Revista de la Facultad de Ciencias
Agrarias. Universidad Nacional de Cuyo. Tomo 57(1). ISSN (en línea) 1853-8665.
Año 2025.
Original article
Influence
of Apis mellifera in-hive conditions on germination capacity of rapeseed
pollen (Brassica napus)
Influencia
del ambiente interno de la colmena de Apis mellifera sobre la capacidad
germinativa del polen de colza (Brassica napus)
Soledad Camila
Villamil1,
Liliana Maria
Gallez1
1Universidad Nacional del Sur (UNS). Departamento de Agronomía.
Laboratorio de Estudios Apícolas (LabEA-CIC). San Andrés 800. Bahía Blanca.
Buenos Aires. Argentina.
Abstract
Brassica napus L. (rapeseed,
canola) ranks third in worldwide importance among oilseeds. The production of
hybrid rapeseed seed requires an androsterile female parent; therefore,
fertilization is possible through pollinators carrying viable pollen from an
androfertile line. To ensure high pollinator populations, hives are used.
However, little is known about the risk of transporting viable pollen into
hives. The in vitro germinability of pollen exposed to in-hive
conditions was evaluated. Samples of rapeseed pollen obtained from potted
plants were placed in four hives of Apis mellifera L. In-hive conditions
are unfavorable for rapeseed pollen germinability. Brood areas with the highest
temperatures showed no germinated pollen grains within 24 h. Starting at 48 h,
germinability decreased significantly, with germinated grains showing atrophied
tubes. At 72 h, pollen placed away from brood areas lost germinability.
Keywords: canola,
germinability, honey bee, pollination
Resumen
Brassica napus L. (colza, canola)
es la tercera en importancia mundial de las oleaginosas. La producción de
semilla híbrida de colza requiere de una línea parental androestéril; por lo
tanto, su fertilización es posible a través de los polinizadores que portan
polen viable desde una línea androfértil. Para lograr una alta población de
polinizadores se colocan colmenas. Sin embargo, poco se conoce sobre el riesgo
de transportar polen viable en el interior de las mismas. Se evaluó la
germinabilidad in vitro del polen expuesto a las condiciones ambientales
dentro de la colmena. A partir de plantas de colza cultivadas en macetas, se
obtuvieron muestras de polen que se colocaron dentro de cuatro colmenas de Apis
mellifera L. Las condiciones dentro de las colmenas son desfavorables para
la germinabilidad de los granos de polen de colza. En el área de cría con las
temperaturas más altas en 24 horas no se registraron granos de polen capaces de
germinar. A partir de las 48 horas, la germinación decreció significativamente
y los granos germinados mostraron tubos atrofiados. El polen ubicado más lejos
del área de cría mantuvo su germinabilidad por menos de 72 horas.
Palabras claves: colza,
germinabilidad, abeja melífera, polinización
Originales: Recepción: 14/03/2024
- Aceptación: 20/09/2024
Introduction
The productivity of
cultivated plants, especially those that bear fruit, seed, or grain, is highly
correlated with pollen production and viability. This viability can be strongly
influenced by non-optimal environmental conditions, such as drought, heat, and
solar radiation (13, 24, 25), causing reduced
fruit set and yield, as reported for rapeseed crops exposed to high
temperatures during flowering (2, 33).
Brassica napus L. (rapeseed,
canola) ranks first among Brassicaceae and third among oilseeds, after palm and
soybean (19, 31). The seed production of hybrid
rapeseed requires an androsterile female parent and pollinators that transport
viable pollen from an androfertile line (6,
23, 32). Female line productivity, pollen production, and
floral-synchrony management between parental lines are key aspects for
maximizing yield. Under this production scheme, the potential contamination with
foreign pollen poses a major risk. In rapeseed, cross-pollination decreases
with the increase in distance between receptive stigma and pollen source (11). Therefore, in
hybrid seed production, the spatial distribution of plots is carefully defined
to prevent the androsterile line from being fertilized by foreign pollen (32). Typically, such
production considers natural pollen transfer by wind, water, or insects rather
than human-mediated unintentional transfer (21,
30).
