Revista de la Facultad de Ciencias
Agrarias. Universidad Nacional de Cuyo. Tomo 56(2). ISSN (en línea) 1853-8665.
Año 2024.
Original article
Laboratory
evaluation of the feeding behavior of the generalist predatory mirid bug Tupiocoris
cucurbitaceus (Hemiptera: Miridae) for the biological control of Phthorimaea
absoluta (Lepidoptera: Gelechiidae)
Evaluación
en laboratorio del comportamiento alimenticio del mírido depredador generalista
Tupiocoris cucurbitaceus (Hemiptera: Miridae) para el control biológico
de Phthorimaea absoluta (Lepidoptera: Gelechiidae)
Rocío Isabel
Montiel Cáceres1,
1Centro de Estudios Parasitológicos y de Vectores (CEPAVE,
CONICET-UNLP, Asociado CIC-BA), Boulevard 120 e/ 60 y 64. (1900). La Plata.
Argentina.
2Universidad Nacional de San Antonio de Areco (UNSAdA).
Departamento de Ciencias Exactas y Naturales. Avda. Güiraldes 689. (2670) San
Antonio de Areco. Argentina.
*mrocca@cepave.edu.ar
Abstract
The use of
predatory insects has gained interest for reliable and environmentally safe
pest management to control the South American tomato leafminer, Phthorimaea
absoluta (Meyrick) (Lepidoptera: Gelechiidae), a pest of tomato crops
worldwide. Based on video tracking using EthoVision® software and static
feeding multiple-choice tests, we report the prey-searching behavior and
feeding preference of the Neotropical mirid bug Tupiocoris cucurbitaceus Spinola
(Hemiptera: Miridae), a biological control agent of P. absoluta when
presented with its eggs and other two prey species. T. cucurbitaceus exhibits
generalist feeding behavior; the nymphs initially showed a preference for Trialeurodes
vaporariorum Westwood (Hemiptera: Aleyrodidae) nymphs but consumed more P.
absoluta and Ephestia kuehniella Zeller (Lepidoptera: Pyralidae)
eggs after 24 h. T. cucurbitaceus males preferred T. vaporariorum throughout
the experiment while females showed no preference for any prey. Furthermore,
they did not cause significant damage to the leaves. The findings emphasize the
importance of evaluating the simultaneous offer of multiple prey types to
understand the effectiveness of biocontrol agents in the field. Overall, the
research contributes valuable insights into the feeding habits of T.
cucurbitaceus, supporting its potential as a biological control agent for P.
absoluta in tomato crops.
Keywords: diet breadth, mirid
bug, Trialeurodes vaporariorum, Ephestia kuehniella, biocontrol
Resumen
El uso de insectos
depredadores ha ganado interés para el manejo confiable y ambientalmente seguro
de la polilla del tomate, Phthorimaea absoluta (Meyrick) (Lepidoptera:
Gelechiidae), una plaga de este cultivo en todo el mundo. Por medio de un
estudio con el programa EthoVision® y de ensayos de opción múltiple, reportamos
el comportamiento de búsqueda de presas y la preferencia alimentaria del mírido
neotropical Tupiocoris cucurbitaceus Spinola (Hemiptera: Miridae), un
agente de control biológico de P. absoluta, cuando se le presentan
huevos de la plaga y simultáneamente otras dos especies de presas. T.
cucurbitaceus exhibe un comportamiento alimentario generalista. Las ninfas
del depredador mostraron inicialmente preferencia por las ninfas de Trialeurodes
vaporariorum (Westwood) (Hemiptera: Aleyrodidae) pero consumieron más
huevos de P. absoluta y Ephestia kuehniella Zeller (Lepidoptera:
Pyralidae) al cabo de 24 h. Los machos de T. cucurbitaceus prefirieron a
T. vaporariorum mientras que las hembras no mostraron preferencia por
ningún tipo de presa. Además, los individuos no causaron daños directos a las
hojas. Los hallazgos enfatizan la importancia de evaluar la oferta de
diferentes presas para conocer la efectividad de los agentes de control
biológico en el campo. La investigación aporta valiosos conocimientos sobre los
hábitos alimentarios de T. cucurbitaceus, respaldando su potencial como
agente de control biológico para P. absoluta.
Palabras clave: amplitud de dieta,
chinche depredadora, Trialeurodes vaporariorum, Ephestia kuehniella,
biocontrol
Originales: Recepción: 18/03/2024 - Aceptación: 21/08/2024
Introduction
The use of
generalist arthropod predators for biological control has historically received
less attention compared to parasitoids and entomopathogens, given assumed
negative effects. Among those effects, the most reported are omnivory, attack
of non-target species, competition, and intraguild predation on other natural
enemy species present in crops (21, 32).
However, important biocontrol successes have been achieved with mite and
hemipteran predators since they can feed on a variety of prey and plant resources,
ensuring their survival and reproduction and enhancing their establishment (39, 42). For instance, in Spain, biocontrol
against the sweet potato whitefly, Bemisia tabaci (Gennadius)
(Hemiptera: Aleyrodidae), is accomplished by releasing predatory mirid bugs and
mites in protected sweet pepper, Capsicum annuum L. (Solanales:
Solanaceae) crops (9, 33).
A crucial aspect
when planning the use of generalist predators as biocontrol agents is to
determine the range of species on which they effectively feed, i.e., the
diet breadth. Although most predators usually feed on various species, they may
exhibit a degree of acceptance for different prey based on certain
characteristics, such as nutritional quality and stage of development, which
ultimately can affect their success as biocontrol agents (16). Two life traits especially defined to assess
diet breadth of a predatory species are food-searching behavior and preference.
The former implies predator engagement in the activity of prospecting in the
environment, the recognition and acceptance of some prey. Factors such as
developmental stage, sex, age, starvation, and available prey species influence
food-searching behavior. Preference for food is determined by different prey’s
morphological, physiological, and behavioral traits to obtain enough nutrients
and avoid toxic or indigestible food (3, 14, 31).
