Revista de la Facultad de Ciencias
Agrarias. Universidad Nacional de Cuyo. Tomo 56(2). ISSN (en línea) 1853-8665.
Año 2024.
Review
Pseudocereals
dietary fiber. Amaranth, quinoa, and buckwheat fiber composition and potential
prebiotic effect
Fibra
dietaria en pseudocereales. Composición y potencial efecto prebiótico de la
fibra de amaranto, quinoa y trigo sarraceno
Deborah D´amaro2,
1Centro de Investigación y Desarrollo en Criotecnología de
Alimentos (CIDCA-CONICET-CIC-UNLP). Calle 47 y 116. La Plata. Buenos Aires.
Argentina.
2Universidad Nacional de La Plata. Facultad de Ciencias Exactas.
Calle 47 y 115. La Plata. Buenos Aires. Argentina.
*acsabbione@gmail.com
Abstract
Amaranth (Amaranthus), buckwheat (Fagopyrum
esculentum), and quinoa (Chenopodium quinoa) crops have limited production and agro-industrial
development both in Argentina and globally. As the demand for functional
ingredients and foods grows, developing products from these pseudocereals could
offer substantial economic benefits. This study aims to analyze the dietary
fiber content and composition of amaranth, quinoa, and buckwheat, and to
investigate the relationship between dietary fiber structure and its potential
prebiotic effects. Gaining insights into these aspects would provide valuable
information for developing foods based on these pseudocereals and could enhance
their future applications in the food industry.
Keywords: Pseudocereals,
dietary fiber, prebiotic effect, microbiota
Resumen
Los cultivos de
amaranto (Amaranthus), el trigo
sarraceno (Fagopyrum
esculentum) y la quinoa (Chenopodium quinoa) tienen una producción y desarrollo
agroindustrial limitados tanto en Argentina como a nivel mundial. Dado que la
demanda de ingredientes y alimentos funcionales está en aumento, el desarrollo
de productos a partir de estos pseudocereales podría ofrecer beneficios
económicos sustanciales. Este estudio tiene como objetivo analizar el contenido
y la composición de la fibra dietética del amaranto, la quinoa y el trigo
sarraceno, y examinar la relación entre la estructura de la fibra dietética y
sus posibles efectos prebióticos. Obtener información sobre estos aspectos
proporcionaría datos valiosos para el desarrollo de alimentos basados en estos
pseudocereales y podría potenciar sus aplicaciones futuras en la industria
alimentaria.
Palabras clave: Pseudocereales, fibra
dietaria, efecto prebiótico, microbiota
Originales: Recepción: 12/04/2024 - Aceptación: 07/08/2024
Pseudocereals
The pursuit of
healthy lifestyles and more nutritious foods adaptable to various climatic conditions
has spurred interest in underutilized or alternative crops, leading to a
resurgence in pseudocereals. The World Health Organization (WHO), the Food and
Agriculture Organization of the United Nations (FAO), and the scientific
community are working together to identify foods that can meet the needs of a
growing global population. With a current population of 8 billion and
projections reaching 10.9 billion by 2050, urgent solutions are needed to
address the impending food crisis (7). The agroindustry
faces the challenge of ensuring a sufficient food supply while upholding high
productivity and quality standards.
According to the
FAO, "food security exists when all people, at all times, have physical, social,
and economic access to sufficient, safe, and nutritious food that meets their
daily energy needs and dietary preferences for an active and healthy
life." Currently, global food security relies on a few cereal varieties,
with over 50% of caloric intake provided by wheat, maize, and rice (50). Pseudocereals are
nutritionally superior to traditional cereals. They have higher protein content
and are rich in essential amino acids, including lysine, arginine, tryptophan,
and histidine. Furthermore, pseudocereals exhibit higher digestibility,
bioavailability, and protein efficiency ratios (PER), comparable to milk
casein (50). Unlike wheat, oats, barley, and rye,
which contain gliadin, pseudocereals are gluten-free and safe for celiac
patients (43).
Ongoing research in food science uncovers new, healthy food
components. Bioactive peptides, found in various foods including pseudocereals,
exemplify this discovery. These peptides, along with other beneficial
components, classify pseudocereals as functional foods (45).
Lipids, another crucial nutritional component, exhibit high unsaturation
levels in pseudocereals (75-86%). Linoleic acid (omega-6) is the predominant
fatty acid, followed by oleic and palmitic acids, with notable amounts of
linolenic acid (omega-3) (54).
Both linoleic and linolenic acids are essential for the body, offering benefits
such as cardiovascular disease prevention and improved insulin sensitivity (43).
Pseudocereals also provide substantial dietary fiber, akin to whole grains.
This fiber supports gastrointestinal health, aids in weight management, and reduces
the risk of non-communicable diseases like diabetes and cardiovascular
conditions (2).
Additionally, pseudocereals are richer in minerals such as magnesium, calcium,
zinc, iron, copper, and phosphorus compared to cereals (table 1) (50).
Table 1. Centesimal
composition and mineral content of amaranth, quinoa, buckwheat pseudocereals,
and wheat.
Tabla 1. Composición
centesimal y contenido de minerales de amaranto, quinoa, trigo sarraceno y
trigo.
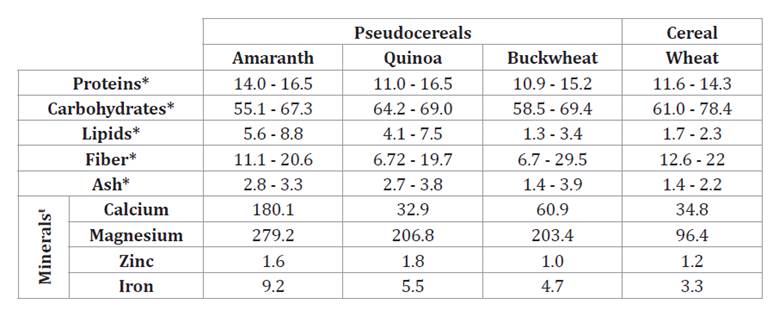
*Data
taken from Haros and Schoenlechner (2017),
and Serna-Saldívar and Sanchez-Hernandez
(2020b). Data expressed as g/100 g of dry weight.
*Data
taken from Alvarez-Jubete et al. (2010).
Data expressed as mg/100 g of dry weight.
*Valores
tomados de Haros y Schoenlechner (2017) y Serna-Saldívar y Sanchez-Hernandez (2020b).
Datos expresados como g/100 g en peso seco.
*Datos tomados
de Alvarez-Jubete et
al. (2010). Datos
expresados como mg/100 g en peso seco.
Despite the
potential benefits that pseudocereals offer, several factors still hinder the
incorporation of these crops into global agri-food systems. These factors are
diverse and include social aspects, economic factors like low market
participation and lack of integration into mass consumer products, as well as
agronomic factors like yield and lack of technology applied to these crops.
Knowledge regarding pseudocereals yield and quality is restricted to
small-scale systems with low investment cultivated in rustic ways, and
therefore cannot be compared to mass crops knowledge. The abundance of
technology and research available for traditional cereals, combined with
numerous and established marketing channels, leads producers to choose not to
invest in underutilized crops.
Pseudocereals
centesimal composition
Table 1 presents the
proximate composition ranges for wheat and pseudocereals such as amaranth,
quinoa, and buckwheat for comparison. Their compositional values and technological
and culinary behaviors are similar (4). Starch, which
forms semi-crystalline granules, is the primary component in both pseudocereals
and wheat. Although cereals generally have lower protein content, pseudocereals
lack gluten-forming proteins, making their flour unsuitable for traditional
baked goods.
Lipid content is
typically higher in amaranth and quinoa compared to cereals. These
pseudocereals have stable lipids due to high concentrations of tocopherols (4). As shown in table 1, dietary fiber
content is similar between pseudocereals and wheat. Fiber levels largely depend
on whether the seed is hulled, as most fiber is located in the outer coverings.
Both cereals and pseudocereals can be consumed as whole grains, whole grain flour,
or processed products. While whole seeds are becoming more popular, most wheat
and rice consumed by humans are dehulled, resulting in lower fiber content.
Table 1
displays the mineral content in wheat and pseudocereals. Pseudocereals
generally contain higher levels of calcium, magnesium, and iron. According to
the Argentine Food Code (CAA) Recommended Daily Intake (RDI) values, a 50 g
serving of wheat provides only 2% of the RDI for calcium, while the same
serving of amaranth provides 9%. For magnesium, a 50 g serving of wheat
provides 19% of the RDI for men (260 mg/day), whereas 50 g of pseudocereals
provide 40% to 54% of the RDI. Amaranth is particularly notable for its iron
content, offering 16% of the RDI for women (29 mg/day) and 33% of the RDI for
men (14 mg/day) in a 50 g serving. These values demonstrate that pseudocereals
are a valuable source of minerals.
Dietary
Fiber
Dietary fiber
intake is increasingly recognized for its importance in human nutrition.
Research shows that fiber supports proper intestinal function and helps prevent
cardiovascular diseases, obesity, diabetes, and certain cancers (2).
The Food and Drug
Administration (FDA) recommends adults consume 25 g of fiber daily in a 2000
kcal diet, which aligns with the National Cancer Institute (NCI) recommendation
of 20-30 g/day to prevent colon cancer (36). However,
statistics from Argentina show that the population fails to meet these
recommendations. The 2007 National Nutrition and Health Survey reported that
97.2% of women aged 10 to 49 did not meet the daily fiber intake
recommendation, with a median intake of 9.4 g/day. Similar deficiencies were
observed in children, with 97.8% not meeting adequate fiber intake (42). Data from the
2019 National Nutrition and Health Survey suggest that the situation has not
improved. Food consumption patterns reveal that 30-40% of respondents consume
only one fruit or vegetable per day, indicating that dietary fiber intake
remains significantly below recommended levels (42).