Unlike naturally
occurring pollen transfer, the anthropic movement of pollen has scarcely been
studied. In the case of entomophily, there is limited information on the
duration of pollen viability on insect bodies (12,
20). Colonies dedicated to pollination in hybrid seed production
are commonly moved from one field to another in the same crop. Pollen in hives
or on bees could contaminate production if the androfertile lines in successive
fields are different. Pollen already processed by bees (corbicular pollen,
pressed pollen, or bee bread) is of no concern; rather, pollen grains that
remain on their bodies or are free in the hive are in question (20).
The production of
hybrid rapeseed seed requires large quantities of pollinators in a relatively
short period, specifically during crop flowering. Native pollinators are highly
efficient in pollen transfer, but their low and unpredictable population
density requires incorporation of human-managed pollinators. The species most
commonly used for pollinating crops, including rapeseed, is Apis mellifera L.
(32).
Despite its global presence, honey bees maintain consistent
colony traits, notably brood nest temperature (14).
For proper development, A. mellifera larvae need a stable temperature,
which adults keep between 32 and 36°C (4, 5, 8, 27, 28,
29). Unlike temperature, worker bees have limited control of hive
humidity. The optimal humidity varies across the brood nest and fluctuates with
external conditions. Because temperature regulation takes priority, it
inevitably impacts humidity levels (9, 10).
Pollen grains on
bee bodies are exposed to in-hive conditions. Temperature can affect canola
pollen germinability during pre-anthesis (17), and germinability
and pollen tube length can be reduced when germinated at 33°C (18). Beekeepers must
follow strict hive movement rules to prevent potential contamination, even
without clear evidence of whether the pollen in the hives retains its
germination capacity. To date, there are no records of rapeseed pollen
germinability under actual in-hive conditions. This study aimed to determine
how long rapeseed pollen maintained germination capacity in the hive, helping
to establish safe intervals before moving hives between hybrid rapeseed fields,
thus minimizing the risk of contamination.
Materials
and Methods
Experimental
site
To assess in
vitro pollen germinability under hive conditions, experiments were
conducted once each spring (October/November) of 2017, 2018, and 2019 at the
Laboratorio de Estudios Apícolas, Universidad Nacional del Sur, Bahía Blanca,
Argentina (-38.694944, -62.253293). Four Langstroth-type A. mellifera hives
were selected each year, with nine frames per brood chamber. The seven central
frames held brood, while the two outer frames stored honey. No signs of disease
were observed. The queen was ovipositing prolifically.
Pollen
samples
Pollen samples were obtained from 20 Hyola 433 rapeseed plants
grown in 10-liter pots. When plants reached full bloom, mature flower buds
(close to opening) were labeled. The following day, at 7:30 AM (-3 GMT), 80 of
these marked flowers were harvested, ensuring that anthers had been fully
developed, as pollen is most fertile immediately after the flower opens (16).
Anthers from all harvested flowers were pooled into one sample. Three random
anthers were placed in 60 tubes (0.5 ml) and crushed with a histological needle
to release pollen. Tubes were left open in the hives, secured with two pins to
prevent movement due to bee activity (figure 1).
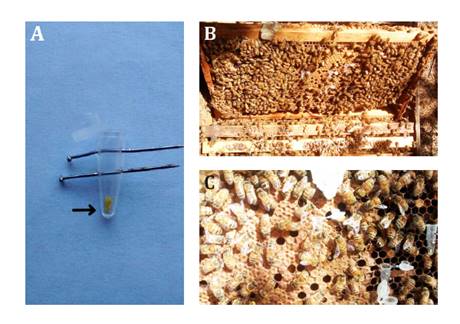
Figure 1. A:
Tube with Brassica napus anther tissue and pollen samples (arrow)
secured with pins to prevent movement from bee activity; B: Distribution of
pollen samples in an Apis mellifera hive; C: Detail of pollen samples on
a brood frame (Frame 5).