Miridae bugs (Hemiptera) are important predatory insect species.
Most of them show high preying rates and are capable of finding and colonizing
new habitats due to their dispersing capacity (27).
Approximately 20 species are currently commercialized worldwide as biocontrol
agents against whiteflies, eggs and small larvae of lepidopterans, among other
horticultural pests (41). Particularly
for the biological control of the South American tomato pinworm, Phthorimaea
absoluta (Meyrick) (Lepidoptera: Gelechiidae), a Neotropical pest that has
invaded the European, African, and Asian continents, several mirid species are
main predatory agents (4, 7, 9, 10, 19, 23, 28, 38,
40).
Tupiocoris
cucurbitaceus Spinola (Hemiptera: Miridae) has been reported in the Americas
preying mainly on whiteflies (17, 29).
High predation rates and pest kill rates were later examined for this predatory
bug over a range of prey species: the whiteflies B. tabaci and Trialeurodes
vaporarioum (Westwood) (Hemiptera: Aleyrodidae), the green peach aphid, Myzus
persicae (Sulzer) (Hemiptera: Aphididae), the two-spotted spider mite, Tetranychus
urticae Koch (Acari: Tetranychidae), and eggs of three lepidopteran
species, the Mediterranean flour moth, Ephestia kuehniella Zeller
(Lepidoptera: Pyralidae), the Angoumois grain moth, Sitrotroga cereallela Olivier
and P. absoluta (Lepidoptera: Gelechiidae) (8,
22, 23, 45). Besides, E. kuehniella eggs are also applied
under a prey-enrichment technique to allow the establishment of mirids, as for
the use of N. tenuis to control P. absoluta in Spain (40, 41). T. cucurbitaceus has been
recently developed as a commercial biocontrol agent in Argentina and Uruguay
against whiteflies and P. absoluta. To improve its establishment,
releases are performed along with E. kuehniella eggs as supplementary
food (1, 18, 35). In this research, we
aimed to explore T. cucurbitaceus prey-searching behavior and feeding
preference when offered simultaneously P. absoluta and E.
kuehniella eggs, and T. vaporariorum nymphs. This information could
help better assess the performance of this generalist predatory mirid in tomato
crops as biocontrol agent of P. absoluta.
Materials
and methods
Plant
and insect materials
Tomato plants, Solanum
lycopersicon L. (Solanales: Solanaceae), Elpida variety Enza Zaden, The
Netherlands, were cultivated at the Centro de Estudios Parasitológicos y de
Vectores (CEPAVE, CONICET-UNLP-Asociado CICPBA). Tomato seedlings were
individually transplanted to 1 l-plastic pot, watered daily, and kept free of
insect pests by protecting them inside 60 x 40 x 40 cm (length x width x
height) white voile cages (BioQuip Inc., USA). Plants used in P. absoluta,
T. cucurbitaceus, and T. vaporariorum colonies and experiments
had 4-5 expanded leaves.
Tupiocoris cucurbitaceus colony was initiated with individuals
collected from organic tomato crops in farms located near the La Plata
Horticultural Belt (N Buenos Aires province, Argentina). Insects were
maintained in a controlled environment walk-in rearing room at 25 ± 2°C, 60 ±
10% RH, 14:10 L:D. Identifications of T.
cucurbitaceus were confirmed by taxonomists at La Plata Museum (Entomology
Department, School of Natural Sciences and Museum, National University of La
Plata). To obtain individuals of T. cucurbitaceus of known age for the
tests, 30 cohorts were reared in a white voile cage along with a potted tomato
plant (as described above). Ephestia kuehniella eggs provided by Brometán
SRL (Argentina) and commercial bee pollen were spread ad libitum on the
leaves as food, and distiller water was provided in soaked sponge pieces inside
containers. Oviposition on leaves was allowed for 24 h, and later the plant was
placed in a new cage to start a cohort and replaced with a fresh one. Once
nymphs emerged, they were fed as adults until they reached the developmental
stages needed for the trials (4-5th instar nymphs or adults) (6). To obtain P. absoluta eggs, a colony
was initiated by maintaining bouquets of tomato leaves infested with moth
larvae collected from the field placed in white voile cages. Once pupae were
formed, they were transferred to a new cage until moths emerged. Adults were
provided with a honey solution (70%) and allowed to mate. Potted tomato plants
were offered daily as oviposition substrate and those replaced were held in
clean voile cages (30). A uniform cohort
of 24 h-old eggs was used in the experiments. T. vaporariorum nymphs
used in the feeding preference experiment were obtained from adults captured in
tomato crops using a manual aspirator and transported to the laboratory for
identification (26). Then, they were
released in white voile cages and provided with potted tomato plants, to lay
eggs (37). Once they hatched, development
was checked and after 15 days revised every 24 h to collect late instar nymphs
for the assays.
Prey
searching behavior assay
To assess T.
cucurbitaceus food searching capacity, a three-treatment experiment was set
up, considering developmental stage and sex: 1) late (4-5th instars) nymph, 2) 7-d
adult coupled female, and 3) 7-d adult coupled male. Individuals were isolated
from the cohort and kept starved for 24 h before performing the assays in plastic
Petri dishes (diameter: 5 cm, height: 1 cm), provided with small, moistened
cotton pieces as water sources.
