of translocated D. muscoides cushions was evidenced by both growth and
the accumulation of metals in their tissues. Thus, it was demonstrated that translocation
is a technique with potential for the recovery of impacted wetlands, as also
corroborated by Luna
(2018),
who collected and transplanted D. muscoides cushions to artificial
wetlands and determined a growth of 3.3 cm in roots and 2.8 cm in foliage after
9 months. Additionally, the pH of ARD at the inlet of the wetland was 2.9-3.6
and at the outlet was 3.87-5.30, indicating a decrease in water acidity,
although the level achieved was lower than the EQS for water (6.5-8.5).
Cushions
before and after translocation
The t-Student analysis for related samples revealed
statistically significant differences (p<0.05) in the concentrations of Al,
Fe, and Mn in peat, root tissue, and foliage before and after transplantation.
The concentrations of these metals were higher after transplantation (figure
2).
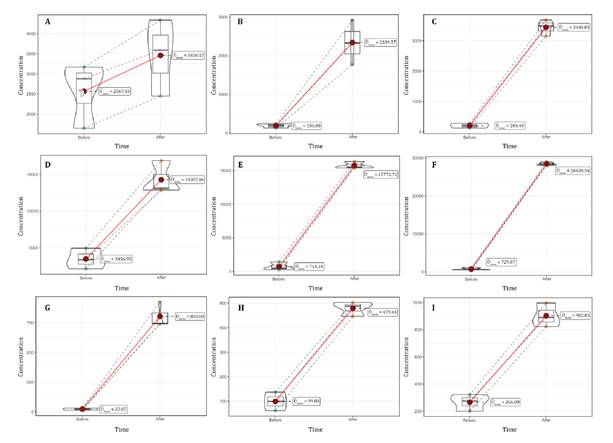
Figure 2. Concentration
of heavy metals (mg/kg) in D. muscoides before and after transplantation
(five months). A. Al (peat). B. Al (root). C. Al (foliage). D. Fe (peat). E. Fe
(root). F. Fe (foliage). G. Mn (peat). H. Mn (root). I. Mn (foliage).
Figura 2. Concentración
de metales pesados (mg/kg) en D. muscoides antes y después del
trasplante (cinco meses). A. Al (turba). B. Al (raíz). C. Al (follaje). D. Fe
(turba). E. Fe (raíz). F. Fe (follaje). G. Mn (turba). H. Mn (raíz). I. Mn
(follaje).
In contrast, the
concentrations of Cr and Ar in peat, root tissue, and foliage before and after
transplantation were statistically equal (table 2, p > 0.05). On
the other hand, concentrations of Pb were statistically different in peat;
those of Zn were statistically different in root and aerial tissues; and those
of Cu differed in peat and foliage. However, the Wilcoxon analysis showed that
Cu concentrations in root tissue before and after transplantation were
statistically equal (p > 0.05).
Table 2. Concentrations
of heavy metals (mg/kg) before and after transplantation of D. muscoides.
Tabla 2. Concentraciones
de metales pesados (mg/kg) antes y después del trasplante de D. muscoides.
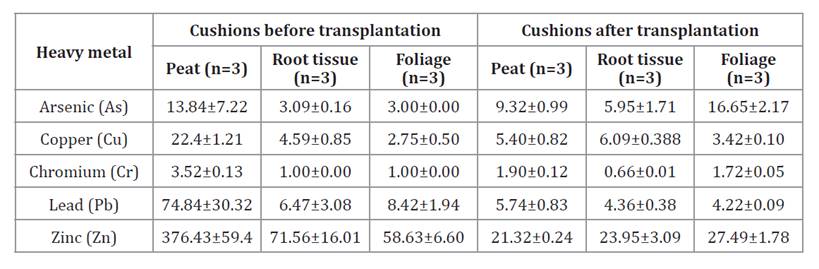
The presence of
metals in D. muscoides tissues before and 5 months after translocation
demonstrated that macrophytes can easily absorb bioavailable metals (As, Cd,
Cu, Se, Ni, Zn), moderately bioavailable metals (Co, Fe, Mn, Hg), and poorly
bioavailable metals (Cr, Pb) from water sediments, accumulating, translocating,
and eventually storing them (4, 35). This capacity
persisted in the translocated D. muscoides cushions. The ability to
accumulate metals in translocated D. muscoides cushions corresponds with
Luna
(2018),
who quantified Al, Cd, Cu, Fe, Mn, Ni, and Zn in plants of this species 9
months after transplantation to artificial wetlands.