Figura 1. A:
Tubo con tejido de anteras y muestras de polen (flecha) de Brassica napus,
sujetado con alfileres para evitar su movimiento por la actividad de las
abejas; B: Distribución de muestras de polen dentro de una colmena de Apis
mellifera; C: Detalle de muestras de polen en un cuadro de cría (Cuadro 5).
Treatments
Out of 60 samples, the germinability of 12 freshly harvested
pollen samples was analyzed as the 0 h treatment. The remaining 48 tubes were
randomly grouped in fours and placed in three different frames in each hive,
with 12 samples per hive per year. Frame 5, in the center of the brood chamber,
had abundant brood. Frame 7, located between the edge and the center, had brood
in the center and honey and pollen on the margins. Frame 9, on the outer edge,
contained only honey (figure 2).

Figure 2. A:
Identification of frames in one of the four A. mellifera hives used for
the experiment; B: Frame 5, center of the brood chamber, completely covered by
brood; C: Frame 7, with brood in the center and stored honey and pollen on the
edges; D: Frame 9, outer edge of the brood chamber only with stored honey.
Figura 2. A:
Identificación de los cuadros de una de las cuatro colmenas de A. mellifera utilizada
para el ensayo; B: Cuadro 5, centro de la cámara de cría, cubierto
completamente por cría; C: Cuadro 7, compuesto por cría en el centro, miel y
polen en los bordes; D: Cuadro 9, externo de la cámara de cría compuesto por
reservas de miel.
Each year, temperature sensors (Onset HOBO UA-002-64
Temperature/Light Data Logger 64K spa) were placed to record in-hive conditions
every hour. During 2019, two sensors (HOBO Onset H08-032-IS Temperature/HR Data
Logger 64K HOBO), one in the brood area and another in the honey reserves, were
added to record humidity hourly in one of the hives. Over three consecutive
days, one tube per frame was removed every 24 h from each of the hives,
totaling 12 tubes per day, for analysis.
Technique
to determine germinability
The hanging drop technique was used to assess the percentage of in
vitro pollen germination (25). Brassica
napus pollen grains were incubated in a culture medium at 25°C and 90%
relative humidity for two hours. A drop of culture medium was placed on a glass
slide, and pollen samples were added. The glass slide was then inverted in a
sealed humid chamber with wet absorbent paper at the bottom. After two hours, a
cover slip was placed over the drop, and 500 pollen grains per sample were
counted under a microscope at 400X magnification. Germination was determined by
the percentage of grains with a pollen tube longer than grain diameter (15).
Culture
medium
The culture medium
used to measure in vitro pollen germinability, originally described for
sunflower by Astiz
(2012),
proved suitable for rapeseed pollen. It contained 150 g/L polyethylene glycol
6000 (PEG6000) in distilled water, 100 g/L sucrose, 240 mg/L calcium nitrate,
and 100 mg/L boric acid. The pH was maintained between 6.5 and 7.0, adjusted
with 0.1 N sodium chloride. PEG6000, which is inert to pollen metabolism and
unable to enter cells (22), was included to
enhance the development of pollen tubes by regulating plasma membrane
permeability and providing stability to the pollen tube membrane (22).
Statistical
analysis
The experimental design consisted of randomized complete blocks
with four replicates. Data were subjected to analysis of variance (ANOVA), and
if differences were detected at p-values < 0.05, means were compared using
Fisher’s LSD test. Statistical analyses were performed using Infostat software (7).
Results
In-hive
temperature and humidity
Over the three
years and during the experiments (three days), differences in hourly
temperature were observed among the hive sectors studied (p < 0.0001; n =
72). Frame 5 had the highest temperatures (around 35°C), followed by Frame 7
and 9 (figure
3).