The experimental unit consisted of a plastic container
(diameter: 9 cm, height: 5 cm) with a tomato leaflet on 1-cm layer of water
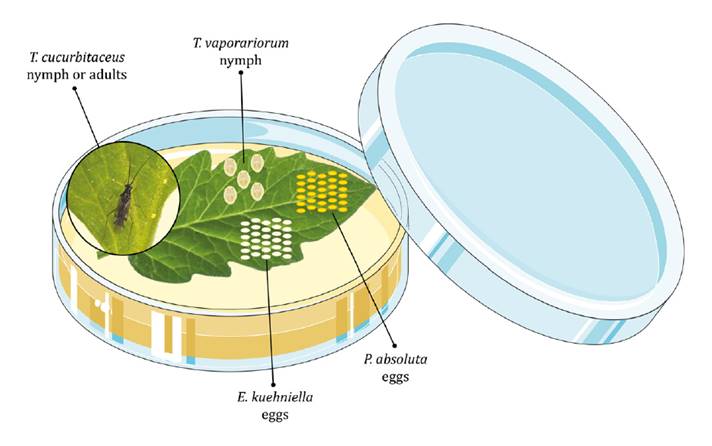
agar (1%) to maintain its turgidity. Three different prey patches were offered
simultaneously to one T. cucurbitaceus individual: the target patch (TP)
containing 30 P. absoluta eggs (24 h old) and two non-target patches
(NTP) with 30 E. kuehniella eggs and 5 T. vaporariorum nymphs
(late instar), respectively (figure 1).
Figure
1. Assembly of the experimental unit to analyze the
predation behavior of Tupiocoris cucurbitaceus on three types of prey
exposed in patches on a fresh tomato leaflet and maintained on a layer of
agar-water.
Figura
1. Esquema de la unidad experimental
para analizar el comportamiento de depredación de Tupiocoris cucurbitaceus sobre
tres tipos de presas expuestas en parches sobre un folíolo fresco de tomate y
mantenidos con una capa de agar-agua.
Since we were interested in determining the predatory action of T.
cucurbitaceus on P. absoluta, we considered this species as a TP
while the other prey species were treated as NTP. To avoid food depletion
during the experiment, the quantity of P. absoluta and E. kuehniella eggs
offered was estimated based on previous diet reports by Burla
et al. (2014), López et al. (2019)
and Duarte et al. (2022) for T.
cucurbitaceus, Macrolophus basicornis (Stål) and Engytatus
varians (Distant) (Hemiptera: Miridae), respectively. The number of T.
vaporariorum nymphs used in the experiment was based on T. cucurbitaceus
food uptake registered by Burla et al. (2014).
Patches of prey were carefully deposited on the leaflet using a fine brush with
the aid of a stereoscope microscope and placed at equidistant points. The
quality of all prey items was checked before starting the trial to discard
collapsed eggs or dead whiteflies. Each treatment was replicated 15 times and
experimental units were not re-utilized.
Prey searching behavior was studied for 30 min using the
software EthoVision® XT (Noldus, The Netherlands) which videotapes and analyzes
animal activity inside an arena. Various steps were followed to calibrate the
recording of T cucurbitaceus when visiting the food patches or the time
they spent outside the patches (i.e., clean parts of the leaflet).
Observations were made between 10 am and 2 pm. Environmental conditions
remained similar for all replicates during the trial (25±2°C and 60±10% RH).
Four behavioral
descriptors were evaluated: 1) time spent in each of the three food patches or
on the clean leaflet, 2) accumulated time of T. cucurbitaceus nymphs and
adults in movement or non-movement, 3) visit frequency (first visit to TP and NTP),
i.e., the number of times that the predator entered the patch, and
recurrent visit or re-visits to TP, and 4) the maximum number of times
predators alternated among patches. Given our interest in mirid behavior
concerning that prey, only revisits to the TP (i.e., with P. absoluta)
were considered. Thus, we set out the trial to analyze whether T.
cucurbitaceus, after choosing TP as the first option, decided to revisit
more frequently, i.e., whether the predator preferred that food, or not.
Feeding
preference assay
The feeding
preference of T. cucurbitaceous nymphs, females, and males at 30 min and
then for 24 h was evaluated by registering the number of preyed lepidopteran
eggs or whitefly nymphs (figure 1). The number of preys
consumed at 30 min was counted by removing predators from the experimental unit
and keeping in labelled Eppendorf tubes, then restored to its unit. Later, all
units were placed in a rearing chamber (I501PF, SEMEDIX, Argentina) at
controlled temperature, relative humidity, and photoperiod conditions (25 ±
1°C, 65 ± 5% RH, 14:10 L:D) to check prey consumption
at 24 h, without food replacement. After the end of the trial, the experimental
units were checked to record preyed food using a stereoscopic microscope (Nikon
SMZ1270) to observe and count the remains of eggs and nymphs caused by the
stylets of the mirid. The occurrence of phytophagous behavior was checked by
observing the presence of feeding punctures in leaflets (38). Preference was observed at 30 min since we aimed
to discern whether the starvation period could influence the first food
election for the predator. Instead, consumption at 24 h could bring information
on prey choice when prey density decreased.
Statistical
analysis
The time spent by
individuals in the clean leaflet or prey patches was analyzed with a
Generalized Linear Mixed Model (GLMM) using lme4 package and glmer function (2) with the type of patches and developmental
stage, i.e., late nymph, female and male adults as fixed factors and the
individual as a random factor. The accumulated time of T. cucurbitaceus nymphs
and adults in movement or non-movement was measured as the proportion of the
time in movement / the total time of the experiment (1800 s) (response variable)
and analyzed using Beta regression with betareg package (12), being the stage of predatory individuals
(nymphs, female, and male adults of T. cucurbitaceus) the predictive
factor. The frequency of the first visit to TP or NTP was analyzed using 2 x 2
contingency tables with Fisher’s exact test for each developmental stage and
sex of T. cucurbitaceus separately. Then, since the study was aimed to
evaluate the feeding preference of this predator on P. absoluta,
compared with other two possible prey items, we evaluated the frequency of
those individuals who revisited the TP after visiting that TP as its first
choice using 2 x 2 contingency tables with Fisher’s exact test. The maximum
number of food patch alternations was analyzed by Kruskal-Wallis test because
the data was not normal. Then, Dunn’s test checked for significant differences
among factor levels with a p-value adjusted by the Benjamini-Hochberg method
for multiple comparisons.
