Revista de la Facultad de Ciencias
Agrarias. Universidad Nacional de Cuyo. En prensa. ISSN (en línea) 1853-8665.
Original article
Physiological
and biochemical responses of Neltuma ruscifolia under Na2SO4
stress
Respuestas
fisiológicas y bioquímicas de Neltuma ruscifolia bajo estrés con Na2SO4
José Aliçandro
Bezerra da Silva2,
María Judith Ochoa1,
Julia Andrea
Lescano1
1 Universidad Nacional de Santiago del Estero. Facultad de
Agronomía y Agroindustrias. INDEAS. Av. Belgrano (S) 1912. 4200- Santiago del
Estero. Argentina.
2 Universidade Federal do Vale São Francisco. Av. Antônio Carlos
Magalhães 510. Juazeiro. BA. Brazil.
* dmeloniunse@gmail.com
Abstract
Salt stress limits
plant production in arid and semi-arid zones. Although Na2SO4
is frequent in saline soils, most studies on plant physiological
responses to salt stress were conducted using NaCl. This study aimed to
determine the effect of Na2SO4
salt stress on physiological and biochemical responses in Neltuma
ruscifolia. Increasing concentrations of Na2SO4
were added to 6-month-old plants grown hydroponically in 25%
Hoagland nutrient solution. After 60 days of saline treatments, biomass,
cysteine concentration, gas exchange, mineral composition, abscisic acid and
salicylic acid concentrations, and antioxidant enzyme activity were determined.
It is concluded that 200 mmol L-1 Na2SO4
is the threshold for N. ruscifolia seedling growth. Growth
inhibition can be attributed to altered ionic homeostasis and photosynthesis
inhibition after stomatal closure. Nevertheless, the species shows adaptive
responses to this salt. Stomatal closure and increased foliar concentrations of
abscisic acid contribute to water economy, while cysteine synthesis reduces
sulfate toxicity. In parallel, salt stress induces salicylic acid accumulation in
leaves, increasing the activity of antioxidant enzymes that prevent oxidative
stress.
Keywords: ion homeostasis,
phytohormones, antioxidant enzymes, photosynthesis, salinity stress
Resumen
El estrés salino
limita la producción vegetal en zonas áridas y semiáridas. Aunque el Na2SO4 es muy frecuente en
suelos salinos, la mayoría de los estudios sobre las respuestas fisiológicas de
las plantas al estrés salino se realizaron utilizando NaCl. El objetivo de este
trabajo fue determinar el efecto del estrés salino con Na2SO4, sobre las
respuestas fisiológicas y bioquímicas en Neltuma ruscifolia. Se
adicionaron concentraciones crecientes de Na2SO4
a plantas de 6 meses, cultivadas hidropónicamente en solución
nutritiva de Hoagland al 25%. Después de 60 días de tratamientos salinos, se
determinó la biomasa, la concentración de cisteína, el intercambio gaseoso, la
composición mineral, las concentraciones de ácido abscísico y salicílico, y la
actividad de enzimas antioxidantes. Se concluye que el umbral para el crecimiento
de plántulas de N. ruscifolia es 200 mmol L-1 Na2SO4. La inhibición del
crecimiento puede atribuirse a alteraciones en la homeostasis iónica y a la
inhibición de la fotosíntesis, debido al cierre de los
estomas. Sin embargo, la especie muestra respuestas adaptativas a esta sal. Por
lo tanto, el cierre de los estomas, asociado con mayores concentraciones
foliares de ácido abscísico, contribuye a la economía del agua. La síntesis de
cisteína reduce la toxicidad del ion sulfato absorbido por las raíces. Por otro
lado, el estrés salino induce la acumulación de ácido salicílico en las hojas y
un aumento de la actividad de enzimas antioxidantes, que pueden prevenir el
estrés oxidativo.