There are several definitions of dietary fiber. According to the
CAA, "dietary fiber is any edible material not hydrolyzed by the
endogenous enzymes of the human digestive tract." Compaore-Sereme
et al. (2022) define it as "the edible parts of plants or carbohydrates
resistant to digestion and absorption in the human small intestine, with
complete or partial fermentation in the large intestine," highlighting its
fermentability by large intestine microorganisms. Dietary fiber consists
mainly of non-starch polysaccharides in plant cell walls and can be classified
based on water-holding capacity into insoluble and soluble fiber. Insoluble
fiber includes cellulose and hemicelluloses, while soluble fiber encompasses
pectins, β-glucans, gums, mucilages, oligosaccharides, and inulin.
Additionally, dietary fiber includes indigestible non-polysaccharide compounds
such as lignin, proteins resistant to gastrointestinal digestion, phenolic
compounds, waxes, saponins, phytates, and phytosterols (52).
Health benefits are linked to the solubility of dietary fiber. Soluble dietary
fiber (SDF) forms viscous gels upon contact with water, which delays gastric
emptying and nutrient absorption in the intestines. This increases satiety and
reduces caloric density, lowering the long-term risk of obesity (72).
The delay in nutrient absorption also helps prevent glycemic spikes in diabetic
patients. Additionally, SDF improves insulin sensitivity in both type 2
diabetes patients and healthy individuals (69),
and lowers blood cholesterol levels, particularly LDL cholesterol, thereby
reducing cardiovascular disease risk (45).
This effect may result from SDF binding bile acids, altering micelle formation,
preventing bile acid reabsorption in the enterohepatic circulation, and
promoting their elimination in feces (74).
Consequently, new bile acid synthesis in the liver is stimulated, which lowers
blood cholesterol levels (8).
Another important aspect of fiber is its fermentability by intestinal
microorganisms. The degree of fermentability correlates with fiber solubility
and particle size. For example, fructooligosaccharides are highly fermentable,
whereas large cellulose or lignin polymers remain unchanged throughout the
large intestine. Clinical studies show that intestinal microorganisms utilize
different types of dietary fiber, with the remainder excreted in feces (18).
Soluble polysaccharides fermented by intestinal microbiota produce short-chain
fatty acids (SCFAs) such as acetate, propionate, and butyrate. SCFAs offer
various health benefits, primarily at the intestinal level. Butyrate, for
instance, strengthens the intestinal epithelial barrier by inducing tight
junction protein expression and redistribution within the membrane. A loss of
intestinal barrier integrity, leading to increased permeability, is linked to
chronic inflammation associated with obesity, insulin resistance, and type 2
diabetes (9).
Propionate also benefits individuals with obesity by inhibiting hepatic
cholesterol synthesis, decreasing lipogenesis in adipose tissue, and reducing
appetite (9).
Insoluble dietary fiber (IDF) travels through the gastrointestinal tract with
minimal modification. Its effects are largely due to mechanical interactions (69).
IDF retains water and adds bulk to feces, enhancing intestinal regularity.
Additionally, it reduces caloric density by acting as a physical barrier that
slows the transit of digestive products through the enterocytes' brush border (69).
This characteristic of insoluble dietary fiber (IDF) also impedes the
absorption of other components, such as cholesterol, and promotes its excretion
via feces. Studies have shown that the lignin fraction, commonly found in the
outer layers of seeds, enhances bile acid binding capacity, leading to reduced
blood cholesterol levels (46). Sabbione
et al. (2023a) reported that amaranth IDF has significant bile acid binding
capacity, suggesting that its adsorptive effect may contribute to a potential
hypocholesterolemic effect by sequestering bile acids. Additionally, IDF
reduces the concentration and contact time of potentially carcinogenic compounds
with the colon mucosa, thereby lowering the risk of colon cancer (5).
Regarding other cancers, research indicates that total or insoluble dietary
fiber from legumes reduces prostate cancer risk, while soluble dietary fiber
decreases breast cancer risk (64).
Pseudocereals
dietary fiber composition
Numerous studies have assessed the relative amounts of total
dietary fiber (TDF), soluble dietary fiber (SDF), and insoluble dietary fiber (IDF)
in pseudocereal whole grains and flours (table 2).
Table 2. Amaranth,
quinoa, and buckwheat dietary fiber content, expressed as total dietary fiber
(TDF), soluble dietary fiber (SDF), and insoluble dietary fiber (IDF).
Tabla 2. Contenido
de fibra dietaria total (TDF), soluble (SDF) e insoluble (IDF) en amaranto,
quinoa, y trigo sarraceno.
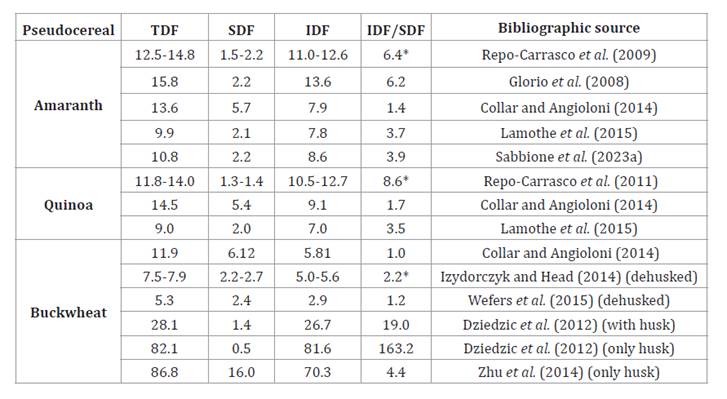
Data
are expressed as g fiber/100 g of seeds on a wet basis. *IFDaverage/ SFDaverage
Valores
expresados como g fibra/100 g de semillas. *IFDmedia/ SFDmedia
These values vary widely due to differences in genotypes,
environmental conditions, and laboratory techniques used for fiber
quantification and characterization. While dietary fiber content is crucial,
the ratio between insoluble and soluble fractions is also significant, as an
appropriate balance enhances health benefits. The FDA recommends an IDF/SDF
ratio close to 3 for optimal fiber balance (45).
Understanding the polysaccharide structures in pseudocereal cell walls is
important, as these structures influence both techno-functional and biological
properties. This information can predict the behavior of fiber in the body and
its effects on various food matrices.
Over the past
decades, various studies have focused on extracting and quantifying dietary
fiber (DF) from fruits and vegetables to identify rich sources, assess their
health benefits, and evaluate their functional properties for product
development. Notable achievements have been observed in citrus fruits, tropical
fruits, berries, and various vegetables.
Cell
wall constituents
Dietary fiber
components are primarily located in the cell wall, which provides shape and
structural integrity to plant cells. The cell wall consists of a complex mixture
of polysaccharides and other polymers arranged in a three-dimensional network.
It also includes structural proteins, enzymes, phenolic polymers, and other
materials that influence its physical and chemical properties. The
characteristics of the cell wall, such as thickness, matrix arrangement, and
the types and proportions of molecules, vary depending on the plant tissue (41). Among the cell
wall constituents is cellulose, a linear polysaccharide made up of
D-glucose molecules linked by β-D (1-4) bonds. Its degree of polymerization
varies significantly, with molecules consisting of 2,000 to 15,000 units,
depending on the specific region of the cell wall (66). These linear
polymers form microfibrils through intermolecular hydrogen bonds, which
aggregate into larger structures and interact with other components such as
hemicelluloses, pectins, and lignin. This interaction creates strongly
hydrated matrices with high mechanical resistance. Hemicelluloses are a
diverse group of polysaccharides with long linear chains that may have short
side chains as substituents. They have a lower molecular weight than cellulose
and are generally more soluble. However, their physicochemical properties,
such as solubility, viscosity, and gel-forming abilities, vary based on their
chemical structure, molecular size, interactions, and spatial arrangement. At
least 250 types of hemicellulose polymers have been identified, including
xyloglucans, xylans, mannans, glucans, and β-(1-3,1-4) glucans (53). Lignins,
another cell wall component, are complex biopolymers and are the second most
abundant in the plant kingdom after cellulose. These polyphenolic biopolymers
consist of phenylpropane units and form a matrix through the condensation of
three primary phenolic alcohols (47). Pectins are
structural polysaccharides primarily composed of α-D-galacturonic acid. They
form chains that can be linear, known as homogalacturonans (HG), or include
rhamnose. Two typical structures containing rhamnose are rhamnogalacturonan I
(RG-I) and rhamnogalacturonan II (RG-II), which differ in structure, linkage
types, and complexity. Although pectins vary in solubility, the pectin in the
middle lamella of cell walls is insoluble and considered calcium pectate (75). These compounds
have significant nutritional and technological value for the food industry due
to their prebiotic potential and gelling ability (66). Fructans,
which are reserve carbohydrates and the most abundant non-structural
polysaccharides in nature, include inulin, oligofructose, and
fructooligosaccharides (FOS). They are relatively soluble in water and remain
intact in the upper gastrointestinal tract. However, in the colon, fructans are
fully utilized by microorganisms, providing a prebiotic effect. Inulin, FOS,
and oligofructose are commonly used as functional ingredients (70). These compounds
also offer valuable technological properties, such as sweetening ability and
gel formation, which enhance the body and palatability of certain foods. Oligosaccharides,
which are short chains of sugars containing 3 to 20 monosaccharides, cannot be
digested by the human body and are classified as dietary fiber. Due to their
small size and capacity to form hydrogen bonds with water, they are highly
soluble. The raffinose family of oligosaccharides (RFOs), including
raffinose, stachyose, and verbascose, as well as related compounds, have shown
potential prebiotic effects by promoting the selective growth of beneficial
bifidobacteria (35). Resistant
starch (RS) is a type of starch that retains its structural characteristics
and remains undigested as it passes through the gastrointestinal tract. It
reaches the colon, where it can be fermented by the microbiota or excreted in
feces. Although RS is not a cell wall component, it functions similarly to
fermentable soluble fiber, providing associated health benefits (34).