Translocated
and non-translocated cushions in the ARD-impacted wetland
The t-Student
analysis of a single sample demonstrated that the concentration of heavy metals
was statistically different (p<0.05) in the peat, root tissue, and foliage
of D. muscoides in the ARD-impacted wetland (figure 3).
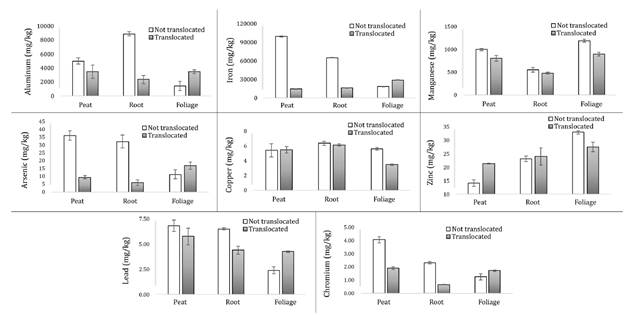
Figure 3. Concentration
of heavy metals (mg/kg) in non-translocated and translocated D. muscoides cushions.
Figura
3. Concentración de metales pesados (mg/kg) en cojines
de D. muscoides no translocados y translocados.
The concentrations
of Al, Fe, Cr, As, Zn, Cu, and Mn were higher in the foliage of translocated D.
muscoides cushions compared to the peat and root tissue. In contrast, the
concentrations of Al, Fe, Pb, Cr, and As in the peat and root tissue of
non-translocated cushions were higher than in the foliage. These results
indicate the absorption and movement of metals within the plant tissues of
translocated D. muscoides cushions, where the concentrations of Cu in
peat, root tissue, and foliage; Zn in peat and root tissue; and Al, Fe, Pb, Cu,
and As in foliage were higher than in non-translocated cushions. The
accumulation and translocation of Al, Cd, Cu, Fe, Mn, Ni, and Zn have
previously been demonstrated in translocated D. muscoides to an
artificial wetland (17). In contrast, the
concentration of Mn was lower in the peat, root tissue, and foliage of
translocated cushions compared to non-translocated cushions, a result that may
be related to the duration of non-translocated cushions in the ARD-impacted
wetland 2, where the concentration of Mn in water, root tissue, and foliage was
higher than in wetland 1, which was not impacted.
Behavior
of translocated cushions in the ARD-impacted wetland
The bioconcentration and translocation factors used to evaluate
bioremediation capacity (1, 9, 10, 14)
demonstrated the absorption of heavy metals, as well as their translocation to
the foliage in transplanted D. muscoides cushions (17),
highlighting their potential for phytoremediation of these contaminants (14).
The BAF indicates the phenotypic trait of heavy metal accumulation in plant
tissues and their potential for phytoextraction (14),
while the TF provides information on contaminant mobility within plants (10).
The BAF of heavy metals in foliage classified both translocated and
non-translocated cushions as excluders (BAF < 1) of Al, Cu, Cr, Pb, and
accumulators (BAF 1-10) of Mn and Zn (figure 4).