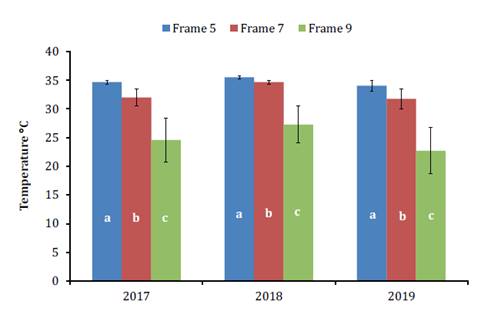
Las
barras muestran valores medios (± DE). Letras iguales indican que no se
detectaron diferencias significativas (p > 0,05) entre los grupos
analizados.
Figure
3. In-hive temperature variation in Frames 5, 7, and 9.
Figura
3. Variación de la temperatura dentro
las colmenas experimentales, Cuadro 5, 7 y 9.
Throughout the period of pollen exposure to in-hive conditions,
humidity showed no significant differences between brood and honey storage
areas (p = 0.1099; n = 138). In the area with stored honey, the average (± SD)
relative humidity was 42.09% (±10.47), and in the brood area it was 44.23% (±
5.29).
Pollen
germinability
In all years, the highest germination percentages (X̅ = 57.9%)
were obtained with freshly collected pollen (0 h) (p < 0.01). In-hive
conditions reduced rapeseed pollen germinability, with pollen in brood areas
losing its germination ability within 24 h. No significant differences were
found between brood frames (5 and 7) (p > 0.05).
Pollen in Frame 9, near honey reserves, retained its germination
capacity longer than that in the brood areas (p < 0.05) (figure 4).
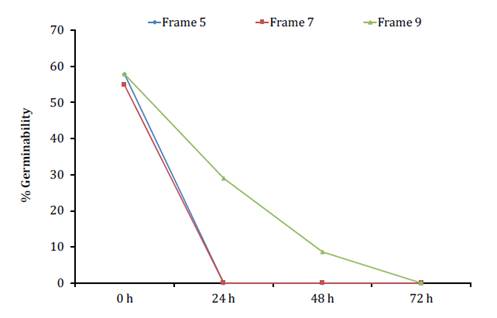
The curve
corresponding to Frame 5 overlaps with that of Frame 7.
La curva
correspondiente al Cuadro 5 se superpone con la del Cuadro 7.
Figure
4. Mean percentages of in vitro germinability of
rapeseed pollen grains after storage in different locations in an A.
mellifera hive.
Figura 4. Porcentajes
promedios de la germinabilidad in vitro de los granos de polen de colza
luego de permanecer en diferentes ubicaciones dentro de una colmena de A.
mellifera.
Across all trials, germination capacity dropped similarly, with
some pollen in Frame 9 still viable at 48 h, but falling below 20%. After 72 h,
no pollen germinated, in most cases with a significant reduction occurring
within 24 h.
Temperature averages in Frame 9 were 24.6 ± 3.8°C, 27.3 ± 3.2°C,
and 22.7 ± 4.0°C in 2017, 2018, and 2019, respectively. Despite these
differences, pollen germinability decreased substantially after 48 h of
exposure to in-hive conditions (figure 5).
Pollen germination capacity was completely lost after 72 h.
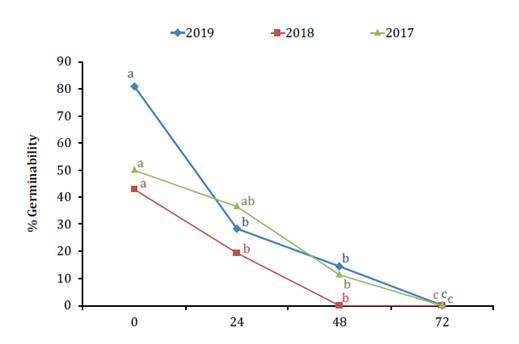
Equal letters
indicate no significant differences (p > 0.05) between the groups analyzed.
Letras iguales
indican que no se detectaron diferencias significativas (p > 0,05) entre los
grupos analizados.
Figure
5. Percentage of pollen germinability in Frame 9 in
2017, 2018, and 2019.
Figura 5. Porcentaje
de germinabilidad de los granos de polen en el Cuadro 9 en 2017, 2018 y 2019.
Fresh pollen had the thickest and most developed pollen tubes.