Amaranth
Table 2 presents the
values of total dietary fiber (TDF), insoluble dietary fiber (IDF), and soluble
dietary fiber (SDF) for amaranth, quinoa, and buckwheat, expressed on a wet
basis. The TDF values align with those reported for pseudocereals in table 1. The data show a
predominance of IDF over SDF. Specifically, 12% to 42% of the TDF corresponds
to soluble fiber, while 58% to 86% corresponds to insoluble fiber.
Bunzel et
al. (2005) reported that the primary cell walls of dicotyledons like
amaranth are rich in pectins, xyloglucans, and cellulose. Lamothe
et al. (2015) examined the composition of the insoluble dietary fiber (IDF)
and soluble dietary fiber (SDF) fractions in amaranth. They found that 5% of
the IDF on a dry basis was lignin. Their analysis revealed that the IDF
primarily consists of galacturonic acid, arabinose, xylose, glucose, and
galactose. A significant portion of the glucose was attributed to cellulose
and xyloglucans, which retained the characteristic bonds of these structures.
Glucose from cellulose accounted for 7% of the IDF, though this value might be
underestimated due to the analytical method used. The estimated proportion of
xyloglucans was 30%, with a high degree of branching indicated by the elevated
Xyl/Glc ratio. This finding is consistent with Bunzel et al. (2005), who noted a high
number of terminal xylose units in amaranth, likely part of the xyloglucan side
chains. Sabbione
et al. (2023a) confirmed that galacturonic acid is the primary monosaccharide
in insoluble dietary fiber (IDF) and reported the same monosaccharides
identified by Lamothe
et al. (2015) (60). Lamothe
et al. (2015) found that pectins constitute 59% of the IDF, with rhamnose
present in low amounts. This suggests that the pectins are predominantly homogalacturonans
(HG) with small amounts of rhamnogalacturonan I (RG-I). The bonding patterns in
galactose and arabinose residues suggest they are part of RG-I pectic side
chains. The predominance of HG over RG-I is consistent with Mohnen
(2008),
who noted that HG makes up to 65% of the pectin in plant cell walls, while RG-I
represents 25-35%. Additionally, Lamothe et al. (2015) indicated that
xylose is involved not only in xyloglucan structures but also in
arabinoxylans, another type of hemicellulose found in the IDF, together with
pectic polysaccharides that would be the majority. Regarding amaranth soluble
dietary fiber (SDF), Lamothe et al. (2015) reported that the
primary monosaccharides are galacturonic acid, galactose, and arabinose,
indicating the presence of pectic substances. These pectic substances
contribute 34% to the SDF (38). The predominance
of galacturonic acid and the absence of rhamnose suggest that homogalacturonans
(HG) are the major pectin component. Significant amounts of mannose were also
reported, though it is notably lower compared to other monosaccharides. This
mannose is attributed to galactomannans, with an estimated content of 0.3%.
Additionally, xylose and glucose units from xyloglucans also contribute to the
SDF, comprising 60-70% of this fiber fraction. Lamothe et al. (2015) describe a high
Xyl/Glc ratio in these samples, reflecting a high level of branching. The side
chains of these polysaccharides include di- and tri-saccharides, which may
consist of xylose, glucose, and possibly arabinose. Sabbione
et al. (2023a) detected galacturonic acid, xylose, arabinose, mannose, and
glucose/galactose in amaranth SDF, with xylose and arabinose being the most
prevalent. Villacrés
et al. (2013) also reported significant amounts of soluble arabinoxylans in
the SDF.
Capriles
et al. (2008) studied resistant starch content in amaranth seeds and found
that raw seeds possess 0.5% RS on a dry basis. However, they observed that
after cooking, this value reduced to 0.2%. The authors concluded that the RS
decrease might have occurred due to the starch granules' small size and their
tendency to completely lose the crystalline structure during thermal treatment.
Since a food considered a good source of resistant starch should possess an
RS/total starch ratio of at least 4.5% (58),
amaranth cannot be included in that group of foods since its ratio is 0.86%.
Guzmán-Maldonado and Paredes-López (1998) reported low
levels of raffinose and stachyose in amaranth, with concentrations of 1.65% and
0.15%, respectively. Gamel et al. (2006) found even lower
levels of raffinose, around 0.4%, and similar stachyose content. Both studies
confirm that amaranth seeds are not a significant source of raffinose family
oligosaccharides. Various authors have also assessed the presence of FODMAPs,
which include fermentable oligosaccharides, disaccharides, monosaccharides, and
polyols like lactose, fructose, sorbitol, and mannitol. These non-digestible
carbohydrates can trigger symptoms in individuals with conditions such as
irritable bowel syndrome, causing abdominal distension, diarrhea, and pain. The
impact of these carbohydrates is limited to those with specific intolerances or
diseases. Békés
et al. (2017) classified pseudocereals, including amaranth, buckwheat, and
quinoa, as low in FODMAPs, indicating that their FOS content is also low.
Furthermore, Habus et al. (2022) analyzed the
FODMAPs content in amaranth bran and reported that the sum of fructans and
galacto-oligosaccharides (GOS) is 0.96% on a dry weight basis.
Quinoa
Table 2 presents the total
dietary fiber (TDF), insoluble dietary fiber (IDF), and soluble dietary fiber
(SDF) content in quinoa seeds. Across different studies, IDF consistently
exceeds SDF, with soluble fiber ranging from 10% to 37% of TDF, and insoluble
fiber ranging from 63% to 90% of TDF. Zhang et al. (2020)
analyzed the dietary fiber content of quinoa seeds, reporting that it contains
41% hemicellulose, 52% cellulose, 4.7% pectins, and 1.7% lignin (77). Lamothe et al. (2015)
studied the soluble and insoluble fiber composition in quinoa seeds, finding
that the IDF contained 9% lignin. This finding aligns with the data from Repo-Carrasco-Valencia and Serna (2011), who reported
lignin values between 6% and 7%. Based on the types of bonds present in the IDF
xylose and glucose units, these monosaccharides are likely part of
xyloglucans, as suggested by Serna Saldívar and Ayala
Soto (2020). These authors identified xyloglucans as the primary
hemicellulose components in quinoa seeds. The xyloglucans found in quinoa are
similar to those in amaranth, characterized by branching with di- and
trisaccharide side chains and a significant degree of branching. In their
analysis of IDF, Lamothe et al. (2015)
reported that glucose associated with cellulose constituted 6%, a value
comparable to that found in amaranth. However, this finding is much lower than
the 52% TDF described by Zhang et al. (2020),
suggesting that the lower cellulose content could be attributable to the
analytical methods employed (38).
The primary
monomeric unit identified in quinoa TDF by Lamothe et
al. (2015) is galacturonic acid with β-(1,4) linkages, indicating that
pectic polysaccharides are predominant in both IDF and SDF fractions. According
to these authors, these pectic polymers constitute approximately 55% of IDF,
predominantly as homogalacturonans (HG) and, to a lesser extent, as
rhamnogalacturonan I (RG-I) compounds, which are branched with arabinans and
galactans. Cordeiro et al. (2012) also
described similar pectic structures in quinoa dietary fiber, reinforcing these
findings. Furthermore, the pectic content in SDF constitutes 55%, but unlike
the pectins found in IDF, the SDF pectins are composed exclusively of branched
homogalacturonans (HG), as rhamnose was absent from this fraction. Lamothe et al. (2015) identified arabinose,
glucose, galactose, and, to a lesser extent, xylose and mannose in SDF. Most of
the arabinose is part of the pectic branches, specifically as arabinans.
Galactomannans in quinoa SDF account for 0.5%, while xyloglucans range from
40-60%, exhibiting a low Xyl/Glc ratio and a relatively low degree of
branching. Additionally, Villacrés et al. (2013)
reported significant amounts of soluble arabinoxylans as part of quinoa SDF.
The resistant
starch (RS) content in quinoa seeds, as reported by Kraic
(2006), is 12.6 g/kg on a dry basis, translating to an RS/total starch
ratio of approximately 2%. Consequently, quinoa is not considered a
significant source of resistant starch. However, a study by Linsberger‐Martin et al. (2012) demonstrated
that applying high hydrostatic pressures to quinoa seeds could increase the RS
content by up to 18 times, presenting a promising method for developing
functional ingredients in the food industry. In their analysis of the FODMAP
profile of various grains, Ispiryan et al. (2020)
found that quinoa has a low content of fructans and oligofructans (OFR).
Fructans were below the detection limit of their method, and the RFOs were
present in minimal amounts (0.09% on a dry basis).