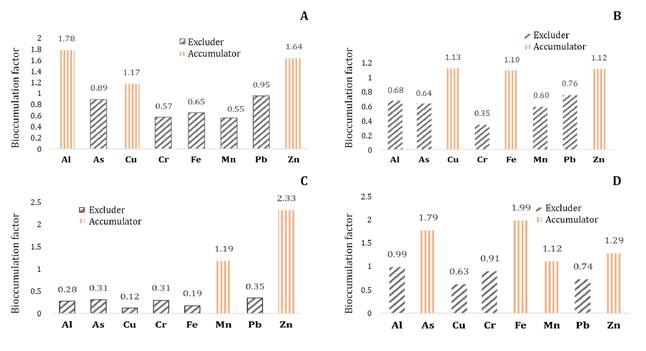
Figure 4. Bioaccumulation
factor in root tissue in non-translocated cushions (A), translocated cushions
(B), and in foliage in non-translocated cushions (C) and translocated cushions
(D) of D. muscoides.
Figura 4. Factor
de bioacumulación en tejido radicular en cojines no translocados (A),
translocados (B), y en follaje en cojines no translocados (C) y translocados
(D) de D. muscoides.
A previous study identified D. muscoides as an
accumulator of Zn and a hyperaccumulator of Al and Mn (17).
The difference between that study and the present one may be attributed to the
use of an artificial wetland in the former, where plants were exposed to a
constant flow of ARD for 9 months, unlike the natural wetland used in the
present study over 5 months. Additionally, manure was applied during the
implementation of the artificial wetland, providing microorganisms that may
promote plant growth and activity (13).
The BAF of metals
in root tissue classified both translocated and non-translocated cushions as
excluders of As, Cr, Mn, and Pb, and accumulators of Cu and Zn. The highest BAF
values in roots corresponded to Fe (1.10), Cu (1.13), and Zn (1.12), which were
higher than for other heavy metals (0.34-0.75). This contrasts with the values
observed in T. latifolia, where BAF values for Ni and Zn drastically
decreased, metals that reached higher concentrations (42.2 and 107 mg/kg) in
wetland sediments compared to Cr (30.1 mg/kg), Cu (33.2 mg/kg), and Pb (39.3
mg/kg). This decrease is related to negative regulation or the plant’s capacity
to reduce or suppress a response to stimuli. At low environmental
concentrations of metals, the plant can retain them; however, when
concentrations increase chronically, tissues may not effectively control
bioaccumulation (9). While an inverse
relationship between BAF and the concentration of major metals was observed in T.
latifolia, this relationship was not observed in D. muscoides regarding
Fe, Cu, and Zn, metals that were found in higher concentrations in wetland 2
impacted by ARD.
The translocation
factor of metals equally classified transplanted and non-transplanted cushions
as phytoextractors (TF > 1) of Mn and Zn, and phytostabilizers (TF < 1)
of Pb and Cu (figure
5).
This aligns with the classifications attributed to T. latifolio as a
phytoextractor of Zn, but contrasts with these plants as phytostabilizers of
As, Cr, and Ni (9).
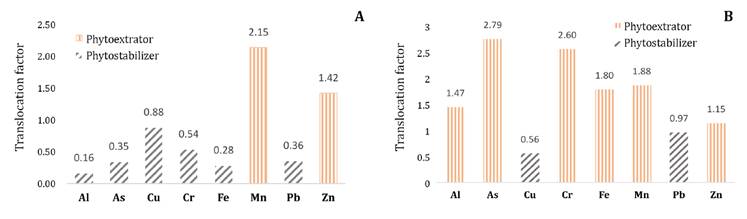
Figure 5. Translocation
factor of non-translocated (A) and translocated (B) cushions of D. muscoides.
Figura
5. Factor de traslocación de cojines no traslocados (A)
y traslocados (B) de D. muscoides.
Regarding Al, As,
Fe, and Cr, the translocation factor classified transplanted cushions as
phytoextractors, whereas non-transplanted cushions were classified as
phytostabilizers. In phytoremediation, metals absorbed by the roots are
transported and accumulated in the foliage, thereby permanently reducing these
contaminants in peat or soil. In phytostabilization, metal mobilization is
reduced, preventing migration into groundwater; however, contaminants remain in
the peat or soil (24, 30).