After 24 h in the hive, pollen grains had thinner, shorter, and convoluted
pollen tubes. After 48 h, pollen tubes were atrophied (figure 6).
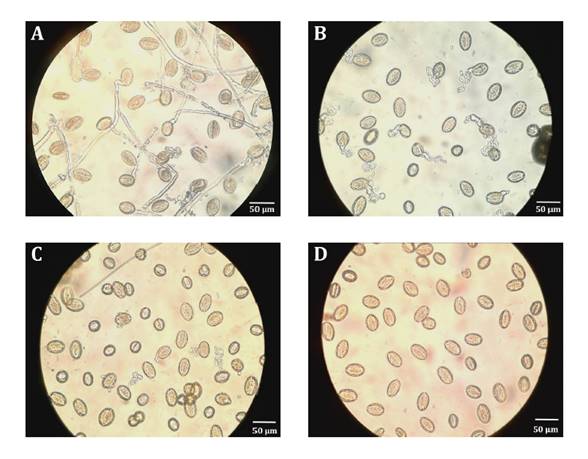
Figure 6. Development
of pollen tubes from rapeseed pollen grains in Frame 9 after different in-hive
exposure periods (400X). A: 0 h; B: 24 h; C: 48 h; D: 72 h.
Figura 6. Desarrollo
de los tubos polínicos de granos de polen de colza en el Cuadro 9 luego de
diferentes períodos de exposición en una colmena (400X). A: 0 h ; B: 24 h; C: 48 h; D: 72 h.
Discussion
The outer frames of
the brood chamber showed wider temperature variations and lower average
temperatures than those of the central frames. This was expected because the
brood is mainly in the center, while the outer frames, typically filled with
honey, have less temperature regulation from bees. Honey acts as an insulator
against external temperature variations, and nurse bees concentrate on
controlling the temperature of brood frames. Humidity levels showed no
significant differences between honey and brood areas, consistent with findings
reported by Alburaki
& Corona (2021) and Human (2006).
Pollen grains in the brood area lost their germination capacity
after 24 h of exposure to in-hive conditions, markedly reducing the risk of
contaminating new seed production plots. Temperature affects pollen not only
during transport to the stigma but also during its Temperature affects pollen
not only during transport to the stigma but also during its development in the
anther (17, 24, 26).
Notably, pre-anthesis temperature affects canola pollen germinability (17),
with significant reductions in germinability and pollen tube length at 33°C (18).
Our findings support this, as the temperature of the brood area remained close
to 35°C.
Pollen tubes from
fresh pollen and those exposed to in-hive conditions showed similar results to
those reported by Young
et al. (2004), who found that pollen germinated at 35°C had abnormal growth,
being thinner and shorter than those germinated at 23°C. These abnormalities in
pollen tubes could hinder proper ovule fertilization and, even if successful,
would be in significant disadvantage compared to fresh pollen grains in the new
plot.
Conclusions
In-hive conditions
at the experimental site significantly reduced the germination capacity and
pollen tube development of rapeseed. To minimize contamination risks, hives
should remain outside the new production plot for 72 h.
These studies provide a starting point for understanding the
potential risks of Apis mellifera hives carrying viable pollen and
contaminating hybrid rapeseed seed production plots. Further research is
recommended to confirm the required waiting period before relocating hives
between plots. Similar studies on other seed crops would also be beneficial.
Acknowledgements
The authors are
particularly thankful to Ana Andrada for all her help during the writing
process.
This study was financed by “Desafíos de la apicultura en el sur
bonaerense”, PGI 24/A248, Universidad Nacional del Sur. CIC (Comisión de
Investigaciones Científicas, Argentina) and CONICET (Consejo Nacional de
Investigaciones Científicas y Técnicas) provided financial support for graduate
studies.
1.
Alburaki, M.; Corona, M. 2021. Polyurethane honey bee hives provide better
winter insulation than wooden hives. Journal of Apicultural Research. 61(2):
190-196.
2.
