Revista de la Facultad de Ciencias
Agrarias. Universidad Nacional de Cuyo. Tomo 56(2). ISSN (en línea) 1853-8665.
Año 2024.
Original article
In
vitro micropropagation and physiological assessment of Senecio
bonariensis
Micropropagación
in vitro y evaluación del estado fisiológico de plantas de Senecio
bonariensis
Patricia Andrea
Uchiya3,
Víctor Andrés
Ramos-Duarte1,
2,
Luisa Fernanda
Mendoza-Morales1,
2,
José Alberto
Corigliano4,
Mariana Georgina
Corigliano1,
2*
1Instituto Tecnológico de Chascomús (CONICET-UNSAM). Intendente
Marino Km 8,2. C. C. 164 (B7130IWA). Chascomús. Provincia de Buenos Aires.
Argentina.
2Instituto Tecnológico de Chascomús (CIC).
3Escuela de Bio y
Nanotecnologías (UNSAM). Campus Miguelete. 25 de Mayo y Francia. C. P. 1650.
San Martín. Provincia de Buenos Aires. Argentina.
4Universidad Nacional de Río Cuarto (UNRC). Departamento de
Ecología Agraria, Uso y Manejo de Suelos. Ruta Nacional 36. Km 601. Río Cuarto.
Córdoba. Argentina.
*marianacorigliano@intech.gov.ar
Abstract
Senecio bonariensis
is
a plant native to South American wetlands. This plant has ecological
importance, is used in traditional medicine, and is also popular for ornamental
purposes. This study aimed to develop the first in vitro propagation
protocol for S. bonariensis. Leaf explants were disinfected and placed
on Murashige and Skoog (MS) agar medium supplemented with different
combinations of growth regulators. We tested the effect of two different
cytokinins: Kinetin (KIN) and 6-benzylaminopurine (BAP), in the presence of the
auxin α-naphthalene acetic acid (NAA). All treatments with KIN resulted in root
production, but only treatments with BAP induced shoot formation. As results,
we determined the optimal concentration for maximum shoot production, achieving
a 100% success in rustication while finding similar physiological traits among
micro-propagated and wild-type plants. In conclusion, we developed a protocol
for the large-scale production of S. bonariensis plants, providing an
alternative source of bioactive compounds for medical and pharmaceutical
purposes while preserving the natural habitat of this native plant.
Keywords: Margarita de
bañado, Senecio bonariensis, plant growth regulator, in vitro tissue
culture, conservation, OJIP-test
Resumen
Senecio bonariensis
Hook.
y Arn. es una planta nativa
que se encuentra principalmente en zonas de humedales de América del Sur. Esta
planta tiene importancia ecológica y también se utiliza en la medicina
tradicional. Además, es una opción popular con fines ornamentales. El objetivo
de este estudio fue desarrollar el primer protocolo de propagación in vitro de
S. bonariensis. Los explantos de hojas se desinfectaron y luego se
colocaron en medio agar Murashige y Skoog (MS) suplementado con diferentes
combinaciones de reguladores de crecimiento. Se probó el efecto de diferentes
citoquininas: kinetina (KIN) y 6-bencilaminopurina (BAP) en presencia de la
auxina ácido α-naftalenoacético (NAA). Encontramos que todos los tratamientos,
incluido KIN, dieron como resultado la producción de raíces, pero solo los
tratamientos que incluyeron BAP mostraron inducción de brotes. Como resultado,
determinamos la concentración óptima para la mayor producción de brotes y la
tasa de éxito del proceso de rusticación fue del 100%. También evaluamos el
estado fisiológico de las plantas micropropagadas y observamos que los
parámetros probados eran similares a los de las plantas silvestres. En
conclusión, hemos desarrollado un protocolo para la producción a gran escala de
plantas de S. bonariensis. Esto proporcionará una fuente alternativa de
compuestos bioactivos para fines médicos y farmacéuticos y al mismo tiempo
preservará el hábitat natural de esta planta nativa.