This study had several limitations, including aspects of
experimental design (such as the study area and sample size), challenges during
experiment execution (such as inaccessibility to the experimental zone due to
flooding and the slow growth of D. muscoides), and logistical issues
(such as entry and handling of plants in the protected area and transportation
of samples to the laboratory). Despite these challenges, the data obtained and
analyzed were sufficient to determine the experimental positive effect of
translocation and adaptation of D. muscoides cushions in a ARD-impacted
wetland.
Conclusion
The translocated cushions of D. muscoides adapted to the
ARD-impacted wetland, promoting the phytoextraction and phytostabilization of
heavy metals over the five months of evaluation. Particularly notable is the
accumulation of Mn and Zn in the aerial plant tissue, alongside the
accumulation and stabilization of Pb and Cu in the root tissue. The concentrations
of some metals at certain points showed a higher standard deviation from the
mean; however, these outliers had little effect on the overall results. This
confirms D. muscoides’ phytoremediation activity in extracting heavy
metals from the environment, thereby preventing their deposition in the soil
and surrounding water. They are proposed as suitable candidates for
bioremediating ARD-impacted wetlands. However, larger-scale studies are needed
that encompass a broader sample size, evaluate the concentration of heavy
metals in water throughout the study period, and cover both wet and dry periods
of the wetland to obtain more definitive results. Finally, special attention is
emphasized on the concentration of Mn, which was the metal with the highest
concentration in the evaluated wetlands.
Acknowledgement
This work was supported by the Instituto Nacional de
Investigación en Glaciares y Ecosistemas de Montaña (INAIGEM). We also thank
the biology laboratory of the Centro de Investigación para el Fomento
Sustentable (CIFOS) for allowing the use of its facilities.
1. Alcalá Jáuregui,
J. A.; Rodríguez Ortiz, J. C.; Filippini, M. F.; Martínez Carretero, E.;
Hernández Montoya, A.; Rojas Velázquez, A. N.; Méndez Cortés, H.; Beltrán
Morales, F. A. 2022. Metallic elements in foliar material and fruits of three
tree species as bioindicators. Revista de la Facultad de Ciencias Agrarias.
Universidad Nacional de Cuyo. Mendoza. Argentina. 54(2): 61-72. DOI:
https://doi.org/10.48162/rev.39.083
2. Arsenic, A. M.;
Beryllium, A. M.; Chromium, B. N.; Cobalt, C. P.; Iron, C. S.; Lead, C. S.;
Thallium, I. Z. 1996. Method 3050B acid digestion of sediments, sludges, and
soils 1.0 scope and application. United States Environmental Protection Agency
(EPA). Washington. DC. USA. 1-12.
3. Autoridad
Nacional del Agua (ANA). 2011. Protocolo Nacional de Monitoreo de la calidad en
cuerpos naturales de agua superficial. Ministerio de Agricultura. Perú.
https://hdl. handle. net/20.500.12543/215 (Accessed
May, 2023).
4. Awa, S.;
Hadibarata, T. 2020. Removal of heavy metals in contaminated soil by
phytoremediation mechanism: A Review. Water, air, Soil Pollut. 231-47.
https://doi.org/10.1007/s11270- 020-4426-0
5. Benfield, A.;
Yu, Z.; Benavides, J. 2021. Environmental controls over Holocene carbon
accumulation in Distichia muscoides-dominated peatlands in the eastern
Andes of Colombia. Quaternary Science Reviews. 251: 106687.
https://doi.org/10.1016/j.quascirev.2020.106687
6. Chávez, J.;
Gonzales, J.; Iparraguirre, J. 2023. El impacto del cambio climático en la
evolución glaciar desde la pequeña edad de hielo, en la vertiente occidental de
la subcuenca Ranrahirca (Cordillera Blanca, Ancash). Tesis de grado en
Ingeniería Ambiental y Recursos Naturales. Facultad de Ingeniería Ambiental y
Recursos Naturales. Universidad Nacional del Callao. Perú. 156 p.