Angadi, S. V.; Cutforth, H. W.; Miller, P. R.; McConkey, B. G.; Entz, M. H.;
Brandt, S. A.; Volkmar, K. M. 2000. Response of three Brassica species
to high temperature stress during reproductive growth. Canadian Journal of
Plant Science. 80(4): 693-701. https://doi.org/10.4141/P99- 152
3.
Astiz, V. 2012. Biología polínica, fecundación y rendimiento en dos genotipos
híbridos de girasol (Helianthus annuus L.) alto oleico. Tesis de
Magister. Departamento de Agronomía, Universidad Nacional del Sur, Bahía
Blanca, Argentina. 80 p.
4.
Bujok, B.; Kleinhenz, M.; Fuchs, S.; Tautz, J. 2002. Hot spotsinthebeehive.
Naturwissenschaften. 89: 299-301. https://doi.org/10.1007/s00114-002-0338-7
5.
Crane, E. 1990. Bees and beekeeping science, Practice and World Resources.
Cornell University Press. 640 p.
6.
Delaplane, K. S.; Mayer, D. F. 2000. Crop pollination by bees. CABI.
Wallingford, U.K. 344 p.
7.
Di Rienzo, J. A.; Casanoves, F.; Balzarini, M. G.; Gonzalez, L.; Tablada, M.;
Robledo, C. W. 2018. Infostat versión 2018. Grupo Infostat, FCA, Universidad
Nacional de Córdoba. Argentina. http:// www.infostat.com.ar
8.
Fahrenholz, L.; Lamprecht, I.; Schricker, B. 1992. Calorimetric investigations
of different castes of honey bees, Apis mellifera carnica. Journal of
Comparative Physiology B. 162: 119-130. https://doi.org/10.1007/BF00398337
9.
Gil-Lebrero, S.; Navas González, F. J.; Gámiz López, V.; Quiles Latorre, F. J.;
Flores Serrano, J. M. 2020. Regulation of microclimatic conditions inside
native beehives and its relationship with climate in Southern Spain.
Sustainability. 12(16): 6431.
10.
Human, H. 2006. Evaluation of the floral rewards of Aloe greatheadii var.
davyana (Asphodelaceae), the most
important indigenous South African bee plant (Doctoral dissertation, University
of Pretoria).
11.
Hüsken, A.; Dietz-Pfeilstetter, A. 2007. Pollen-mediated intraspecific gene
flow from herbicide resistant oilseed rape (Brassica napus L.).
Transgenic Research. 16: 557-569.
12.
Kendall, D. A. 1973. The viability and compatibility of pollen on insects
visiting apple blossom. The Journal of Applied Ecology. 10(3): 847.
https://doi.org/10.2307/2401873
13.
Khatun, S.; Flowers, T. J. 1995. The estimation of pollen viability in rice.
Journal of Experimental Botany. 46(1): 151-154.
https://doi.org/10.1093/jxb/46.1.151
14.
Koeniger, N. 1978. Das Wärmen der Brut bei der Honigbiene (Apis mellifera L.).
Apidologie. 9(4): 305-320.
15.
Li, C.; Yu, M.; Chen, F.; Wang, S. 2010. In vitro maturation and
germination of Jatropha curcas microspores. International Journal of
Agriculture and Biology. 12(4): 541-546.
16.
Mesquida, J.; Renard, M.; Mesquida, B. 1987. Etude préliminaire sur la
germination «in vitro» du pollen de colza (Brassica napus L. var. oleifera
Metzger) et sur l’évolution dans le temps de son aptitude à germer.
Agronomie. 7(6): 409-416. https://doi.org/10.1051/ agro:19870606
17.
Morrison, M. J. 1993. Heat stress during reproduction in summer rape. Canadian
Journal of Botany. 71(2): 303-308. https://doi.org/10.1139/b93-031
18.
Morrison, M. J.; Gutknecht, A.; Chan, J.; Miller, S. S. 2016. Characterising
canola pollen germination across a temperature gradient. Crop and Pasture
Science. 67(4): 317-322.