Buckwheat
Table 2 presents the total
dietary fiber (TDF), soluble dietary fiber (SDF), and insoluble dietary fiber
(IDF) contents of both husked and dehusked buckwheat seeds, as well as those of
the husk alone. The values for TDF, SDF, and IDF vary among studies, partly due
to the inclusion or exclusion of the husk in the analyzed samples. The
buckwheat husk is particularly rich in insoluble fiber, and flours made from
buckwheat that include the husk typically have high levels of insoluble fiber (19). According to Zhang et
al. (2020), the fiber composition of buckwheat seeds includes 39.2%
hemicellulose, 38.8% cellulose, 20.2% lignin, and 1.8% pectin. Based on the
data presented in table
2,
it is inferred that the buckwheat seeds analyzed likely contained the husk,
given the high content of cellulose and lignin, which are characteristic of the
husk's insoluble polysaccharides. Wefers et al. (2015)
analyzed the IDF monosaccharides in dehusked buckwheat and identified
significant amounts of galacturonic acid, arabinose, galactose, and to a lesser
extent, rhamnose. Many of the monosaccharides found in buckwheat IDF, such as
galacturonic acid, arabinose, and galactose, are attributed to the presence of
pectic arabinans and galactans, which are linked to rhamnogalacturonan-I (RG-I)
segments. The observed galacturonic acid/rhamnose ratio indicates that
homogalacturonan (HG) also contributes to buckwheat IDF pectin, though to a
lesser extent compared to quinoa and amaranth. This study also suggests a
relatively low content of cellulose in buckwheat. The presence of terminal
xylose and glucose with β-(1-4) linkages implies that xyloglucans may be
present, although xylose could also be part of xylans, albeit in low
proportions. Regarding SDF, Izydorczyk and Head (2010)
noted that it primarily consists of pectic polysaccharides, xyloglucans, and
arabinogalactans. The SDF monosaccharide composition analyzed by Wefers et al. (2015) supports the presence of
RG-I and indicates higher amounts of HG compared to the IDF. The authors also
identified arabinans in the SDF fraction, which are part of the pectin
branches. Additionally, glucose and xylose monosaccharides with linkages
consistent with xyloglucan structures were found. A high amount of mannose
could be attributed to mannan content. Dziedzic et
al. (2012) further reported that buckwheat husks are predominantly
composed of fiber, with high proportions of insoluble fiber, particularly
lignin and cellulose.
Regarding
buckwheat's resistant starch content, Kraic (2006) reported
an RS content of 38 g/kg on a dry basis and an RS/total starch ratio of 6.5%.
Thus, buckwheat can be considered a good source of resistant starch compared to
other pseudocereals. The high RS values are likely due to buckwheat's elevated
amylose content (27).
Ispiryan et al. (2020) found that buckwheat is
low in FODMAPs, indicating that it contains a low proportion of both fructans
and raffinose family oligosaccharides (RFOs).
Table 3 summarizes the
composition of IDF and SDF in amaranth, quinoa, and buckwheat seeds.
Table 3. Polysaccharides
in the soluble dietary fiber (SDF) and insoluble dietary fiber (IDF) fractions
in amaranth, quinoa, and buckwheat.
Tabla 3.
Polisacáridos presentes en la fibra dietaria soluble (SDF) y en la fibra
dietaria insoluble (IDF) de amaranto, quinoa y trigo sarraceno.
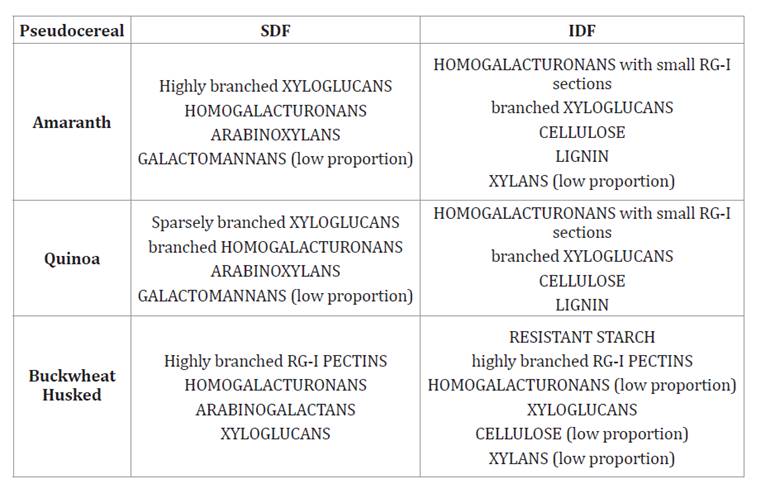
*Data from Bunzel et al. (2005);
Cordeiro et
al. (2012);
Dziedzic et
al. (2012);
Izydorczyk and
Head (2010);
Lamothe et
al. (2015);
Repo-Carrasco-Valencia
and Serna (2011);
Sabbione et
al. (2023a);
Serna Saldívar
and Ayala Soto (2020a);
Villacrés et
al. (2013);
Wefers et al.
(2015);
Zhang et al. (2020).
The data on dietary fiber content in pseudocereals show
considerable variability among studies. However, some general trends can be
identified. Pseudocereals have TDF levels comparable to those found in wheat (table
1). The predominant fiber fraction is IDF, reflected in an
IDF/SDF ratio greater than 1 (table 2).
An ideal balance, suggested to be around 3, is approached by amaranth, quinoa,
and cereals, with some husked buckwheat samples exhibiting even higher ratios.
Regarding fiber composition, amaranth and quinoa share similarities in the
proportion and types of dietary fiber structures (table 2
and table
3). The main fiber components in these pseudocereals are pectic
polysaccharides, with a lesser amount of xyloglucans. These polysaccharides
vary in complexity and branching, contributing to the distinct characteristics
of the dietary fiber in each pseudocereal. In contrast, husked buckwheat is
notable for its high cellulose and lignin content. The removal of the husk
reduces the IDF content, which is reflected in the lower IDF values compared to
buckwheat with the husk (table 2).
Overall, while the general composition of dietary fiber in pseudocereals
follows certain patterns, the specific characteristics and proportions can vary
significantly depending on the pseudocereal and its processing.
Probiotics
and prebiotics
The human
gastrointestinal tract hosts a vast number of microorganisms that interact
symbiotically with the host. These microorganisms perform essential functions,
such as utilizing non-digestible components to produce health-beneficial
compounds, maintaining the epithelial barrier, regulating host metabolism,
preventing pathogen colonization, and modulating the immune and nervous systems
(65). Certain microorganisms, known as
probiotics, are used as food supplements to improve health. Probiotics must
survive gastrointestinal transit, establish in the intestine, and proliferate.
The International Scientific Association for Probiotics and Prebiotics (ISAPP)
defines probiotics as "live strains of strictly selected microorganisms
that, when administered in adequate amounts, confer a health benefit on the
host" (30). Foods containing
probiotics are classified as functional foods. Common probiotic microorganisms
include Lactobacillus acidophilus, Lactobacillus casei, and Bifidobacterium
spp. However, other probiotic species also exist, such as Lactococcus
spp., Streptococcus spp., and certain strains of Saccharomyces yeast
(65). Probiotics and many intestinal
microbes utilize dietary fiber. Different types of fiber selectively promote
the growth of beneficial microbial colonies and are known as prebiotics. The
ISAPP defines prebiotics as "substrates that are selectively utilized by
host microorganisms and confer a health benefit" (21). This broad definition encompasses
non-digestible dietary carbohydrates and other types of substrates. Prebiotics
and probiotics enhance host health by modulating intestinal flora. Research
aimed at developing healthier foods has led to the emergence of new functional
foods, including synbiotics. Synbiotics are defined as "a mixture of
probiotics and prebiotics intended to increase the survival of health-promoting
bacteria and to modify intestinal flora and its metabolism" (48).
Human
intestinal microbiota
The term
"human microbiota" refers to the community of microorganisms residing
in the body. Over 70% of the human microbiota is found in the digestive tract,
which displays significant variability in microbial diversity and quantity
across different regions. The intestinal microbiota contains approximately 100
trillion microorganisms from at least 1,000 different species, and it is
estimated to weigh about 200 grams in adults. Only one-third of these
microorganisms are common to most individuals, while the remaining two-thirds
are unique to each person (17).
According to data
from the Human Microbiome Project (National Institutes of Health, USA), the
phyla Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria,
which together account for over 90% of the total microbiota (table 4), predominantly inhabit
the intestine.
Table 4. Main
bacterial phyla and genera present in the human intestine (17).
Tabla
4. Principales phylum y géneros de bacterias
presentes en el intestino humano (17).
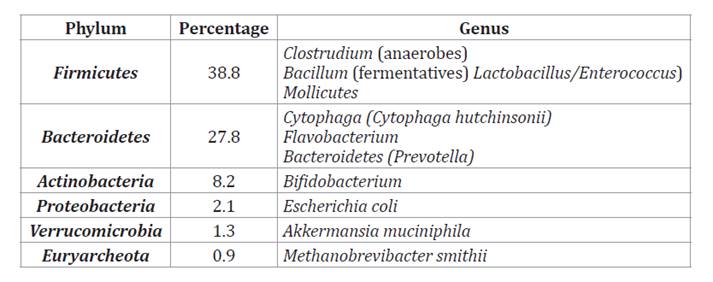
The
remaining microbiota includes Fusobacteria, Verrucomicrobia,
archaea, yeasts, phages, and protists.
The
human colon also contains low quantities of pathogens such as Campylobacter
jejuni, Salmonella enterica, Vibrio cholerae, and certain
strains of Escherichia coli.
Digestive tract
colonization begins at birth. The quantity and type of microorganisms evolve
until age 3, influenced by environmental factors and dietary patterns. After
this period, the intestinal microbiota resembles that of adults in composition,
diversity, and functionality. It can be altered during adolescence due to
hormonal changes but remains relatively stable in adulthood. After age 65, the
microbiota composition shifts, with increased abundance of the Bacteroidetes
phylum and Clostridium from the Ruminococcaceae family, in
contrast to younger individuals, where Clostridium from the Lachnospiraceae
family is more common. In older adults, the microbiota becomes less diverse
and more dynamic, characterized by a higher Bacteroides/Firmicutes ratio,
an increase in Proteobacteria, and a decrease in Bifidobacterium (68). The intestinal microbiota performs numerous
functions and influences various biological processes, both locally and
distantly through its metabolites. The bioactive products and metabolites
produced by microorganisms during fermentation, known as postbiotics, include
short-chain fatty acids (SCFAs), enzymes, vitamins, bioactive peptides, and
components of microorganisms or their remnants (63).