7. Cooper, D.;
Kaczynski, K.; Slayback, D.; Yager, K. 2015. Growth and organic carbon
production in peatlands dominated by Distichia muscoides, Bolivia, South
America. Artic, Antarctic, and Alpine Research. 47(3): 505-510.
https://doi.org/10.1657/AAAR0014-060
8. Ding, X.; Qian,
L.; Juang, W.; Liu, H.; Yiming, A.; Zha, M.; Qu, J.; Jiang, Z. 2024. Review of
bacterial sulfate reduction in lacustrine deposition and its identification in
the Jimsar Sag, Junggar Basin. Marine and Petroleum Geology. 163: 106801. https://doi.org/10.1016/j. marpetgeo.2024.106801
9. Haghnazar, H.;
Sabbagh, K.; Johannesson, K.; Pourakbar, M.; Aghayani, E. 2023.
Phytoremediation capability of Typha latifolia L. to uptake sediment
toxic elements in the largest coastal wetland of the Persian Gulf. Marine
Pollution Bulletin. 188: 114699. https://doi.
org/10.1016/j.marpolbul.2023.114699
10. Heisi, H.;
Awosusi, A.; Nkuna, R.; Matambo, T. 2023. Phytoextraction of anthropogenic
heavy metal contamination of the Blesbokspruit wetland: Potential of wetland
macrophytes. Journal of Contaminant Hydrology. 253: 104101.
https://doi.org/10.1016/j.jconhyd.2022.104101
11. Hirsh, M. 2016.
Compilación sistemática de la situación actual en bofedales, bosques, paisajes,
calidad de agua y praderas en la reserva de biósfera Huascarán, región Áncash.
USAID, Instituto de montaña conservación, cultura, comunidad
AID-527-A-14-00001. 39 p.
12. Hussein, S.;
Qurbani, K.; Ahmed, S.; Tawfeeq, W.; Hassan, M. 2024. Bioremediation of heavy
metals in contaminated environments using Comamonas species: A narrative
review. Bioresource Technology Reports. 25: 101711.
https://doi.org/10.1016/j.biteb.2023.101711
13. Janaki, M.; Kirupanantha, P.; Senthil, S.; Stanley, V.; Al
Farraj, D.; Aljeidi, R.; Arokiyaraj. S. 2024. Beneficial role of Burkholderia
cepacia in heavy metal bioremediation in metal-polluted soils and enhance
the tomato plant growth. Biocatalysis and Agricultural Biotechnology. 57:
103032. https://doi.org/10.1016/j.bcab.2024.103032
14. Jara-Peña, E.;
Gómez, J.; Montoya, H.; Sánchez, T.; Tapia, L.; Cano, N.; Dextre, A. 2017.
Acumulación de metales pesados en Calamagrostis rigida (Kunth) Trin ex
Steud. (Poaceae) y Myriophyllum quitense Kunth (Haloragaceae) evaluadas
en cuatro humedales altoandinos del Perú. Arnaldoa. 24(2): 583-598.
http://dx.doi.org/10.22497/arnaldoa.242.24210
15. La Matta, F.
2020. Influencia del drenaje ácido de roca en la comunidad de
macroinvertebrados bentónicos, índices bióticos de calidad de agua y grupos
funcionales alimenticios en ríos y cabeceras de La Cordillera Blanca (Subcuenca
de Quillcay, Áncash). Tesis de grado en Biología. Facultad de Ciencias y
Filosofía. Universidad Peruana Cayetano Heredia. Lima. Perú. 118 p.
16. López, M.;
Morales, O. 2022. Fitorremediación de suelos contaminados por metales pesados:
una revisión. Revista de Ciencia y Tecnología El Higo. 12(2): 15-28.
https://doi.org/10.5377/ elhigo.v12i2.15197
17 Luna, E. 2018.
Biorremediación utilizando Distichia muscoides y Calamagrostis
glacialis del drenaje ácido de roca proveniente del nevado de
Pastoruri-2015. Tesis de grado en Ingeniería Ambiental. Facultad de Ciencias
del Ambiente. Universidad Nacional Santiago Antúnez de Mayolo. Ancash. Perú.