Palabras clave: homeostasis iónica,
fitohormonas, enzimas antioxidantes, fotosíntesis, estrés salino
Originales: Recepción: 22/04/2024 - Aceptación: 15/03/2025
Introduction
Approximately 7% of
world land surface (7 million hectares) is affected by salinization (16). This situation
worsens in arid and semi-arid areas due to global climate change, inadequate
irrigation practices, and deforestation (9,
22, 26). Arid and semi-arid areas with sodic saline soils present high
concentrations of NaCl, Na2SO4, or Na2CO3 (30). Given that NaCl
is the most abundant salt in these soils, it has starred numerous studies on
plant physiological responses to salt stress (31). However, sulfates
strongly affect several countries like Canada, USA, Mexico, Australia and
central Argentina (10, 15).
Neltuma ruscifolia (ex Prosopis
ruscifolia) is a colonizing species with many ecotypes, from shrubby forms
to 16m tall trees. It is distributed in the southeast of Bolivia, west of
Paraguay, north and center of Argentina, and the extreme south of the State of
Mato Grosso do Sul in Brazil (14, 34). Its wood is used
to manufacture poles and tool handles, and its fruits are suitable as fodder
and for human consumption (14).
N. ruscifolia tolerance to NaCl
has been previously studied (18). N. ruscifolia seedlings
can develop up to concentrations of 400 mmol L-1 NaCl (equivalent to
seawater), showing higher aerial and root biomass than the control. Higher
concentrations caused seedling death after seven days. This high tolerance to
NaCl was attributed to canopy exclusion abilities, compartmentalizing the salt
in the roots, and to the activity of antioxidant enzymes (17). However, the
species tolerance to Na2SO4
and its associated physiological responses are unknown.
Salinity can
inhibit plant growth through osmotic or ion-specific effects. It can produce
nutrient imbalance, alterations in endogenous levels of phytohormones and
oxidative damage (37). Greater
sensitivity to Na2SO4
than to NaCl has been reported in Prosopis strombulifera,
a species phylogenetically related to N. ruscifolia (27). This response was
correlated with decreased K, Ca, P, and Mg leaf concentrations and damaged
photosynthetic apparatus (27, 30). SO4-2
increased the concentrations of abscisic and salicylic acids and
the activity of antioxidant enzymes (10). The accumulation
of abscisic acid would have a protective role against dehydration, whereas
salicylic acid would signal SO4-2
damage.
This study aimed to determine the effect of Na2SO4
salt stress on physiological and biochemical responses in Neltuma
ruscifolia. We hypothesize that N. ruscifolia tolerates high Na2SO4 concentrations. Affected
ion homeostasis and photosynthesis determine growth thresholds. Hormonal
(increased abscisic and salicylic acid concentrations) and antioxidant
responses seem to contribute to Na2SO4
tolerance in this species.
Materials
and methods
Study
site
N. ruscifolia seeds were
harvested in January 2022 in Maco village, Santiago del Estero (27°51’20” S -
64°13’27” W). The region has a subtropical climate with a dry season and
absolute maximum and minimum temperatures of 45°C and -10°C, respectively (5). In this region, N.
ruscifolia forms secondary forests (vinalares), naturally distributed in
flooding areas of the Dulce and Salado rivers and on the margins of salt
marshes (7). Soils originate from loessial silts
and are saline-sodic, with a higher proportion of sodium chlorides and sulfates
(12).
Plant
material
Seeds were
scarified in concentrated sulfuric acid for 10 min and rinsed with running
water for 30 min. Then, they were sown with paper towels, watered with 25%
Hoagland nutrient solution, and incubated in a growth chamber at 26°C and 12 h
photoperiod. The seedlings were grown hydroponically in 15 L containers with
25% Hoagland nutrient solution (12 seedlings per container). The pH was
adjusted daily to 6.5 by adding HCl or KOH 1N. The trial was conducted under
greenhouse conditions, with 26°C and 6 MJ m-2 solar irradiance. After
six months of age, Na2SO4
was added, initiated by pulses of 50 mmol L-1 every 24 h, until concentrations
of 50, 100, 150, 200, 250, or 300 mmol L-1 Na2SO4
were achieved. The control consisted of a 25% Hoagland nutrient
solution. After 60 days of cultivation, gas exchange was measured, and roots
and aerial parts were separated and dried at 60°C in a forced ventilation oven
for biomass and mineral composition determinations. Cysteine, antioxidant
enzymes, and phytohormones were determined with leaf samples.