Palabras clave: Margarita de
bañado, Senecio bonariensis, regulador de crecimiento vegetal, cultivo
de tejidos in vitro, conservación, prueba OJIP
Originales: Recepción: 07/03/2024 - Aceptación: 05/07/2024
Introduction
The Asteraceae
family is one of the largest families of dicotyledonous plants (5, 10, 14). Senecio bonariensis is a
native plant species primarily found in wet zones of central and northern
Argentina, Bolivia, Uruguay, and southern Brazil (10).
This shrub is also known as Margarita de bañado, Pillahuincó, Bálsamo,
Lampacillo, Lampaso, Lampazo, Lengua de ciervo, Margarita del agua, Margaritón
de bañado, or Sanguinaria in Spanish; Margarida do banhado in Portuguese; and
butterweed, groundsel, or ragwort in English (10).
Considering S. bonariensis is traditionally used to treat skin,
respiratory and osteoarticular diseases (5, 10, 14),
there is growing interest in its cultivation to obtain bio-compounds for
medicinal use and basic or applied research. Plant tissue culture is widely
accepted for propagating native species. This technology has been adopted for
conservation purposes by organizations such as The Botanic Gardens Conservation
International (BGCI), which represents botanical gardens in 120 countries (4, 6, 11). However, only a few micropropagation
and in vitro propagation protocols for other Senecio species have
been previously reported (7, 16, 18) with
protocols presenting bottlenecks at various stages of the propagation process,
highlighting the need for specific protocols tailored to this genus.
Considering that precise regulation of cytokinins and auxin levels strongly
affects growth of stems, roots, and leaves, determining type and concentrations
of particular plant growth regulators (PGRs) is essential. Poorly established
protocols typically result in low shoot multiplication, low rooting frequency,
morphological abnormalities, and high production costs (1). This study aimed to develop a protocol, particularly
considering S. bonariensis for successful plant micropropagation and
rustication. We assessed the effects of two cytokinins, Kinetin (KIN) and
6-benzyl aminopurine (BAP), in conjunction with the α-naphthalene acetic acid
(NAA) auxin, on shoot production from leaf explants, aiming to identify the
optimal combination for maximizing yield. Additionally, we evaluated
physiological parameters of the micro-propagated plants utilizing the
non-destructive and cost-effective OJIP test. This test analyzes the OJIP curve
providing insights into thermal and photochemical phases of electron transport
chain (17).
In conclusion, our study developed a protocol for large-scale
production of S. bonariensis plants as an alternative source of
bioactive compounds for medical and pharmaceutical purposes, while contributing
to the preservation of the species´ natural habitat.
Material
and methods
Plant
material and surface sterilization of explants
Leaves of Senecio
bonariensis were collected during springtime (2018-2022) from Laguna de
Chascomús (35°35’22.92’’ S 58°1’20.14’’ W, Buenos Aires, Argentina). A voucher
specimen was deposited in the Herbarium of the Museo de Ciencias Naturales de
La Plata (Buenos Aires, Argentina), under the collection number M. G.
Corigliano 1, LP 082432. Healthy young leaves were meticulously washed under
running tap water to prevent damage. Subsequently, explants underwent surface
sterilization using a 20% v/v solution of commercial bleach for 30 minutes,
followed by four rinses with sterile distilled water in a biosafety cabinet.