The microbiota contributes to maintaining mucosal barrier integrity, providing
essential nutrients like vitamins, and protecting against pathogens (17). Additionally, the interaction between the
microbiota and the immune system in the colonic mucosa is essential for proper
immune function. Microbial molecular pattern recognition receptors or
microbiota-derived metabolites activate barrier functions and mediator
synthesis, regulating intestinal immune cell responses to tolerate beneficial
microorganisms and prevent pathogen overgrowth (3).
The interaction between the human microbiota and the gut-brain axis is a
bidirectional and dynamic communication pathway between the intestine and the
brain, mediated through nervous, endocrine, and immune signaling mechanisms (62). The enteric nervous system (ENS) in the
intestine comprises over 100 million neurons responsible for basic digestive
functions, including motility and mucosal secretion. It communicates with the
central nervous system (CNS) primarily via the vagus nerve. The intestinal
microbiota stimulates vagus nerve afferent pathways, promotes cytokine release,
and modulates the production of neurotransmitters, hormones, and metabolites
such as SCFAs (23). Microbiota influences
the hypothalamic-pituitary-adrenal axis, regulating cortisol release. Research
indicates that high levels of Lactobacillus rhamnosus are associated
with lower corticosterone levels and improved stress and depression management.
Conversely, stress can alter the microbiota profile (23).
Dysbiosis may disrupt molecules essential for proper CNS function, potentially
linking microbiota imbalances to neurological diseases such as Alzheimer's,
Parkinson's, multiple sclerosis, autism spectrum disorders, depression, and
anxiety (1).
Colon
microorganisms primarily ferment dietary carbohydrates that resist digestion in
the gastrointestinal tract. Species such as Bacteroides, Roseburia,
Bifidobacterium, and Enterobacter produce SCFAs, which serve as
an energy source for enterocytes or enter the bloodstream to affect distant
organs. Many intestinal anaerobes produce acetate, while Bacteroidetes predominantly
produce propionate and Firmicutes produce butyrate (68). Butyrate is recognized for its
anti-inflammatory and anticancer properties; it promotes the proliferation of
colonocytes in the crypts and enhances apoptosis and exfoliation in the areas
closer to the lumen. Additionally, butyrate supports barrier function
regulation and reduces bacterial translocation by contributing to tight
junction assembly and mucin synthesis (68).
SCFAs also impact hepatic lipid and glucose homeostasis. Propionate, besides
serving as an energy source, regulates blood glucose levels by modulating
gluconeogenesis in the liver. It enhances insulin sensitivity and reduces
cholesterol synthesis rates (29).
Acetate, used as an energy source in the intestine, can be transported to
peripheral organs or the liver, where it serves as a precursor for cholesterol
and long-chain fatty acids. Additionally, SCFAs increase intestinal hormone
levels that promote satiety and enhance insulin action on glucose uptake in
muscle and adipose tissue. They inhibit de novo lipogenesis and
lipolysis, reducing plasma-free fatty acids and aiding in body weight control (29).
Microbiota
Modulation: Probiotics and Prebiotics
Microbiota
modulation involves deliberately altering the composition and activity of
intestinal microorganisms to improve health. This can be achieved through
dietary changes, probiotics, prebiotics, synbiotics, or, occasionally,
antibiotics, either alone or in combination. Such interventions can enhance the
diversity and abundance of beneficial bacteria, restore balance, or reduce
pathogenic microorganisms.
Probiotics are
incorporated into the diet through fermented foods or dietary supplements.
Commonly used probiotic genera include Lactobacillus and Bifidobacterium.
Strains such as Lactobacillus acidophilus, Lactobacillus rhamnosus,
Bifidobacterium bifidum, and Bifidobacterium lactis are naturally
present in the intestine. These strains, along with those from Saccharomyces,
can reduce pathogen adherence to the mucosa (39).
Additionally, the metabolic activity of beneficial microorganisms increases
SCFA concentrations and decreases colonic pH, which inhibits the growth of
pathogenic bacteria such as Escherichia coli, Staphylococcus aureus,
Klebsiella pneumoniae, and Salmonella enteritidis (59).
Prebiotics can naturally occur in foods or be added to enhance
functional properties. Various dietary fibers serve as energy sources for
intestinal microorganisms, but only some selectively promote the growth of
beneficial microbiota and probiotics. Prebiotics exert health benefits by
stimulating beneficial microorganisms' growth, modulating immune function, and
protecting against pathogens. They primarily increase populations of Bifidobacterium
and Lactobacillus, but also stimulate less well-studied beneficial
bacteria such as Akkermansia, Eubacterium, Propionibacterium,
Roseburia, and Faecalibacterium (59).
The rate and extent of fiber fermentation by microorganisms are crucial for
their prebiotic potential. These factors are influenced by fiber solubility,
chain size, total surface area, and the structure of the cell wall or food
matrix containing the fiber (59).
Fructans such as inulin, oligofructose, and FOS, as well as galactans (GOS),
are well-recognized prebiotics due to their low degree of polymerization and
high solubility. Additionally, oligosaccharides derived from hemicelluloses,
such as mannanoligosaccharides (MOS), arabinoxylanoligosaccharides (AXOS), and
xylooligosaccharides (XOS), have been proposed as prebiotics because they
enhance the growth of beneficial microorganisms and promote SCFAs production (33). Conversely, RFOs have shown prebiotic
effects by increasing Bifidobacteria and Lactobacillus populations
while reducing the adhesion and colonization of enteric pathogens (35). The degree of polymerization of
polysaccharides and their interactions with other polysaccharides in food
matrices can affect their metabolism. Some non-starch polysaccharides, such as
pectins and certain hemicelluloses, are considered potentially prebiotic (59). Microorganisms must possess enzymes to
degrade glycosidic bonds in polysaccharides into smaller molecules for
utilization as carbon sources. For example, xyloglucans are degraded by Clostridium
in the colon due to its microbiota-specific enzymes, converting them into
fermentable oligosaccharides that positively impact the colon microbiota (24). β-glucans enhance
the growth of Bifidobacterium and Lactobacillus strains and
modulate SCFA production by increasing Clostridium histolyticum and Prevotella
(65). Arabinoxylans in cereals and
pseudocereals can generate AXOS and XOS through enzymatic hydrolysis with
xylanases and arabinofuranosidases. Although hydrolysis yields various
structures depending on the plant source, arabinoxylans are considered
potential prebiotics. Their consumption has been linked to positive
immunomodulation and selective growth of probiotic microorganisms such as Lactobacillus
cellobiosus, Lactobacillus paracasei, and Bifidobacterium spp.
(65). Pectins, a complex family of
fermentable polysaccharides, can also promote the growth of Lactobacillus and
Bifidobacterium in the intestine. The effectiveness of pectin
utilization by microorganisms depends largely on colon pH and the degree of
methoxylation, with low methoxyl pectins fermenting more rapidly (49). In vivo studies have shown an
increase in Clostridium species capable of producing acetate and
butyrate in the presence of pectin (65).
Additionally, research in rats fed with pectins revealed increased SCFAs and
the presence of pectic oligosaccharides as intermediates. These
oligosaccharides result from the action of bacterial enzymes such as pectate
lyase, polygalacturonase, and pectin esterase (49).
Resistant starches are also potentially prebiotic, with promising results from
both in vitro and in vivo studies. Several studies indicate that
dietary RS increases the number of beneficial microorganisms, particularly Bifidobacterium,
and elevates SCFA concentration in the colon (13).
This proliferation is attributed to the fermentation of resistant starch
degradation products. Ruminococcus bromii is a key species for
initiating RS degradation, enabling other bacteria to utilize its fermentation
products (65).
Potential
modulatory and prebiotic effect of pseudocereals' dietary fiber
Amaranth
The prebiotic
potential of amaranth remains under investigation. Although literature is
limited, results are promising and have generated increased interest in these
seeds. Gullón et al. (2014) assessed the in
vitro prebiotic potential of amaranth by quantifying SCFAs, monitoring pH
changes, and evaluating microbial population dynamics using adult women's fecal
inoculums for fermentation. Amaranth seeds were cooked in water and subjected
to simulated gastrointestinal digestion. The study revealed significant
modifications in bacterial composition, with notable growth of Bifidobacterium
spp., Lactobacillus, and Enterococcus, which are beneficial,
as well as Bacteroides and Prevotella, which produce propionate.
An increase in Clostridium coccoides and Eubacterium rectale,
both butyrate producers, was also observed. SCFA production showed a
progressive increase over time, with higher concentrations of acetate,
followed by propionate and butyrate, indicating high fermentability of the
medium where amaranth carbohydrates serve as a carbon source. pH decreased over time, correlating with the production of
lactate and formate SCFAs (24). Sabbione et al. (2023b) investigated the ability
of dietary fibers from three amaranth products to modulate children's fecal
microbiota using an in vitro fermentation model. They observed changes
in fecal microbiota and SCFAs after 24, 48, and 72h of fermentation. Sequencing
results at 24h revealed a significant decrease in Fusobacterium and
enterobacteria compared with the basal medium, accompanied by a notable
increase in Bacteroides and Parabacteroides. These findings
confirm that amaranth fibers are fermented by children's fecal microbiota,
leading to changes indicative of a potentially prebiotic effect.