Considering the feeding preference assay, the proportions of
prey eaten at 30 min were compared using Manly’s Alpha index without prey
replacement (25), as follows:
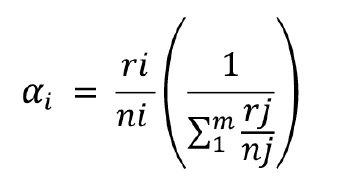
where
αi = Manly’s Alpha
index for prey i
ri, rj = Proportion of
prey type i
j in the diet (i and
j = 1, 2,…, m)
ni, nj = Proportion of
prey type i and j in the environment
m = number of prey types possible
The Manly’s Alpha
index varies between 0 and 1, and because in this study three types of prey
were offered, values of α = 0.33 indicated no preference, greater than 0.33 a
preference, and lower than 0.33 a rejection. The number of prey of each food
type consumed at 24 h was calculated as the number of prey alive after 24 h -
the initial number of offered prey. Then the proportion of consumed prey (i.e.,
the number of individuals of each prey type consumed / the number of
individuals of that prey type alive after 24 h) was analyzed using a logistic
model (binomial family, logit link function), with the individuals (4-5th
instar nymphs, females, and males of T. cucurbitaceus) and
the type of prey (P. absoluta eggs, T. vaporariorum nymphs, and E.
kuehniella eggs) as the predictor variables. All analyses were carried out
using R software (36).
Results
Prey
searching behavior
Predators spent significantly more time on the clean leaflet
than in any of the food patches (χ2= 57.44; df= 3; P<0.001), with females
spending more time outside of food patches than 4-5th nymphs and males (χ2=
6.5; df= 2; P= 0.04). When present on food patches, all predators spent a
similar amount of time on any of the three sources (figure 2).
All
bar graphs are represented by mean ± SE. Different letters show significant
differences (p-value<0.05).
Las barras
indican el promedio ± EE. Letras diferentes indican diferencias significativas
(p valor<0,05).
Figure
2. Time (s) elapsed in the different food patches (P.
absoluta eggs, E. kuehniella eggs, and T. vaporariorum nymphs)
by T. cucurbitaceus individuals (4-5th instar nymphs, females, and males) in 30 min.
Figura 2. Tiempo
(seg) transcurrido por individuos de T. cucurbitaceus (ninfas de 4-5°
estadio, hembras y machos) en los diferentes parches de alimento (huevos de P.
absoluta, huevos de E. kuehniella y ninfas de T. vaporariorum)
en 30 min de observación.
Regarding walking
activity, all predatory individuals remained still for almost the 30 min
tested, except for more active males (χ2= 25.07; df= 2; P<0.001). Given the
small size and coloration of these insects -particularly nymphs-, on some
occasions, the software was unable to detect activity responses (either moving
or not moving). Failure was about 180 s for nymphs, while for adults of both
sexes, the error was lower (<50 s).
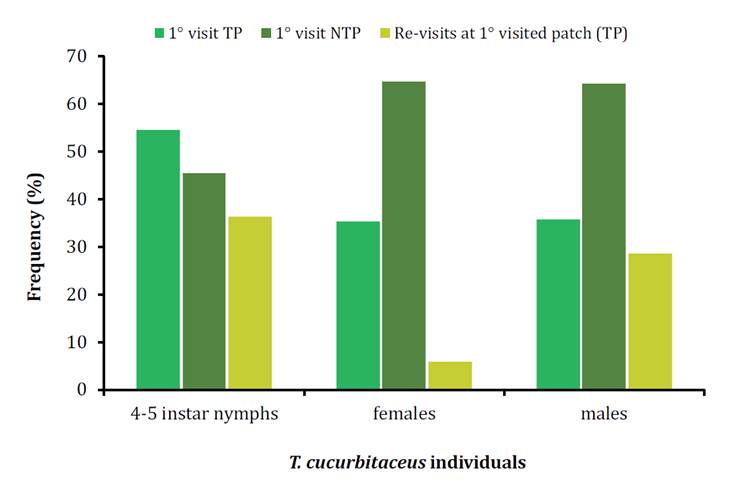
The frequency of first visits to the TPs (P. absoluta eggs)
and NTPs (E. kuehniella eggs and whitefly nymphs) was similar for all T.
cucurbitaceus individuals (females: P= 0.08, males: P= 0.13, nymphs: P=
0.5). Mirid nymphs and males returned to the TPs independently of their first
visit to that patch (males: P= 0.13, nymphs: P= 0.5). Females did not re-visit
TPs (P= 0.04) (figure 3). In addition, males of T.
cucurbitaceus showed a higher frequency of interchanges among patches than
females and nymphs (χ2= 15.25; df= 2; P<0.001).
Figure
3. Frequency (%) of first visit to the target patch (P.
absoluta eggs) and non-target patch (E. kuehniella eggs and T.
vaporariorum nymphs), and re-visits to the target patch when it was the
first patch visited by individuals of T. cucurbitaceus (4-5th instar
nymphs, females, and males) in 30 min. TP: target patch, NTP: non-target patch.
Figura 3. Frecuencia
(%) de primera visita al parche blanco (huevos de P. absoluta) y parche
no blanco (huevos de E. kuehniella y ninfas de moscas blancas), y
revisitas al parche blanco cuando este fue el primer parche visitado por
individuos de T. cucurbitaceus (ninfas de 4-5° estadio, hembras y
machos) en 30 min de observación.