Callus
induction
Surface-sterilized
explants were dissected into small pieces (1 cm2)
without disrupting serrated margins and subsequently placed onto sterile
Murashige and Skoog (MS) basal medium (13)
supplemented with 3% (w/v) sucrose and 0.8% (w/v) agar. To induce callus
formation, the auxin α-naphthalene acetic acid (NAA) was tested at three
different concentrations (0.1, 0.5, and 1 μg ml-1)
in combination with two different cytokinins, BAP or KIN, each at three
different concentrations (0.5, 1, and 2 μg ml-1),
totalling eighteen combinations. Eight leaf fragments from distinct plants were
incubated in culture flasks in a growth chamber set to a 16-hour day/8-hour night
photoperiod, with a photosynthetic photon flux density (PPFD) of *350 μmol
quanta m-2 s-1 provided by cool-white
fluorescent lamps, at a constant temperature of 24/21 ± 2°C. The percentage of
callus induction (PCI) was assessed 30 days after the initial culture
(d.a.i.c.), and calculated by dividing the number of explants with calli by the
total cultured explants, x 100. Three independent experiments were performed.
Shoot
growth study
Calli were
sub-cultured onto fresh MS medium with the same hormone combination 30 d.a.i.c.
The number of shoots produced per explant was evaluated at 90 d.a.i.c. We also
measured shoot length and registered the tallest shoot for each treatment.
Acclimatization
Shoots obtained at
90 d.a.i.c. were transplanted into plastic pots filled with a sterile mixture
of sand, soil, and perlite (1:1:1 ratio) and watered with Hoagland nutrient
solution (8) every 2 days. The pots were
placed in a growth chamber with a 16-hour day/8-hour night photoperiod,
provided by cool-white fluorescent lamps, and maintained at a temperature of
24/21 ± 2°C. Plant survival and phenotypic variation were recorded. After 12
weeks, the plants were transferred to field conditions and flowering ability
was assessed.
Chlorophyll
fluorescence fast-transient analysis
The non-invasive OJIP test (16)
was conducted on 6 to 7-month-old plants using a portable chlorophyll
fluorometer (Pocket PEA v.1.1, Hansatech Instruments Ltd.), as described by Corigliano et al. (2019). Briefly, the youngest
fully developed leaf was dark-adapted for 20 minutes before analysis.
Subsequently, leaf samples were exposed to a 3-second pulse of light at an
intensity of 3500 μmol photons m-2 s-1 (peak wavelength: 637
nm). Data were analyzed using PEA Plus software (Hansatech Instruments Ltd.).
Maximum quantum yield of primary PSII photochemistry (Fv/Fm) and dissipation
energy flux per active reaction center of PSII (DIo/RC) were determined.
Additionally, we analyzed the contribution to photosynthesis regulation of two
functional steps, namely ABS (absorption of light energy) and TRo (trapping of
excitation energy) by RC (reaction center), and CSo (cross-section).
Results
Callus
induction
Effective surface sterilization was achieved for S.
bonariensis leaf explants. The effect of two cytokinins, BAP and KIN, in
combination with the NAA auxin, was tested. Figure 1 shows
calli induction 30 d.a.i.c. Although MS medium without PGR did not lead to
callus generation (data not shown), callus induction was observed for all
hormone combinations, either BAP-NAA (figure 1A) or KIN-NAA (figure 1C).
Bars
represent percentage of one experiment. Blue represents callus induction, while
orange indicates no callus induction. The assay was performed in triplicate.
Significant differences were observed between NAA 1 μg ml-1 - KIN
0.5 μg ml-1 and NAA 0.1 μg ml-1 - KIN 1 μg ml-1
(P= 0.04, X2= 16.1,8).
Las barras
representan los promedios de un experimento y se graficaron como porcentajes.
El color azul representa la inducción de callos, mientras que el anaranjado
indica que no hay inducción de callos. Este ensayo se realizó por triplicado.
Se observaron diferencias significativas entre NAA 1 μg ml-1 - KIN
0,5 μg ml-1 y NAA 0,1 μg ml-1 - KIN 1 μg ml-1
(P= 0,04, X2= 16,1,8).