Currently, no
studies confirm which specific amaranth fiber carbohydrates can beneficially
modulate the microbiota or increase SCFA production. However, several
components show potential prebiotic effects. Given the high content of pectin
and arabinoxylans in amaranth, these compounds could be considered potential
prebiotics. Additionally, xyloglucans are abundant in both soluble and
insoluble fractions of amaranth dietary fiber, suggesting various hemicellulose
sizes and conformations (10, 38). The
observed prebiotic effects may be linked to xyloglucans, which can be
hydrolyzed by microbiota enzymes into smaller carbohydrates used as a carbon
source by beneficial microorganisms.
Quinoa
Quinoa, extensively
studied as a pseudocereal, has a well-documented prebiotic effect on its
dietary fiber. Gullón et al. (2014) reported
results similar to those observed with amaranth. Authors described an increase
in beneficial bacterial groups, although Faecalibacterium prausnitzii grew
less in quinoa compared to amaranth. SCFA levels increased significantly over
time, with a higher concentration in quinoa compared to amaranth. The study
concluded that quinoa provides a highly fermentable medium conducive to the
growth of SCFA-producing beneficial bacteria. In agreement, Zeyneb et al. (2021) found a marked increase in
SCFAs and a decrease in pH during in vitro fermentation of cooked and
raw quinoa following simulated gastrointestinal digestion. The authors found
that propionate and butyrate were the SCFAs present in the highest
concentrations after 24h of fermentation, while acetate was present in lower
amounts. This low concentration of acetate, which is typically the most
abundant SCFA, may suggest its degradation by other bacteria (76). Additionally, the study reported a positive
shift in microbial diversity post-fermentation, with increased levels of
beneficial species such as Bifidobacterium and Collinsella,
indicating a potential prebiotic effect. Zeyneb et
al. (2021) also examined polysaccharides extracted from quinoa and
found a greater prebiotic effect compared to digested samples, particularly
enhancing the growth of Bifidobacterium.
Several
carbohydrates in quinoa may contribute to potential prebiotic effects. Cao et al. (2020) isolated a quinoa fiber
carbohydrate composed of glucose and arabinose units, which exhibited a
modulatory effect on the microbiota of rats fed a high-fat diet. A decrease in
the Firmicutes/Bacteroidetes ratio (F/B) was noted, which is
favorable as a high F/B ratio is linked to metabolic diseases. Additionally,
levels of Clostridium and Proteobacteria, associated with
inflammation and metabolic disorders, also decreased. The authors attributed
these effects to the reduction in hyperlipidemia induced by the high-fat diet (42). Other polysaccharides in quinoa, such as
pectin or xyloglucans, may also be fermented by colon microorganisms following
enzymatic hydrolysis in the intestine. Multiple carbohydrates can contribute to
the observed modulatory effects, with SCFAs produced from fermentation playing
a role in this process.
Buckwheat
The prebiotic
potential of buckwheat has been investigated in various studies. Préstamo et al. (2003) examined the effects of
incorporating buckwheat into the diet of rats. Their findings revealed a
significant increase in Lactobacillus and Bifidobacteria, while potentially
pathogenic strains, such as Clostridium and enterobacteria, decreased,
suggesting a prebiotic effect of buckwheat. In a more recent study, Ren et al. (2021) showed that buckwheat
supplementation in rats on a high-fat diet positively modulates the microbiota,
reducing the Firmicutes/Bacteroidetes ratio and increasing
microbial diversity, which helps reverse dysbiosis. Zhou
et al. (2019) explored the impact of buckwheat RS on the microbiota
by supplementing a high-fat diet with this component. The authors reported
increased levels of Lactobacillus, Bifidobacteria, and Enterococcus,
alongside inhibition of Escherichia coli. Additionally, supplementation
with buckwheat RS led to increased SCFA production and significantly lower
plasma levels of cholesterol, triglycerides, and glucose. The study concluded
that buckwheat RS supplementation inhibited inflammation and prevented insulin
resistance and hypertriglyceridemia. Given these findings, RS, a prominent
component in buckwheat, may play a key role in its prebiotic effects. RS can be
degraded by bacterial amylases into simpler carbohydrates that are fermented by
intestinal microorganisms. This fermentation can directly influence the
microbiota or produce by-products that benefit other microorganisms, leading to
positive modulation. Additionally, other polysaccharides in buckwheat, such as
pectins, arabinogalactans, and xyloglucans, may also contribute to these
effects.
Conclusions
An exhaustive review was conducted on the structure of dietary
fiber in amaranth, quinoa, and buckwheat, focusing on the relationship between
their components and potential prebiotic effects. The current literature is
limited regarding the modulatory effects of dietary fiber from these
pseudocereals on human microbiota. Nonetheless, both in vitro and in
vivo studies have evaluated the prebiotic potential of pseudocereal dietary
fiber, showing promising results. These studies consistently demonstrate
significant increases in beneficial microbial species, reductions in
potentially pathogenic species, and enhanced SCFA production. Overall, the
findings underscore the potential prebiotic effects of dietary fiber from
amaranth, quinoa, and buckwheat. However, Argentine legislation, specifically
Article 1390 of the Argentine Food Code (CAA, Chapter XVII), mandates the
identification of functional components with potential prebiotic effects.
Promoting and incorporating pseudocereals into processed food products would
not only enhance the nutritional value of consumers' diets but also diversify
raw material sources. Their consumption could offer health benefits, boost the
regional economy of pseudocereal-producing areas, support food sovereignty, and
provide consumers with options that align with their health needs and personal
values.
1. Akbari, E.;
Asemi, Z.; Daneshvar Kakhaki, R.; Bahmani, F.; Kouchaki, E.; Tamtaji, O. R.;
Ali Hamidi, Gholam; Salami, M. 2016. Effect of probiotic supplementation on
cognitive function and metabolic status in Alzheimer's disease: a randomized,
double-blind and controlled trial. Frontiers in Aging Neuroscience. 8: 256.
10.3389/fnagi.2016.00256
2.
Almeida-Alvarado, S. L.; Aguilar-López, T.; Hervert-Hernández, D. 2014. La fibra
y sus beneficios a la salud. Anales Venezolanos de Nutrición. Fundación Bengoa.
27: 73-76.
3. Álvarez, J.; Real, J. M. F.; Guarner, F.; Gueimonde, M.;
Rodríguez, J. M.; de Pipaon, M. S.; Sanz, Y. 2021. Microbiota intestinal y
salud. Gastroenterología y Hepatología. 44(7): 519-535. 10.1016/j.
gastrohep.2021.01.009
4. Alvarez-Jubete,
L.; Arendt, E. K.; Gallagher, E. 2010. Nutritive value of pseudocereals and
their increasing use as functional gluten-free ingredients. Trends in Food
Science & Technology. 21(2): 106-113. 10.1016/j.tifs.2009.10.014
5. Arayici, M. E.;
Mert-Ozupek, N.; Yalcin, F.; Basbinar, Y.; Ellidokuz, H. 2022. Soluble and
insoluble dietary fiber consumption and colorectal cancer risk: a systematic
review and meta-analysis. Nutrition and Cancer, 74(7): 2412-2425.
10.1080/01635581.2021.2008990
6. Békés, F.;
Schoenlechner, R.; Tömösközi, S. 2017. Ancient wheats and pseudocereals for
possible use in cereal-grain dietary intolerances. In Cereal Grains. p.
353-389. Woodhead Publishing. 10.1016/B978-0-08-100719-8.00014-0
7. Bekkering, C.
S.; Tian, L. 2019. Thinking outside of the cereal box: breeding underutilized
(pseudo) cereals for improved human nutrition. Frontiers in Genetics. 10: 1289.
10.3389/ fgene.2019.01289
8. Bellesi, F. A.;
Pilosof, A. M. R. 2021 Potential implications of food proteins-bile salts
interactions. Food Hydrocolloids. 118: 106766. 10.1016/j.foodhyd.2021.106766
9. Bengoa, A. A.;
Dardis, C.; Gagliarini, N.; Garrote, G. L.; Abraham, A. G. 2020.
Exopolysaccharides from Lactobacillus paracasei isolated from kefir as
potential bioactive compounds for microbiota modulation. Frontiers in
Microbiology. 11: 583254. 10.3389/fmicb.2020.583254
10. Bunzel, M.;
Ralph, J.; Steinhart, H. 2005. Association of non‐starch polysaccharides and ferulic acid in grain amaranth (Amaranthus
caudatus L.) dietary fiber. Molecular Nutrition & Food Research. 49(6):
551-559. 10.1002/mnfr.200500030
11. Cao, Y.; Zou,
L.; Li, W.; Song, Y.; Zhao, G.; Hu, Y. 2020. Dietary quinoa (Chenopodium
quinoa Willd.) polysaccharides ameliorate high-fat diet-induced
hyperlipidemia and modulate gut microbiota. International Journal of Biological
Macromolecules. 163: 55-65. 10.1016/j.ijbiomac.2020.06.241
12. Capriles, V.
D.; Coelho, K. D.; Guerra‐Matias, A. C.; Arêas, J. A. G. 2008. Effects of processing
methods on amaranth starch digestibility and predicted glycemic index. Journal
of Food Science. 73(7): 160-164. 10.1111/j.1750-3841.2008.00869.x
13. Cho, S. S.;
Finocchiaro, E. T. 2010. Natural resistant starches as prebiotics and
synbiotics. Handbook of prebiotics and probiotics ingredients: health benefits
and food applications. CRC Press, USA. p. 124-138.
14. Collar, C.;
Angioloni, A. 2014. Pseudocereals and teff in complex breadmaking matrices:
Impact on lipid dynamics. Journal of Cereal Science. 59(2): 145-154.
0.1016/j.jcs.2013.12.008
15.