Feeding
preference
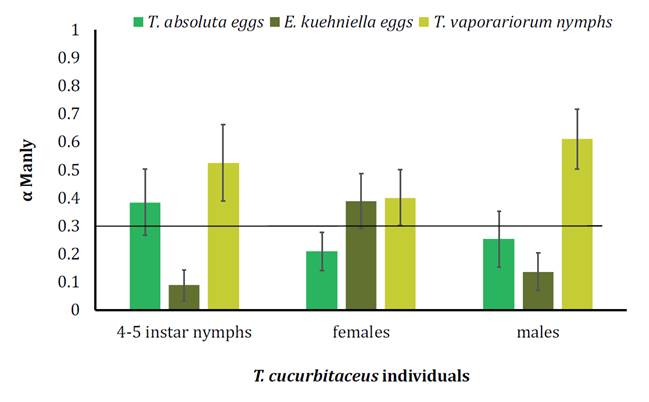
During the 30 min
trial, all T. cucurbitaceus individuals tested consumed more T.
vaporariorum nymphs, while predatory nymphs also fed on P. absoluta eggs
and females did on E. kuehniella eggs. Besides, T. cucurbitaceus nymphs
and males rejected feeding on E. kuehniella eggs (figure
4).
The
black line indicates indifference (α= 0.33). All bars indicate mean ± SE.
La
línea negra indica indiferencia (α= 0,33). Las barras indican el promedio ± EE.
Figure
4. Preference Index (Manly’s α) of 4-5th instars nymphs, females, and males of T.
cucurbitaceus for P. absoluta and E. kuehniella eggs, and T.
vaporariorum nymphs, after the first 30 m of the trial.
Figura
4. Índice de preferencia (α de Manly) de las ninfas de
4-5° estadio, hembras y machos de T. cucurbitaceus por huevos de P. absoluta
y E. kuehniella y ninfas de T. vaporariorum luego de 30 min
de iniciado el ensayo.
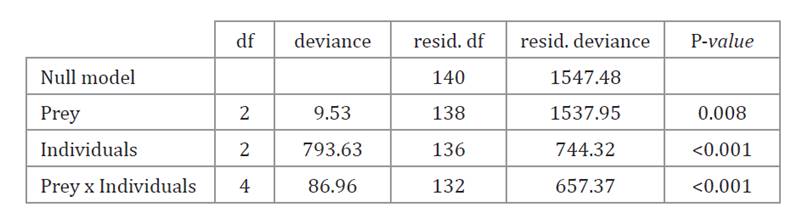
After 24 h trial, T.
cucurbitaceus nymphs ate more eggs of P. absoluta than females and
males. Mirid nymphs also consumed a greater proportion of E. kuehniella eggs
and T. vaporariorum nymphs than females and males except for the latter
which mainly fed on whitefly nymphs (table 1; figure 5).
Table 1. Results
of the ANOVA of the logistic model (binomial family) to analyze the proportion
of different prey eaten by T. cucurbitaceus (4-5 instar nymphs, females,
and males) after 24 h.
Tabla
1. Resultados del ANOVA del modelo
logístico (familia binomial) para analizar la proporción consumida de las
diferentes presas por T. cucurbitaceus (ninfas de 4-5° estadio, hembras
y machos) al cabo de 24 h.
All
bar graphs indicate mean ± SE. Asterisks denote significant differences between
individuals (late instar nymphs, females, and males), and different letters
show significant differences among prey items (p-value<0.05).
Las
barras indican el promedio ±EE. Los asteriscos muestran diferencias
significativas entre individuos (ninfas de 4-5° estadio, hembras y machos), y
letras diferentes indican diferencias significativas entre ítems de presas (p
valor<0,05).
Figure
5. Proportion (mean) of each prey item eaten by T.
cucurbitaceus individuals (4-5th instars nymphs, females, and males) in 24 h.
Figura
5. Proporción (promedio) de cada ítem
de presa consumido por individuos de T. cucurbitaceus (ninfas de 4-5° estadio,
hembras y machos) en 24 h.
At the beginning of the experiment, all 24 h starved nymphs and
adults of T. cucurbitaceus practiced phytophagy on the leaflet
immediately after being placed in the experimental unit even in the presence of
prey. However, no direct leaf injury was registered after feeding.
Discussion
In this study, we
present novel knowledge on the feeding habits of T. cucurbitaceus, a
biocontrol agent of P. absoluta and whiteflies. The results confirmed
the generalist feeding behavior of T. cucurbitaceus since the different
developmental stages tested did not show clear patterns for food search. Mirid
males searched for and consumed more T. vaporariorum. Tupiocoris cucurbitaceus
nymphs also initially preferred whiteflies nymphs, but after 24 h consumed
more lepidopteran eggs. Notably, T. cucurbitaceus females avoided P.
absoluta eggs when first presented and ate all prey items in a similar
proportion. As a result, the depletion of the mostly consumed prey across the
experiment (observed in the 24 h trial) could force them to choose other
available prey. This may indicate that, in the field, T. cucurbitaceus will
consume the more abundant prey species. Likewise, Jaworski
et al. (2013) showed that Macrolophus pygmaeus Rambur
(Hemiptera: Miridae) was able to switch feeding from B. tabaci to P.
absoluta depending on their relative numbers. These results stress the
importance of evaluating simultaneous prey offers to corroborate the in-field
effectiveness of entomophagous biocontrol agents. Other studies proved that in
a single-prey system, T. cucurbitaceus showed a greater consumption rate
of P. absoluta eggs than those fed on B. tabaci and M.
persicae nymphs, and T. urticae adults (23).
Urbaneja et al. (2009) also found high
consumption of P. absoluta eggs by other two mirids, Macrolophus
pygmaeus Rambur and Nesidiocoris tenuis Reuter.
Zoophytophagy is an important aspect to consider when using
predators as biological control agents (11, 34).
Interestingly, starved 4-5th nymphs and adults of T.
cucurbitaceus consumed plant tissue before feeding on prey but this
behavior did not result in noticeably injury to the leaflet. Similarly,
non-damaging feeding habit was also reported previously for this mirid species
tested on tobacco Nicotiana tabacum L. and tomato plants without adding
any prey (8). However, the potential
plant injury caused by T. cucurbitaceus should be more thoroughly
evaluated to discard effects on the crop yield.