Figure
1. Effect of NAA and BAP on callus induction. A Callus
induction from leaf explants on MS medium supplemented with either 0.5 μg ml-1 NAA and
0.5 μg ml-1 BAP (C),
or with 0.1 μg ml-1 NAA and
1 μg ml-1 KIN, 30
days after culture initiation. The PCI from leaves cultured on MS and
supplemented with NAA at three different concentrations (0.1, 0.5, and 1 μg ml-1)
and combined with either BAP (B) or KIN (D) at three different concentrations
(0.5, 1, and 2 μg ml-1)
was evaluated 30 d.a.c.i.
Figura 1. Efecto
de NAA y BAP en la inducción de callos. (A) Inducción de callos a partir de
explantos de hojas en medio MS suplementado con 0,5 μg ml-1 de NAA y 0,5 μg ml-1 de BAP (C) o con 0,1 μg ml-1 de NAA y 1 μg ml-1 de KIN, 30 días luego de la inducción del
callo (d.l.i.c). Evaluación del porcentaje de inducción de callos en hojas
cultivadas con MS y suplementadas con NAA en tres concentraciones diferentes
(0,1, 0,5 y 1 μg ml-1)
y combinadas con BAP (B) o KIN (D) en tres concentraciones diferentes: 0,5, 1,
y 2 μg ml-1,
a los 30 d.l.i.c.
However, the BAP-NAA combination resulted in a higher calli
percentage compared to KIN-NAA in any combination. The PCI was determined for
various BAP-NAA combinations. The highest PCI obtained was about 90% when calli
were cultured in MS medium supplemented with NAA 0.5 μg ml-1 and BAP 0.5 μg ml-1
(figure 1B). On the other hand, the highest PCI
was only 50% when calli were cultured with NAA 1 μg ml-1 and KIN 0.5 μg ml-1
(figure 1D). Initial callus induction was
observed at the serrated edge of leaves (figure 1A, 1C).
Effects on BAP,
KIN, and NAA on shoot multiplication.
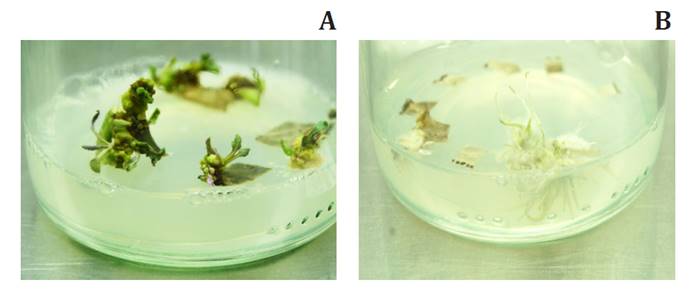
Treatments including BAP led to shoot induction (figure
2A). Conversely, even though different concentrations of KIN induced callus
formation, they did not result in shoot production, but in roots (figure
2B).
(A)
Shoot induction from NAA-BAP treated calli 45 d.a.i.c. (B) Root induction from NAA-KIN
treated calli 45 d.a.i.c.
(A) Inducción de
brotes de callos tratados con NAA-BAP luego de 45 días. (B) Inducción de raíces
tratadas con NAA-KIN luego de 45 días.
Figure
2. Effects of NAA and BAP on shoot multiplication of S.
bonariensis.
Figura 2. Efecto de NAA y BAP en la multiplicación de brotes de S.
bonariensis.