103 p.
18. Magnússon, R.;
Cammeraat, E.; Lücke, A.; Jansen, B.; Zimmer, A.; Recharte, J. 2020. Influence
of glacial sediments on the chemical quality of surface water in the Ulta
valley, Cordillera Blanca, Perú. Journal of Hydrology. 587: 125027.
https://doi.org/10.1016/j.jhydrol.2020.125027
19. Martín, T. D.;
Brockhoff, C. A.; Creed, J. T.; EMMC Methods Work Group. 1994. Methods EMW
200.7: Methods for the determination of metals in environmental samples. U.S.
Environmental Protection Agency Cincinnati, Ohio. Methods Work Group. Revision
4.4. 1-58.
20. Martínez, V.;
Benavides, J. 2023. Carbon balance shift in mountain peatlands along a gradient
of grazing disturbance in the tropical Andes (Colombia). Plant Ecology. 224:
1049-1058. https://doi.org/10.1007/s11258-023-01356-8
21. Ministerio del
Ambiente (MINAM). 2016. El Perú y el cambio climático, tercera comunicación
nacional del Perú a la Convención Marco de las Naciones Unidas sobre cambio
climático. https://www.gob.pe/institucion/minam/informes-publicaciones/2653-el-peru-y-el-cambio-climatico-tercera-comunicacion-nacional-del-peru
(Accessed March, 2023).
22. Ministerio del
Ambiente (MINAM). 2017. Decreto Supremo N° 004-2017. Estándares de Calidad
Ambiental (ECA) para Agua y establecen Disposiciones Complementarias.
https://www.gob. pe/institucion/minam/normas-legales/3671-004-2017-
minam (Accessed March, 2023)
23. Narváez, C.;
Kalinhoff, C.; Sánchez, A. 2024. Potencial del arbusto Lantana camara L.
(Verbenaceae) para la fitoestabilización y volatilización de mercurio. Revista
Internacional de Contaminación Ambiental. 40: 95-103.
https://doi.org/10.20937/RICA.54790
24. Oggero, A.;
Nakayama, H.; Ávalos, C.; Garcia, I.; Benítez, J.; Ayala, J.; Elkhalili, R.,
Peralta, I. 2021. Eficiencia de la absorción de cobre (Cu) y cromo (Cr), una
propuesta de fitorremediación de efluentes mediada por Typha domingensis.
Revista de la Sociedad Científica del Paraguay. 26(2): 100-113.
https://doi.org/10.32480/rscp.2021.26.2.100
25. Orrego, S.
2019. Eficiencia de consorcios de bacterias sulfato reductoras en la
degradación anaerobia de hidrocarburos aromáticos del petróleo en microcosmos,
junio-octubre de 2018. Tesis de maestría en Ciencias en Ingeniería Ambiental.
Universidad Nacional Pedro Ruiz Gallo. Lambayeque. Perú. 83 p.
26. Oyague, E.;
Cooper, D.; Ingol, E. 2022. Effects of land use on the hydrologic regime,
vegetation, and hydraulic conductivity of peatlands in the central Peruvian
Andes. Journal of Hydrology. 609: 127687.
https://doi.org/10.1016/j.jhydrol.2022.127687
27. Pabón, S.;
Benítez, R.; Sarria, R.; Gallo, J. 2020. Contaminación del agua por metales
pesados, métodos de análisis y tecnologías de remoción. Una revisión. Entre
Ciencia e Ingeniería. 14(27): 9-18. https://doi.org/10.31908/19098367.1734
28. Pedraza, M.
2021. Fitorremediación en cuerpos de agua contaminados por metales pesados.
Revista Científica de Biología y Conservación. 1(1): 61-78.