In sum, meticulous
studies on diet breadth of generalist predators should avoid failures in
biocontrol programs (42). Currently, two
mirid species, M. pygmaeus and N. tenuis, have proved to be
successful in control programs in Europe against the tomato moth P. absoluta
(13). In Brazil, several studies
showed other hemipteran species such as Macrolophus basicornis (Stal), Engytatus
varians (Distant), Campyloneuropsis infumatus (Carvalho),
(Hemiptera: Miridae), and Podisus nigrispinus (Dallas) (Hemiptera:
Pentatomidae) as promissory biological control agents of this pest (5, 7, 38, 43). Notably, T. cucurbitaceus is
a dominant predatory species in northern Buenos Aires horticultural crops,
co-occurring with T. vaporariorum and P. absoluta populations (29) allowing strategies for its augmentation and
conservation to improve pest control. This study and others (22, 23, 24, 44) highlight the value of native
beneficial fauna and the importance of preserving their natural presence in
crops to contribute to IPM programs. In that context, we are currently
assessing the potential of other entomophagous insects of P. absoluta as
biological control agents, including intraguild predation interaction studies.
Conclusions
Results confirmed
the generalist feeding behavior of T. cucurbitaceus since the different
developmental stages tested in the laboratory did not show clear patterns when
searching for the prey items offered. This finding could indicate that, in the
field, this predatory species will consume the more abundant prey species,
evidencing the importance of evaluating simultaneous prey offers to corroborate
biocontrol effectiveness under crop conditions. Since we observed modest leaf
consumption by T. cucurbitaceus in laboratory trials, the potential
plant injury should be more thoroughly evaluated to discard effects on crop
yield. We are currently assessing the potential of other entomophagous insects
of P. absoluta as biological control agents, including intraguild
predation interaction studies.
Acknowledgements
We thank Alberto
Urbaneja (IVIA, Spain) for the critical review of the earlier version of the
manuscript, and to Eliana Nieves and Alina Cerquetti (CEPAVE) who kindly helped
with insect rearing. The research was funded by PICT 2019-01745, Director M.
Rocca, and PICT 2020-0764 Director: M. G. Luna (Agencia
Nacional de
Promoción Científica y Tecnológica, Argentina). This study is part of the MSc
thesis dissertation of R. Montiel Cáceres, Facultad de Agronomía, Universidad
de Buenos Aires, Área Producción Vegetal (Argentina).
1. Basso, C.;
Cibils-Stewart, X. 2020. Foundations and developments of pest management in
Uruguay: a review of the 20 lessons and challenges. Agrociencia Uruguay. 24(2):
409. https://doi. org/10.31285/AGRO.24.409
2. Bates, D.;
Mächler, M.; Bolker, B.; Walker, S. 2015. Fitting linear mixed-effects models
using lme4. Journal of Statistical Software 67(1): 1-48.
https://doi:10.18637/jss.v067.i01
3. Bell, W. J.
1990. Searching behavior patterns in insects. Annual Review of Entomology. 35:
447-467. https://doi.org/10.1146/annurev.en.35.010190.002311
4. Biondi, A.;
Guedes, R. N. C.; Wan, F. H.; Desneux, N. 2018. Ecology, worldwide spread, and
management of the invasive South American tomato pinworm, Tuta absoluta:
past, present, and future. Annual Review of Entomology 63: 239-258.
https://doi.org/10.1146/ annurev-ento-031616-034933
5. Bottega, D. B.;
Souza, B. H. S.; Rodrigues, N. E. L.; Eduardo, W. I.; Barbosa, J. C.; Boiça Júnior,
L. A. 2017. Resistant and susceptible tomato genotypes have direct and indirect
effects on Podisus nigrispinus preying on Tuta absoluta larvae.
Biological Control. 106: 27-34.
https://doi.org/10.1016/j.biocontrol.2016.12.0066
6. Bueno, V. H. P.
2009. Biological pest control: Mass production and quality control. 2nd.
Editora da UFLA, Lavras, Brazil.
7. Bueno, V. H. P.;
van Lenteren, J. C.; Calixto, L. A. M. Jr.; Montes, F.; Silva, D.; Santiago, L.
D.; Pérez, L. M. 2013. New records of Tuta absoluta (Meyrick)
(Lepidoptera: Gelechiidae) predation by Brazilian Hemipteran predatory bugs.
Journal of Applied Entomology 137: 29-34. https:// doi.org/10.1111/jen.12017
8. Burla, J. P.;
Grille, G.; Lorenzo, M. E.; Franco, J.; Bonato, O.; Basso, C. 2014. Effect of
different diets on the development, mortality, survival, food uptake and
fecundity of Tupiocoris cucurbitaceus (Hemiptera: Miridae). Florida
Entomology 97(4): 1816-1824.
9. Calvo, F. J.;
Bolckmans, K.; Belda, J. E. 2012. Biological control-based IPM in sweet pepper
greenhouses using Amblyseius swirskii (Acari: Phytoseiidae). Biocontrol
Science and Technology. 22: 1398-1416. https:
//doi.org/10.1080/09583157.2012.731494
10. Castañé, C.;
Alomar, O.; Goula, M.; Gabarra, R. 2004. Colonization of tomato greenhouses by
the predatory mirid bugs Macrolophus caliginosus and Dicyphus
tamaninii. Biological Control 30: 591–597.
https://doi.org/10.1016/j.biocontrol.2004.02.012
11. Castañé, C.; Arnó, J.; Gabarra, R.; Alomar, O. 2011. Plant
damage to vegetable crops by zoophytophagous mirid predators. Biological
Control 59: 22-29.https://doi.org/10.1016/j. biocontrol.2011.03.007
Cribari-Neto, F.;
Zeileis, A. 2010. Beta Regression in R. Journal of Statistical Software. 34(2):
1-24. https://doi:10.18637/jss.v034.i02
13. Desneux, N.;
Han, P.; Mansour, R. Arnó, J.; Brévault, T.; Campos, M.; Chailleux, A.; Guedes,
R.; KarimiJ, J.; Kouassi, A.; Lavoir, A.; Luna, M.; Perez-Hedo, M.; Urbaneja,
A.; Verheggen, F.; Zappalà, L.; Abbes, K.; Ali, A.; Bayram, Y.; Cantor, F.; Cuthbertson,
A.; De Vis, R.; Erler, F.; Firake, D.; Haddi; K.; Hajjar, M.; Ismoilov, K.;
Jaworski, C.; Kenis, M.; Liu, H.; Madadi, H.; Martin, T.; Mazih, A.; Messelink,
G.; Mohamed, S.; Nofemela, R.; Oke, A.; Ramos, C.; Ricupero, M.; Roditakis, E.;
Shashank, P.; Wan, F.; Wang, M.; Wang, S.; Zhang, Y.; Biondi, A. 2021.