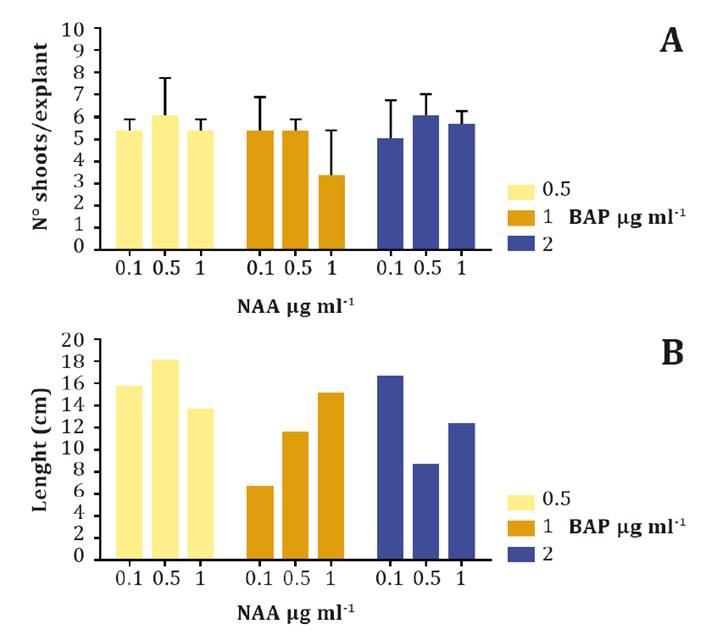
Shoots per cultured explant were counted at 90 d.p.i.c (figure 3). Even without statistical differences among groups
with different BAP-NAA combinations, the highest number of shoots per explant
was observed with BAP 0.5 μg ml-1 and NAA 0.5 μg ml-1
(figure 3A). Interestingly, the longest shoots
were both obtained with the BAP 0.5 μg ml-1 and NAA 0.5 μg ml-1
combination (figure 3B).
(A)
Assessment of the number of shoots obtained per explant using NAA at three
different concentrations (0.1, 0.5, and 1 μg ml-1) and combined with BAP at 0.5 μg ml-1 (yellow),
1 μg ml-1 (orange),
and 2 μg ml-1 (blue)
obtained 60 d.a.i.c. This experiment was conducted in triplicate. Statistical
analysis was performed by one-way ANOVA and no statistical differences were
observed among groups. (B) Shoot length at different hormone combinations
achieved 60 d.a.i.c. The longest shoot per group in three independent
experiments is plotted in the figure.
(A) Evaluación
del número de brotes obtenidos por explanto utilizando NAA en tres
concentraciones diferentes (0,1, 0,5 y 1 μg ml-1) y combinado con BAP a 0,5 μg ml-1 (amarillo),
1 μg ml-1 (anaranjado),
o 2 μg ml-1 (azul)
luego de 60 días. Este experimento se realizó por triplicado. El análisis
estadístico se realizó mediante análisis de varianza unidireccional (ANOVA) y
no hubo diferencias estadísticas entre los grupos. (B) Gráfico de la longitud
máxima de los brotes con diferentes combinaciones de hormonas alcanzada a 60
d.l.i.c. La longitud del brote más largo de cada grupo en tres experimentos
independientes se midió y se representó en la figura.
Figure
3. Effects on BAP and NAA on shoot multiplication.
Figura 3. Efectos de BAP y NAA en la multiplicación de brotes.
Micro-propagated plants displayed
purple phenotype with no physiological disturbances.
Shoots obtained 90
d.a.i.c. were transferred to plastic pots and placed in a plant room with a 16
h day/ 8 h night photoperiod and 24/21 ± 2°C. Plant survival and rustication
were 100% successful (data not shown). A variety of physiological parameters validated
the micropropagation protocol. Some plants grown in vitro showed purple
colorations on leaf abaxial face, prompting a comparative analysis of
physiological traits of green and purplish leaves from micro-propagated plants,
and green leaves from non-propagated (wild-type, WT) control plants (figure 4A).
(A)
Adaxial face (upper left and right) and abaxial face (lower left and right) of
green and purple leaves from micro-propagated plants, respectively. (B) Mean
values of six OJIP parameters are shown in radar charts for WT (black line),
micro-propagated green leaf (light grey line), and micro-propagated purple leaf
(dark grey line). Results are expressed relative to WT, assigned as 1. Each
parameter is defined in the text. (C) Dissipation energy per reaction center
determination in WT (yellow), micro-propagated green leaf (orange), and
micro-propagated purple leaf (blue). Results are means of 7 biological
replicates ± SD. Statistical analysis was performed by one-way ANOVA followed
by Tukey’s Multiple Comparison Test using Prism 5 (GraphPad Software, CA, USA).