Compaore-Sereme, D.; Tapsoba, F. W. B.; Zoénabo, D.; Compaoré, C. S.; Dicko, M.
H.; Sawadogo-Lingani, H. 2022. A review on dietary fiber: definitions, classification,
importance and advantages for human diet and guidelines to promote consumption.
International Journal of Biological and Chemical Sciences. 16(6): 2916-2929.
10.4314/ijbcs.v16i6.36
16. Cordeiro, L.
M.; de Fátima Reinhardt, V.; Baggio, C. H.; de Paula Werner, M. F.; Burci, L.
M.; Sassaki, G. L.; Iacomini, M. 2012. Arabinan and arabinan-rich pectic
polysaccharides from quinoa (Chenopodium quinoa) seeds: Structure and
gastroprotective activity. Food Chemistry. 130(4): 937-944.
10.1016/j.foodchem.2011.08.020
17. Covarrubias
Esquer, J. 2020. Manual de probióticos. Ergon.
18. Cronin, P.;
Joyce, S. A.; O’Toole, P. W.; O’Connor, E. M. 2021. Dietary fibre modulates the
gut microbiota. Nutrients. 13(5): 1655. 10.3390/nu13051655
19. Dziedzic, K.;
Górecka, D. G.; Kucharska, M.; Przybylska, B. 2012. Influence of technological
process during buckwheat groats production on dietary fibre content and
sorption of bile acids. Food Research International. 47(2): 279-283.
10.1016/j.foodres.2011.07.020
20. Gamel, T. H.;
Linssen, J. P.; Mesallam, A. S.; Damir, A. A.; Shekib, L. A. 2006. Effect of
seed treatments on the chemical composition of two amaranth species: oil,
sugars, fibres, minerals and vitamins. Journal of the Science of Food and
Agriculture. 86(1): 82-89. 10.1002/jsfa.2318
21. Gibson, G. R.;
Hutkins, R.; Sanders, M. E.; Prescott, S. L.; Reimer, R. A.; Salminen, S. J.;
Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.; Verbeke, K.; Reid, G. 2017.
Expert consensus document: The International Scientific Association for
Probiotics and Prebiotics (ISAPP) consensus statement on the definition and
scope of prebiotics. Nature reviews Gastroenterology & Hepatology. 14(8):
491-502. 10.1038/nrgastro.2017.75
22. Glorio, P.;
Repo-Carrasco, R.; Velezmoro, C.; Anticona, S.; Huaranga, R.; Martínez, P.;
Melgarejo, S.; Astuhuaman, L.; Huamán, N. E.; Icochea, J. C.; Peña, J. C. 2008.
Fibra dietaria en variedades peruanas de frutas, tubérculos, cereales y
leguminosas. Revista de la Sociedad Química del Perú. 74(1): 46-56.
23. Gómez-Eguílaz,
M.; Ramón-Trapero, J. L.; Pérez-Martínez, L.; Blanco, J. R. 2019. El eje
microbiota-intestino-cerebro y sus grandes proyecciones. Revista de Neurología.
68(3): 111-7.
24. Gullón, P.;
Gullón, B.; González‐Munñoz, M. J.; Alonso, J. L.; Parajó, J. C. 2014. Production and
bioactivity of oligosaccharides from biomass hemicelluloses. Food
oligosaccharides: Production, Analysis and Bioactivity. 88-106.
10.1002/9781118817360.ch6
25. Guzmán-Maldonado, S. H.; Paredes-Lopez, O. 1998. Functional
products of plants indigenous to Latin America: amaranth, quinoa, common beans,
and botanicals. Functional Food: Biochemical and Processing Aspects. 293-328.
26. Habuš, M.;
Mykolenko, S.; Iveković, S.; Pastor, K.; Kojić, J.; Drakula, S.; Curic, D.;
Novotni, D. 2022. Bioprocessing of wheat and amaranth bran for the reduction of
fructan levels and application in 3D-printed snacks. Foods. 11(11): 1649.
10.3390/foods11111649
27. Hallström, E.;
Sestili, F.; Lafiandra, D.; Björck, I.; Östman, E. 2011. A novel wheat variety
with elevated content of amylose increases resistant starch formation and may
beneficially influence glycaemia in healthy subjects. Food & Nutrition
Research. 55(1): 7074. 10.3402/fnr. v55i0.7074
28. Haros, C. M.;
Schoenlechner, R. 2017. Pseudocereals: chemistry and technology. John Wiley
& Sons.
29. He, J.; Zhang,
P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X.;
Zhang, S.; Zhu, L. 2020. Short-chain fatty acids and their association with
signalling pathways in inflammation, glucose and lipid metabolism.
International journal of molecular sciences. 21(17): 6356. 10.3390/ijms21176356
30. Hill, C.;
Guarner, F.; Reid, G.; Gibson, G. R.; Merenstein, D. J.; Pot, B.; Morelli, L.;
Berni Canani, R.; Flint, H.; Salminen, S.; Calder, P.; Sanders, M. E. 2014.
Expert consensus document: The international scientific association for
probiotics and prebiotics consensus statement on the scope and appropriate use
of the term probiotic. Nature reviews Gastroenterology & Hepatology. 10.1038/nrgastro.2014.66
31. Ispiryan, L.;
Zannini, E.; Arendt, E. K. 2020. Characterization of the FODMAP-profile in
cereal-product ingredients. Journal of Cereal Science. 92: 102916.
10.1016/j.jcs.2020.102916
32. Izydorczyk, M.
S.; Head, D. 2010. Characterization and potential uses of functional buckwheat
fractions obtained by roller milling of new Canadian buckwheat genotypes. The
European Journal of Plant Science and Biotechnology. 4: 71-81.
33. Jana, U. K.;
Kango, N.; Pletschke, B. 2021. Hemicellulose-derived oligosaccharides: Emerging
prebiotics in disease alleviation. Frontiers in Nutrition. 8: 670817.
10.3389/fnut.2021.670817
34. Jiang, F.; Du,
C.; Jiang, W.; Wang, L.; Du, S. K. 2020. The preparation, formation,
fermentability, and applications of resistant starch. International Journal of
Biological Macromolecules. 150: 1155-1161.
35. Kanwal, F.;
Ren, D.; Kanwal, W.; Ding, M.; Su, J.; Shang, X. 2023. The potential role of
nondigestible Raffinose family oligosaccharides as prebiotics. Glycobiology, 33(4):
274-288. 10.1093/ glycob/cwad015
36. Korczak, R.;
Slavin, J. L. 2020. Definitions, regulations, and new frontiers for dietary
fiber and whole grains. Nutrition reviews. 78(Supplement_1): 6-12.
10.1093/nutrit/nuz061
37. Kraic, D. M. J.
2006. Natural sources of health-promoting starch. Journal of Food and Nutrition
Research. 45(2): 69-76.
38. Lamothe, L. M.;
Srichuwong, S.; Reuhs, B. L.; Hamaker, B. R. 2015. Quinoa (Chenopodium
quinoa W.) and amaranth (Amaranthus caudatus L.) provide dietary
fibres high in pectic substances and xyloglucans. Food Chemistry. 167: 490-496.
10.1016/j.foodchem.2014.07.022
39. Leccese, G.;
Bibi, A.; Mazza, S.; Facciotti, F.; Caprioli, F.; Landini, P.; Paroni, M. 2020.
Probiotic Lactobacillus and Bifidobacterium strains counteract
adherent-invasive Escherichia coli (AIEC) virulence and hamper
IL-23/Th17 axis in ulcerative colitis, but not in crohn’s disease. Cells. 9(8):
1824. 10.3390/cells9081824
40. Linsberger‐Martin, G.;
Lukasch, B.; Berghofer, E. 2012. Effects of high hydrostatic pressure on the RS
content of amaranth, quinoa and wheat starch. Starch‐Stärke. 64(2).
157-165. 10.1002/ star.201100065
41. Mahmud, S.;
Hasan, K. F.; Jahid, M. A.; Mohiuddin, K.; Zhang, R.; Zhu, J. 2021.
Comprehensive review on plant fiber-reinforced polymeric biocomposites. Journal
of Materials Science. 56: 7231- 7264. 10.1007/s10853-021-05774-9
42. Ministerio de
Salud de la Nación. 2007-2019. Encuesta Nacional de Nutrición y Salud.
Documento de Resultados 2007 (ENNyS
https://cesni-biblioteca.org/archivos/ennys.pdf) y 2019 (ENNyS. 2
https://cesni-biblioteca.org/wp-content/uploads/2019/10/0000001565cnt-ennys2_resumen-ejecutivo-20191.pdf)
(consultado en mayo 2024).
43. Mir, N. A.;
Riar, C. S.; Singh, S. 2018. Nutritional constituents of pseudo cereals and
their potential use in food systems: A review. Trends in Food Science &
Technology. 75: 170-180. 10.1016/j.tifs.2018.03.016
44. Mohnen, D.
2008. Pectin structure and biosynthesis. Current Opinion in Plant Biology. 11(3):
266- 277. 10.1016/j.pbi.2008.03.006
45. Morales de la
Peña, M.; Odriozola-Serrano, I.; Oms-Oliu, G.; Martín-Belloso, O. 2020. Dietary
fiber in fruits and vegetables. In Book Science and Technology of Fibers in
Food Systems. Springer. 123-152.