Integrated pest management of Tuta absoluta: practical implementations
across different world regions. Journal of Pest Science. 95: 17-39. https://
doi.org/10.1007/s10340-021-01442-8
14. Donnelly, B.
A.; Phillips, T. W. 2001. Functional response of Xylocoris flavipes (Hemiptera:
Anthocoridae): effects of prey species and habitat. Environmental Entomology.
30: 617-624. https://doi.org/10.1603/0046-225X-30.3.617
15. Duarte
Martínez, L.; Martínez Rivero, M. A.; Bueno, V. H. P.; Collatz, J. 2022.
Predation behaviour and prey preference of two neotropical mirids against two
key lepidopteran pests in tomato. International Journal of Tropical Insect
Science 42(1): 815-825 https://doi.org/10.1007/ s42690-021-00605-5
16. Enkegaard, A.;
Brødsgaard, H. F.; Hansen, D. L. 2001. Macrolophus caliginosus:
functional response to whiteflies and preference and switching capacity between
whiteflies and spider mites. Entomologia Experimentalis et Applicata 101: 81-88
https://doi.org/10.1046/j.1570- 7458.2001.00893.x
17. Ferreira, P. S.
F.; Henry, T. J. 2011. Synopsis and keys to the tribes, genera, and species of
Miridae (Hemiptera: Heteroptera) of Minas Gerais, Brazil. Part I: Bryocorinae.
Zootaxa. 2920: 1-41. https://doi.org/10.11646/zootaxa.2920.1.1
18. Greco, N.;
Rocca, M. 2020. Depredadores. In: Polack, L. A.; Lecuona, R. E.; López, S. N.
(eds.). Control biológico de plagas en horticultura: experiencias argentinas de
las últimas tres décadas, Ciudad Autónoma de Buenos Aires: Ediciones INTA.
19. Ingegno, B. L.
B.; Ferracini, C.; Gallinotti, D.; Alma, A.; Tavella, L. 2013. Evaluation of
the effectiveness of Dicyphus errans (Wolff) as predator of Tuta
absoluta (Meyrick). Biological Control. 67: 246-252. https://doi.org/10.1016/j.biocontrol.2013.08.002
20. Jaworski, C.
C.; Bompard, A.; Genies, L.; Amiens-Desneux, E.; Desneux, N. 2013. Preference
and prey switching in a generalist predator attacking local and invasive alien
pests. PLoS ONE. 8(12): e82231. https://doi.org/10.1371/journal.pone.0082231
21. Koul, O.;
Dhaliwal, G. S. 2003. Predators and Parasitoids. Taylor & Francis. London.
22. López, S. N.;
Arce Rojas, F.; Villalba, V.; Cagnotti, C. 2012. Biology of Tupiocoris
cucurbitaceus (Hemiptera: Miridae), a predator of the greenhouse whitefly Trialeurodes
vaporariorum (Hemiptera: Aleyrodidae) in tomato crops in Argentina.
Biocontrol Science and Technology. 22(10): 1107-1117.
https://doi.org/10.1080/09583157.2012.705260
23. López, S. N.;
Orozco Muñoz, A.; Andorno, A. V.; Cuello, E. M.; Cagnotti, C. 2019. Predatory
capacity of Tupiocoris cucurbitaceus (Hemiptera: Miridae) on several
pests of tomato. Bulletin of Insectology 72: 201-205.
24. Luft, E.; Luna,
M. G.; Galise, G.; Speranza, S.; Virla, E. 2015. Mortalidad natural de huevos
de la polilla del tomate, Tuta absoluta (Meyrick) (Lepidoptera:
Gelechiidae) en Argentina e Italia, y primera mención de Encarsia porteri (Mercet)
(Hymenoptera: Aphelinidae) afectando sus poblaciones. Revista de la Facultad de
Ciencias Agrarias. Universidad Nacional de Cuyo. Mendoza. Argentina. 47(2):
219-229.
25. Manly, B. F. J.
1974. A model for certain types of selection experiments. Biometrics. 30:
281-294. https://doi.org/10.2307/2529649
26. Martin, J. H.
1987. An identification guide to common whitefly pest species of the world
(Homoptera Aleyrodidae). Tropical Pest Management 33(4): 298–322.
http://dx.doi. org/10.1080/09670878709371174
27. Messelink, G.;
Bloemhard, C. M. J.; Hoogerbrugge, H.; van Schelt, J.; Ingegno, B. L.; Tavella,
L. 2014. Evaluation of mirid predatory bugs and release strategy for aphid
control in sweet pepper. Journal of Applied Entomology 139(5): 333–341
https://doi.org/10.1111/jen.12170
28. Mollá, O.;
Biondi, A.; Alonso-Valiente, M.; Urbaneja, A. 2014. A comparative life history
study of two mirid bugs preying on Tuta absoluta and Ephestia
kuehniella eggs on tomato crops: implications for biological control.