https://doi.org/10.58720/ibs. v1i1.6
29. Rascón, J.;
Corroto, F. 2020. Evolución fisicoquímica y de las bacterias de azufre en
microcosmos de diferentes sistemas acuáticos de la Región Amazonas. Revista de
Investigación Científica Tayacaja. 3(1). https://doi.org/10.46908/rict.v3i1.68
30. Reyna, D.;
Cortés, L.; Sandino, G.; Martínez, M.; Rodríguez, L. 2022. Estudio del
comportamiento de especies vegetales en un proceso de fitoestabilización para
remediación de suelo contaminado por jal minero abandonado. Ciencia, Tecnología
y Salud. 16(32): 52-57. https://doi.org/10.36790/epistemus.v16i32.197
31. Ruthsatz, B.
2022. Vegetación y ecología de los bofedales tropicales altoandinos en el norte
de Chile. Chloris Chilensis. 25(1): 165-214.
32. Suárez, E.;
Chimbolema, S.; Chimner, R.; Lilleskov, E. 2021. Root biomass and production by
two cushion plant species of tropical high-elevation peatlands in the andean
paramo. Mires and Peat. 27. https://doi.org/10.19189/MaP.2020.OMB.StA.2131
33. Wang, H.; Wang, X.; Zhang, Y.; Wang, D.; Long, X.; Chai, G.;
Wang, Z.; Meng, H.; Jiang, C.; Dong, W.; Guo, Y.; Li, J.; Xu, Z.; Lin, Y. 2023.
Sulfate-reducing bacteria-based bioelectrochemical system for heavy metal
wastewater treatment: Mechanisms, operating factors, and future challenges.
Journal of Electroanalytical Chemistry. 915 (15): 117945. https://doi.org/10.1016/j.
jelechem.2023.117945
34. Wanner, C.;
Moradi, H.; Ingold, P.; Cardenas, M.; Mercurio, R.; Furrer, G. 2023. Rock
glaciers in the Central Eastern Alps-How permafrost degradation can cause acid
rock drainage, mobilization of toxic elements and formation of basaluminite.
Global and Planetary Change. 227: 104180.
https://doi.org/10.1016/j.gloplacha.2023.104180
35. Yan, A.; Wang,
Y.; Tan, S.; Yusof, M.; Ghosh, S.; Chen, Z. 2020. Phytoremediation: a promising
approach for revegetation of heavy metal-polluted land. Frontiers in Plant
Science. 30(11): 359. https://doi.org/10.3389/fpls.2020.00359
36. Yaranga, R.
2020. Humedal Altoandino del Perú: Diversidad florística, productividad
primaria neta aérea, condición ecológica y capacidad de carga. Scientia
Agropecuaria. 11(2): 213-221. https://doi.org/10.17268/sci.agropecu.2020.02.08
37. Zheng, H.;
Bingjie, L.; Guocheng, L.; Zhaohui, C.; Chenchen, Z. 2019. Potential toxic
compounds in biochar: Knowledge gaps between biochar research and safety,
biochar from biomass and waste. Biochar from Biomass and Waste. 349-384.
https://doi.org/10.1016/B978-0- 12- 811729-3.00019-4
38. Zimmer, A.;
Brito, M.; Alegre, C.; Sánchez, J.; Recharte, J. 2018. Implementación de dos
sistemas de biorremediación como estrategia para la prevención y mitigación de
los efectos del drenaje ácido de roca en la Cordillera Blanca, Perú. Revista de
Glaciares y Ecosistemas de Montaña. 4(3): 57-76.
39. Zimmer, A.; Beach, T.; Luzzadder, S.; Rabatel, A.; Lopez,
J.; Cruz, R.; Temme, A. 2024. Physico-chemical properties and toxicity of young
proglacial soils in the Tropical Andes and Alps. Catena. 237: 107748.
https://doi.org/10.1016/j.catena.2023.107748