46. Naumann, S.;
Haller, D.; Eisner, P.; Schweiggert-Weisz, U. 2020. Mechanisms of interactions
between bile acids and plant compounds-a review. International journal of
molecular sciences. 21(18): 6495. 10.3390/ijms21186495
47. Okonkwo, C. E.;
Hussain, S. Z.; Onyeaka, H.; Adeyanju, A. A.; Nwonuma, C. O.; Bashir, A. A.;
Farooq, A.; Zhou, C.; Shittu, T. D. 2023. Lignin polyphenol: From biomass to
innovative food applications, and influence on gut microflora. Industrial Crops
and Products. 206: 117696. 10.1016/j.indcrop.2023.117696
48. Olagnero, G.; Abad, A.; Bendersky, S.; Genevois, C.;
Granzella, L.; Montonati, M. 2007. Alimentos funcionales: fibra, prebióticos,
probióticos y simbióticos. Diaeta. 25(121): 20-33.
49. Pascale, N.;
Gu, F.; Larsen, N.; Jespersen, L.; Respondek, F. 2022. The potential of pectins
to modulate the human gut microbiota evaluated by in vitro fermentation:
a systematic review. Nutrients. 14(17): 3629. 10.3390/nu14173629
50. Pirzadah, T.
B.; Malik, B. 2020. Pseudocereals as super foods of 21st
century: Recent technological interventions. Journal of
Agriculture and Food Research. 2: 100052. 10.1016/j. jafr.2020.100052
51. Préstamo, G.;
Pedrazuela, A.; Peñas, E.; Lasunción, M. A.; Arroyo, G. J. N. R. 2003. Role of
buckwheat diet on rats as prebiotic and healthy food. Nutrition Research.
23(6): 803-814. 10.1016/ S0271-5317(03)00074-5
52. Quirós-Sauceda,
A. E.; Palafox-Carlos, H.; Sáyago-Ayerdi, S. G.; Ayala-Zavala, J. F.;
Bello-Perez, L. A.; Alvarez-Parrilla, E.; De la Rosa, L. A.; González-Córdova,
A. F.; González-Aguilar, G. A. 2014. Dietary fiber and phenolic compounds as
functional ingredients: interaction and possible effect after ingestion. Food
& Function. 5(6): 1063-1072. 10.1039/c4fo00073k
53. Rao, J.; Lv,
Z.; Chen, G.; Peng, F. 2023. Hemicellulose: Structure, chemical modification,
and application. Progress in Polymer Science. 101675.
10.1016/j.progpolymsci.2023.101675
54. Reguera, M.;
Haros, C. M. 2017. Structure and composition of kernels. In Book Pseudocereals:
Chemistry and technology. Wiley. Eds. Haros and Schonlechner. 28-48.
55. Ren, Y.; Wu,
S.; Xia, Y.; Huang, J.; Ye, J.; Xuan, Z.; Li, P.; Du, B. 2021.
Probiotic-fermented black tartary buckwheat alleviates hyperlipidemia and gut
microbiota dysbiosis in rats fed with a high-fat diet. Food & Function.
12(13): 6045-6057.
56.
Repo-Carrasco-Valencia, R.; Peña, J.; Kallio, H.; Salminen, S. 2009. Dietary
fiber and other functional components in two varieties of crude and extruded
kiwicha (Amaranthus caudatus). Journal of Cereal Science. 49(2):
219-224. 10.1016/j.jcs.2008.10.003
57.
Repo-Carrasco-Valencia, R. A. M.; Serna, L. A. 2011. Quinoa (Chenopodium
quinoa, Willd.) as a source of dietary fiber and other functional
components. Food Science and Technology. 31: 225-230. 10.1590/S0101-20612011000100035
58. Repo‐Carrasco‐Valencia, R.;
Arana, J. V. 2017. Carbohydrates of kernels. Pseudocereals: Chemistry and
Technology. 49-70. doi.org/10.1002/9781118938256.ch3
59. Rezende, E. S.
V.; Lima, G. C.; Naves, M. M. V. 2021. Dietary fibers as beneficial microbiota
modulators: A proposed classification by prebiotic categories. Nutrition. 89:
111217. 10.1016/j. nut.2021.111217
60. Sabbione, A.
C.; Añón, M. C.; Scilingo, A. 2023a. Characterization and bile acid binding
capacity of dietary fiber obtained from three different amaranth products.
Plant Foods for Human Nutrition. 1-10. 10.1007/s11130-023-01116-z
61. Sabbione, A.
C.; Bengoa, A. A.; Garrote, G. L.; Añon, M. C.; Scilingo, A.; Abraham, A. G.
2023b. Fibra dietaria de harina, aislado proteico y bebida de amaranto: efecto
sobre la microbiota fecal. 11° Simposio Internacional de Innovación y
Desarrollo de Alimentos. Latitud- Fundación Latu, Montevideo. Uruguay.
62. Sacristán
Oliveri, I. 2021. Influencias de la microbiota en el eje intestino-cerebro y el
desarrollo de enfermedades. Tesis de Grado. Universidad de Valladolid.
https://uvadoc.uva.es/handle/10324/48217
63. Salminen, S.;
Collado, M. C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E. M.; Sanders, M. E.;
Shamir, R.; Swann, J.; Szajewska, H.; Vinderola, G. 2021. The International
Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement
on the definition and scope of postbiotics. Nature Reviews Gastroenterology
& Hepatology. 18(9): 649-667. 10.1038/ s41575-021-00440-6
64. Sánchez
Almaraz, R.; Martín Fuentes, M.; Palma Milla, S.; López Plaza, B.; Bermejo
López, L. M.; Gómez Candela, C. 2015. Indicaciones de diferentes tipos de fibra
en distintas patologías. Nutrición Hospitalaria. 31(6): 2372-2383.
10.3305/nh.2015.31.6.9023
65. Senés-Guerrero,
C.; Gradilla-Hernández, M. S.; García-Gamboa, R.; García-Cayuela, T. 2020. Dietary
fiber and gut microbiota. Science and Technology of Fibers in Food Systems.
277-298. 10.1007/978-3-030-38654-2_12
66. Serna Saldívar,
S. O.; Ayala Soto, F. E. 2020a. Chemical composition and biosynthesis of
dietary fiber components. In Science and Technology of Fibers in Food Systems.
Springer. 15-43. 10.1007/978-3-030-38654-2_2
67. Serna Saldívar,
S. O.; Sanchez Hernández, D. 2020b. Dietary fiber in cereals, legumes,
pseudocereals and other seeds. In Science and Technology of Fibers in Food
Systems. Springer. 87-122.
68. Thursby, E.;
Juge, N. 2017. Introduction to the human gut microbiota. Biochemical Journal.
474(11): 1823-1836. 10.1042/BCJ20160510
69. Torres, N.;
Avila-Nava, A.; Medina-Vera, I.; Tovar, A. R. 2020. Dietary fiber and diabetes.
In Science and Technology of Fibers in Food Systems. Springer. 201-218.
70. Verma, D. K.;
Patel, A. R.; Thakur, M.; Singh, S.; Tripathy, S.; Srivastav, P. P.;
Chávez-González, M. L.; Guptar, A. K.; Aguilar, C. N. 2021. A review of the
composition and toxicology of fructans, and their applications in foods and
health. Journal of Food Composition and Analysis. 99: 103884.
10.1016/j.jfca.2021.103884
71. Villacrés, E.; Cuadrado, L.; Falconí, F. 2013. Los granos
andinos: Chocho (Lupinus mutabilis Sweet), quinua (Chenopodium quinoa
Willd), amaranto (Amaranthus caudatus L.) y sangorache (Amaranthus
hybridus L.) fuente de metabolitos secundarios y fibra dietética. Boletín
Técnico N° 165. Instituto Nacional de Investigaciones Agropecuarias de
Ecuador.
72. Vitaglione, P.;
Mennella, I. 2020. Dietary fiber and obesity. In Science and Technology of
Fibers in Food Systems. Springer. 187-199.
73. Wefers, D.;
Tyl, C. E.; Bunzel, M. 2015. Neutral pectin side chains of amaranth (Amaranthus
hypochondriacus) contain long, partially branched arabinans and short
galactans, both with terminal arabinopyranoses. Journal of Agricultural and
Food Chemistry. 63(2): 707-715. 10.1021/jf505283x
74. Yang, I. F.;
Jayaprakasha, G. K.; Patil, B. S. 2017. In vitro bile acid binding
capacities of red leaf lettuce and cruciferous vegetables. Journal of
Agricultural and Food Chemistry. 65: 8054-8062. 10.1021/acs.jafc.7b02540
75. Zdunek, A.;
Pieczywek, P. M.; Cybulska, J. 2021. The primary, secondary, and structures of
higher levels of pectin polysaccharides. Comprehensive Reviews in Food Science
and Food Safety. 20(1): 1101-1117. 10.1111/1541-4337.12689
76. Zeyneb, H.;
Pei, H.; Cao, X.; Wang, Y.; Win, Y.; Gong, L. 2021. In vitro study of
the effect of quinoa and quinoa polysaccharides on human gut microbiota. Food
Science & Nutrition. 9(10): 5735- 5745. 10.1002/fsn3.2540
77. Zhang, D.;
Wang, L.; Tan, B.; Zhang, W. 2020. Dietary fibre extracted from different types
of whole grains and beans: a comparative study. International Journal of Food
Science & Technology. 55(5): 2188-2196. 10.1111/ijfs.14472
78. Zhou, Y.; Zhao,
S.; Jiang, Y.; Wei, Y.; Zhou, X. 2019. Regulatory function of buckwheat‐resistant starch
supplementation on lipid profile and gut microbiota in mice fed with a high‐fat diet. Journal
of Food Science. 84(9): 2674-2681. 10.1111/1750-3841.14747
79. Zhu, F.; Du, B.; Li, R.; Li, J. 2014. Effect of
micronization technology on physicochemical and antioxidant properties of
dietary fiber from buckwheat hulls. Biocatalysis and Agricultural
Biotechnology. 3(3): 30-34. 10.1016/j.bcab.2013.12.009