BioControl 59: 175–183. https://doi.org/10.1007/ s10526-013-9553-8
29. Montiel
Cáceres, R.; Salas Gervassio, N. G.; Minghetti, E.; Dellapé, P.; Luna, M. G.;
Rocca, M. 2023. Heteropteran bugs assemblage associated with organic tomato
farms: knowledge for pest management. Neotropical Entomology 52: 251–262
https://doi.org/10.1007/s13744- 022-01007-z
30. Nieves, E.; Pereyra, P. C.; Luna, M. G.; Medone, P.;
Sánchez, N. E. 2015. Laboratory population parameters and field impact of the
larval endoparasitoid Pseudapanteles dingus (Hymenoptera: Braconidae) on
its host Tuta absoluta (Lepidoptera: Gelechiidae) in tomato crops in
Argentina. Journal of Economic Entomology 108: 1553–1559. https://doi.
org/10.1093/jee/tov115
31. Parajulee, M.
N.; Phillips, T. W.; Hogg, D. B. 1994. Functional response of Lyctocoris
campestris (F.) adults: effects of predator sex, prey species, and
experimental habitat. Biological Control. 4: 80-87.
32. Paula, D. P.;
Andow, D. A.; Barratt, B. I. P.; Pfannenstiel, R.; Gerard, P.; Todd, J.;
Zaviezo, T.; Luna, M.; Cédola, C.; Loomans, A.; Howe, A.; Day, M.; Ehlers, C.;
Green, C.; Arpaia, S.; Yano, E.; Lövei, G.; Hinomoto, N.; Fontes, E.; Pires,
C.; Togni, P.; Nechols, J.; Eubanks, M.; van Lenteren, J. 2021. Integrating
adverse effect analysis into environmental risk assessment for exotic
generalist arthropod biological control agents: A three-tiered framework.
BioControl. 66: 113-139. https://doi.org/10.1007/s10526-020-10053-8
33. Pérez-Hedo, M.;
Urbaneja, A. 2015. Prospects for predatory mirid bugs as biocontrol agents of
aphids in sweet peppers. Journal of Pest Science. 88: 65-73. https://doi.org/10.1007/
s10340-014-0587-1
34. Pérez-Hedo, M.;
Rambla, J. L.; Granell, A.; Urbaneja, A. 2017. Biological activity and
specificity of Miridae-induced plant volatiles. BioControl. 63: 203-213.
https://doi.org/10.1007/ s10526-017-9854-4
35. Polack, L. A.;
López, S. N.; Silvestre, C.; Viscarret, M.; Andorno, A.; del Pino, M.; Peruzzi,
G.; Gómez, J.; Iezzi, A. 2017. Control biológico en tomate con el mírido Tupiocoris
cucurbitaceus. Comunicación INTA.
36. R Core Team.
2020. A language and environment for statistical computing. R Foundation for
Statistical Computing, Vienna. Austria. https://www.Rproject.org/
37. Scopes, N. E.
A.; Biggerstaff, S. M. 1971. The production, handling and distribution of the
whitefly Trialeurodes vaporariorum and its parasite Encarsia formosa for
use in biological control programmes in glasshouses. Plant Pathology 20(3):
111-116. https://doi. org/10.1111/j.1365-3059.1971.tb00525.x
38. Silva, D. B.;
Bueno, V. J. P.; Montes, F. C.; van Lenteren, J. C. 2016. Population growth of
three mirid predatory bugs feeding on eggs and larvae of Tuta absoluta on
tomato. Biological Control. 61: 545-553.
https://doi.org/10.1007/s10526-016-9736-1
39. Symondson, W.
O. C.; Sunderland, K. D.; Greenstone, M. H. 2002. Can generalist predators be
effective biocontrol agents? Annual Review of Entomology. 47: 561-594.
https://doi.org/10.1146/ annurev.ento.47.091201.145240
40. Urbaneja, A.;
Montón, H.; Mollá, O. 2009. Suitability of the tomato borer Tuta absoluta as
prey for Macrolophus pygmaeus and Nesidiocoris tenuis. Journal of
Applied Entomology. 133: 292-296.
https://doi.org/10.1111/j.1439-0418.2008.01319.x
41. van Lenteren,
J. C.; Tommasini, M. G. 2003. Mass production, storage, shipment and release of
natural enemies. In: van Lenteren, J. C. (ed). Quality control and production
of biological control agents. Theory and testing procedures. CABI Publishing,
Wallingford. UK.
42. van Lenteren,
J. C.; Bolckmans, K.; Köhl, J.; Ravensberg, W. J.; Urbaneja, A. 2018a.
Biological control using invertebrates and microorganisms: plenty of new
opportunities. Biological Control. 63: 29-59.
https://doi.org/10.1007/s10526-017-9801-4
43. van Lenteren,
J. C.; Bueno, V. H. P.; Calvo, F. J.; Calixto, A. M.; Montes, F. C. 2018b.
Comparative effectiveness and injury to tomato plants of three neotropical
mirid predators of Tuta absoluta (Lepidoptera: Gelechiidae). Journal of
Economic Entomology. 111: 1080-1086. https://doi.org/10.1093/jee/toy057
44. van Lenteren,
J. C.; Lanzoni, A.; Hemerik, L. Bueno, V.; Bajonero Cuervo, J.; Biondi, A.;
Burgio, G.; Calvo, F.; de Jong, P.; López, S.; Luna, M.; Montes, F.; Nieves,
E.; Aigbedion-Atalor, P.; Riquelme Virgala, M.; Sánchez, N.; Urbaneja, A. 2021.
The pest kill rate of thirteen natural enemies as aggregate evaluation
criterion of their biological control potential of Tuta absoluta. Science
Report. 11(1): 10756. https://doi.org/10.1038/s41598-021-90034-8
Experimental ethics
Permits for insect
collections were obtained from Dirección de Flora y Fauna, Ministerio de
Desarrollo Agrario de la provincia de Buenos Aires. Natural resources involved
in this study are the exclusive property of the Buenos Aires province,
Argentina.
Conflicts of
interest
This manuscript and the authors of the manuscript are not
involved in any potential conflicts of interest, including financial interests
and relationships and affiliations.