** p < 0.01.
(A) Cara adaxial
(arriba a la zquierda y derecha) y cara abaxial (abajo a la izquierda y
derecha) de hojas verdes y moradas de plantas micropropagadas, respectivamente.
(B) Los valores promedios de 6 parámetros OJIP se muestran en gráficos de radar
para WT (línea negra), hoja verde micropropagada (línea gris claro) y hoja
púrpura micropropagada (línea gris oscuro). Los resultados se expresan en
relación con WT, que se asignó a 1. La definición de cada parámetro se
proporciona en el texto. (C) Energía de disipación por determinación del centro
de reacción en WT (amarillo), hoja verde micropropagada (anaranjado) y hoja
morada micropropagada (azul). Los resultados muestran el promedio de 7 réplicas
biológicas ± DS. El análisis estadístico se realizó mediante análisis de
varianza unidireccional (ANOVA) seguido de una prueba de comparación múltiple
de Tukey utilizando Prism 5 (GraphPad Software, CA, EE. UU.). **p<0,01.
Figure
4. Physiological performance of micro-propagated S.
bonariensis plants.
Figura 4. Desempeño
fisiológico de plantas micropropagadas de S. bonariensis.
We assessed maximum quantum yield of primary photochemistry
(Fv/Fm) and examined photosynthesis regulation by the two functional steps,
namely ABS (absorption of light energy) and TRo (trapping of excitation energy)
by RC (reaction center) and CSo (cross-section). The Fv/Fm values for green,
purple, and WT plants were almost 0.8, considered normal (figure
4B). Other energetic parameters (ABS/RC, ABS/CSo, TRo/RC, and TRo/CSo)
showed no statistical differences among plants (figure 4B).
However, the dissipation energy per reaction center (DIo/RC) was statistically
lower in green and purple plants than in WT plants (p<0.01) (figure
4C).
Acclimatization
of regenerated shoots
After 12 weeks, the plants were transferred to field conditions,
and phenotypic variation was visually assessed. Notably, purple colorations on
leaf abaxial sides disappeared after one or two weeks in the field.
Discussion
The growing
interest in medicinal bio-compounds of S. bonariensis underscores the
necessity for a large-scale production protocol. While micropropagation and in
vitro propagation protocols have been documented for other Senecio species (7, 16, 18), a particular protocol for S.
bonariensis is currently lacking. We successfully identified the optimal
conditions for producing S. bonariensis plants by investigating eighteen
combinations of two cytokinins and one auxin on shoot production. All tested
combinations resulted in callus formation, while the percentage of callus
induction (PCI) varied according to hormone group. Remarkably, we found that MS
medium lacking plant growth regulators did not induce shoot formation. However,
combinations of NAA-BAP proved more effective in inducing callus compared to
NAA-KIN. In a study by Hariprasath et al. (2015),
S. candicans exposed to NAA 0.5 μg ml-1 and either 1 or 2 μg ml-1
BAP resulted in 37% and 47% average PCI, respectively. In
contrast, we achieved a 1.7-fold higher callus induction (66% and 80%, as shown
in figure 1B). Notably, MS supplemented with NAA 0.5 μg ml-1
and BAP 0.5 μg ml-1 achieved 90% PCI. Other
authors observed no differences in PCI for S. candicans between NAA-BAP
and NAA-KIN combinations. However, for S. bonariensis, PCI was lower
when MS was supplemented with NAA-KIN (figure 1D). We
observed a twofold lower PCI with NAA-KIN combinations (0.5 μg ml-1
NAA - 2 μg ml-1 KIN and 1 μg ml-1
NAA - 2 μg ml-1 KIN), although similar
PCI was observed when KIN was tested at 1 μg ml-1.