19. Ouvrard, P.; Jacquemart, A. L. 2019.
Review of methods to investigate pollinator dependency in oilseed rape (Brassica
napus). Field Crops Research. 231: 18-29.
20.
Parker, A. J.; Tran, J. L.; Ison, J. L.; Bai, J. D. K.; Weis, A. E.; Thomson,
J. D. 2015. Pollen packing affects the function of pollen on corbiculate bees
but not non-corbiculate bees. Arthropod-Plant Interactions. 9: 197-203.
21.
Pierre, J.; Renard, M. 2002. La longévité du pollen de colza. Oléagineux, Corps
gras, Lipides. 9(1): 11-13.
22.
Read, S. M.; Clarke, A. E.; Bacic, A. 1993. Stimulation of growth of cultured Nicotiana
tabacum W 38 pollen tubes by poly (ethylene glycol) and Cu(II)
salts. Plant Cell Biology Research Centre, School of Botany, University of
Melbourne, Parkville, Victoria, Australia. 177: 1-14.
23.
Robinson, S. V.; Cartar, R. V.; Pernal, S. F.; Waytes, R.; Hoover, S. E. 2023.
Bee visitation, pollination service, and crop yield in commodity and hybrid
seed canola. Agriculture, Ecosystems & Environment. 347: 108396.
24.
Sato, S.; Peet, M. M.; Thomas, J. F. 2002. Determining critical pre- and
post-anthesis periods and physiological processes in Lycopersicon esculentum
Mill. exposed to moderately elevated temperatures.
Journal of Experimental Botany. 53(371): 1187-1195. https://doi.
org/10.1093/jexbot/53.371.1187
25.
Shivanna, K. R.; Cresti, M. 1989. Effects of high humidity and temperature
stress on pollen membrane integrity and pollen vigour in Nicotiana tabacum.
Sexual Plant Reproduction. 2(3): 137-141.
26.
Shivanna, K. R.; Linskens, H. F.; Cresti, M. 1991. Pollen viability and pollen
vigor. Theoretical and Applied Genetics. 81(1): 38-42.
https://doi.org/10.1007/BF00226109
27.
Simpson, J. 1961. Nest climate regulation in honey bee colonies: Honey bees
control their domestic environment by methods based on their habit of
clustering together. Science. 133(3461): 1327-1333.
28.
Stabentheiner, A.; Kovac, H.; Brodschneider, R. 2010. Honeybee colony
thermoregulation-Regulatory mechanisms and contribution of individuals in
dependence on age, location and thermal stress. PLoS ONE. 5(1): e8967.
https://doi.org/10.1371/journal.pone.0008967
29.
Stalidzans, E.; Berzonis, A. 2013. Temperature changes above the upper hive
body reveal the annual development periods of honey bee colonies. Computers and
Electronics in Agriculture. 90: 1-6.
30.
Timmons, A. M.; O’brien, E. T.; Charters, Y. M.; Dubbels, S. J.; Wilkinson, M.
J. 1995. Assessing the risks of wind pollination from fields of genetically
modified Brassica napus ssp. oleifera.
In The Methodology of Plant Genetic Manipulation: Criteria for Decision Making:
Proceedings of the Eucarpia Plant Genetic Manipulation Section Meeting held at
Cork, Ireland from September 11 to September 14: 1994 (417-423). Springer
Netherlands.
31.
USDA, 2022. United States Department of Agriculture. Accessed in June 2023.
https://downloads.usda. library.cornell.edu/usda-esmis/files/tx31qh68h/mc87r456n/f7624t039/oilseeds.pdf
32.
Westcott, L.; Nelson, D. 2001. Canola pollination: an update. Bee World. 82(3):
115-129.
33.
Young, L. W.; Wilen, R. W.; Bonham-Smith, P. C. 2004. High temperature stress
of Brassica napus during flowering reduces micro- and megagametophyte
fertility, induces fruit abortion, and disrupts seed production. Journal of
Experimental Botany. 55(396): 485-495. https:// doi.org/10.1093/jxb/erh038