Notably, these calli did not produce shoots. After obtaining calluses, we evaluated
shoot induction. NAA-BAP combinations resulted in 100% shoot induction for S.
bonariensis (figure 2A), as previously found on S. macrophyllus M. Bieb, with
100% shoot induction (18), and S.
cruentus cv. Tokyo Daruma, with 86.4% to 98.4% shoot induction (16). In contrast, shoot formation in S. candicans ranged
from 48% to 76% (7). All NAA-BAP
combinations were highly effective for inducing S. bonariensis shoots.
In contrast with other Senecio species, S. bonariensis did not produce
shoots from calli treated with NAA-KIN (figure 2B), (7, 16, 18). This may be attributed to KIN being a
weaker cytokinin than BAP (1, 2). Further
studies should consider the effect of different concentrations of KIN to determine
optimal conditions for significant shoot production.
Shoot number per
explant was assessed after 60 days of incubation. Shoot induction occurred in
the presence of NAA-BAP. Mean shoot number per explant ranged from 3.3 to 6,
similar to Trejgell et al. (2010). Shoot
length was comparable to other Senecio species (7,
16, 18).
Leaf purplish
coloration during rustication could constitute a form of plant photoprotection,
of the immature photosynthetic apparatus, dissipating high irradiance and
mitigating potential damage from solar radiation (15).
We evaluated in vitro-propagated plants using a non-invasive OJIP test,
ideal for researching valuable plant material that should not be destroyed.
Considering our results regarding physiological and energetic parameters, i.e.
Fv/Fm, ABS, TRo, RC and Cso in green and purple leaves under high-light
stress, the maximum quantum yield of photosystem II (PSII) (Fv/Fm) decreases
due to photo-oxidative damage, as previously reported (9, 12). However, the non-significantly different
physiological state among leaves, followed by a significant decrease in DIo/RC
in purple leaves of micro-propagated plants compared to unpropagated (WT) and
green leaves, indicated that purple coloration did not provide photoprotection.
This is because dissipation prevents photodamage. Interestingly, some leaves of
micro-propagated plants displaying purple phenotype showed no physiological
disturbances. This phenotype disappears one or two weeks after being
transplanted to the field. Further studies are required to understand these
findings. Notably, micro-propagated plants successfully flowered and attracted
pollinators.
Conclusion
We created a straightforward and efficient procedure for the
large-scale propagation of S. bonariensis. This protocol holds promise
for diverse applications, ranging from medicinal research and ecological
studies to commercial landscaping. Furthermore, it offers valuable insights
into other Senecio species.
1. Amoo, S. O.;
Finnie, J. F.; van Staden, J. 2011. The role of meta-topolins in alleviating
micropropagation problems. Plant Growth Regul. 63: 197-206. doi:
10.1007/s10725-010-9504-7
2. Bogaert, I.; Van
Cauter, S.; Werbrouck, S. P. O.; Doležal, K. 2006. New aromatic cytokinins can
make the difference. Acta Hortic. 725 I: 265–270. doi:
10.17660/ActaHortic.2006.725.33
3. Corigliano, M.
G.; Albarracín, R. M.; Vilas, J. M.; Sánchez López, E. F.; Bengoa Luoni, S. A.;
Deng, B.; Farran, I.; Veramendi, J.; Maiale, S. J.; Sander, V. A.; Clemente, M.
2019. Heat treatment alleviates the growth and photosynthetic impairment of
transplastomic plants expressing Leishmania infantum Hsp83-Toxoplasma
gondii SAG1 fusion protein. Plant Sci. 284: 117-126. doi:
10.1016/j.plantsci.2019.04.011
4. Dallai, D.; Del
Prete, C.; Sgarbi, E.; Grimaudo, M. 2010. Integrated into situ/ex-situ
plant conservation practices managed by the University Botanic Garden of
Modena. Boll. Mus. Ist. Biol. Univ. Genova. 72: 33-42.
5. Dematteis, M.;
Molero, J.; Angulo, M. B.; Rovira, A. M. 2007. Chromosome studies on some
Asteraceae from South America. Bot J Linn Soc. 153: 221-230.
6. Fay, M.;
Clemente, M. 1997. Aplicación de las técnicas de cultivo de tejidos en la
propagación y conservación de especies amenazadas. Monografía del Jardín Botánico
Córdoba. 43-50.
7. Hariprasath, L.;
Jegadeesh, R.; Arjun, P.; Raaman, N. 2015. In vitro propagation of Senecio
candicans DC and comparative antioxidant properties of aqueous extracts of
the in vivo plant and in vitro-derived callus. South African J
Bot. 98: 134-141. doi: 10.1016/j.sajb.2015.02.011
8. Hoagland, D. R.;
Arnon, D. I. 1950. The water-culture method for growing plants without soil.
Calif Agric Exp Stn Circ. 347: 1-32. doi:
citeulike-article-id:9455435
9. Hughes, N. M.;
Lev-Yadun, S. 2015. Red/purple leaf margin coloration: Potential ecological and
physiological functions. Environ Exp Bot . 119: 27-39.
doi: 10.1016/j.envexpbot.2015.05.015
10. Hurrell, J. A.;
Bazzano, D. H.; Delucchi, G. 2006. Dicotiledóneas herbáceas 1. Nativas y
Exóticas. En Biota Rioplatense XI. Lola (Eds.).
11. Kirakosyan, A.;
Kaufman, P. B. 2009. Recent advances in plant biotechnology. Springer.
12. Meloni, D. A.;
Gulotta, M. R.; Moura Silva, D.; Arraiza, M. P. 2019. Effects of salt stress on
germination, seedling growth, osmotic adjustment, and chlorophyll fluorescence
in Prosopis alba G. Revista de la Facultad de Ciencias Agrarias.
Universidad Nacional de Cuyo. Mendoza. Argentina. . 51(1): 69-78.
13. Murashige, T.;
Skoog, F. 1962. A revised medium for rapid growth and bio assays with tobacco
tissue cultures. Physiol Plant. 15: 473-497. doi:
10.1111/j.1399-3054.1962.tb08052.x
14. Portero, A. G.;
González-Coloma, A.; Reina, M.; Díaz, C. E. 2012. Plant-defensive
sesquiterpenoids from Senecio species with biopesticide potential.
Phytochem Rev. 11: 391-403. doi:
10.1007/s11101-013-9279-
15. Ranjan, S.;
Singh, R.; Singh, M.; Pathre, U. V.; Shirke, P. A. 2014. Characterizing
photoinhibition and photosynthesis in juvenile-red versus mature-green leaves
of Jatropha curcas L. Plant Physiol Biochem. 79: 48-59. doi: 10.1016/j.plaphy.2014.03.007
16. Sivanesan, I.;
Jeong, B. R. 2012. Identification of somaclonal variants in proliferating shoot
cultures of Senecio cruentus cv. Tokyo Daruma. Plant Cell Tissue Organ
Cult. 111: 247-253.
17. Strasser, R.
J.; Srivastava, A.; Tsimilli-Michael, M. 2000. The fluorescence transient as a
tool to characterize and screen photosynthetic samples.
18. Trejgell, A.;
Ichalska, M.; Retyn, A. 2010. Micropropagation of Senecio macrophyllus M.
Bieb. Acta Biol Cracoviensia Ser Bot. 52: 67-72.
Funding
This work was
supported by the Agencia Nacional de Promoción Científica y Tecnológica
(Argentina) (grants PICT 2016-0113 and PICT 2020-07032, Dr. Mariana Corigliano)
and from the Ministerio de Educación, Cultura, Ciencia y Tecnología (Argentina)
(UNIVERSIDADES AGREGANDO VALOR 2018). This work also received institutional
support from the Universidad Nacional General de San Martín (UNSAM, Argentina).
Declarations
All the authors declare that they have no conflicts of interest.