Mineral
composition
Plant material was
ground in a Wiley-type mill and sieved through a 40-mesh screen. Subsequently,
digestion was carried out with nitric acid and perchloric acid (87:13 v/v).
Mineral composition was determined by inductively coupled plasma mass
spectrometry. Results were expressed as mg g DW-1 (8).
Cysteine
determinations
Leaves were
homogenized in a mortar with liquid nitrogen and HCl 0.1 N. The homogenate was
centrifuged at 15,000 g for 30 min at 5°C. Cysteine concentration was
quantified in the supernatant according to Riemenschneider et al. (2005) and expressed as
nmol g FW-1.
Gas
exchange
Gas exchange
measurements were performed using an infrared gas analyzer in an open system
(IRGA-LCpro+ System ADC, BioScientific Ltd.), with an Arabidopsis leaf chamber,
under conditions of saturating artificial light (1000 μmol m-2 s-1)
and ambient CO2 concentration. We
measured net photosynthesis (A), stomatal conductance (gs),
intercellular CO2 concentration (Ci), and
transpiration (E).
Enzymatic
determinations
Leaves were
homogenized in a mortar with liquid nitrogen and 25 mM HEPES buffer pH 7.8,
containing 0.2 mM Na2EDTA,
2 mM ascorbate, and 2% (m/v) polyvinylpyrrolidone. The homogenate was
centrifuged at 12,000 g and 4°C for 20 min. Subsequently, the supernatant was
separated, and the soluble protein concentration was determined according to Bradford
(1976).
In this supernatant, enzymatic activities were also quantified.
Superoxide
dismutase activity (SOD, EC1.15.11) was quantified according to Giannopolitis
and Ries (1977). The reaction mixture had 100 mM phosphate buffer pH 7.4, 1 mM
EDTA, 10 mM methionine, 50 μM riboflavin, and 75μM NBT. After inciting 15 min
under a 15-W fluorescent tube, absorbance was read at 560 nm in
spectrophotometer. One unit of SOD consisted of the amount of enzyme required
to inhibit half the photoreduction of nitro blue tetrazolium chloride. SOD
activity was expressed as U mg-1 protein min-1.
Ascorbate
peroxidase activity (APX, EC1.11.11) was determined according to Nakano
and Asada (1981). The reaction mixture contained 50 mM phosphate buffer pH 7.5,
100 μl of each EDTA, ascorbate, enzyme, and H2O2. Absorbance was
recorded at 290 nm for 2 min, and an extinction coefficient of 2.8 mM-1
cm-1 was used for calculation.
APX activity was expressed as μmol ascorbate mg-1 protein min-1.
Abscisic
(ABA) and Salicylic Acids (SA)
Phytohormones were
extracted and quantified according to Durgbanshi et al. (2005). Lyophilized
leaves (50 mg DW) were homogenized in a mortar with liquid nitrogen and 3 mL
ultrapure water. Next, 25 μL of a mixture of standards containing 100 ng [2H6]
ABA and 100 ng [13C6] SA was added and
centrifuged at 8,000 g for 15 min. The supernatant was partitioned with diethyl
ether, and the organic phase was evaporated in a vacuum at 37°C. Dried extracts
were resuspended in 1 mL methanol.
Thirty μL of this
solution were directly injected in the Ultra Performance Liquid Chromatography
(UPLC) system coupled to a Triple Quadrupole Mass Spectrometer (TQD Mass
Spectrometer coupled to an Acquity LC, Waters Milford, MA, USA) through an
orthogonal Z-spray electrospray interface. Separation was performed with a
reverse phase C18 column (Gravity, 50 × 2.1 mm 1.8 μm particle size,
Macherey-Nagel GmbH, Germany), using a methanol: water gradient, both
supplemented with 0.1% acetic acid at a flow rate of 0.3 mL min-1 (11). Calibration
curves were constructed using known amounts of pure standard samples to
determine phytohormone concentrations. ABA and SA concentrations were expressed
in μg g DW-1.
Experimental
design and statistical analysis
A completely
randomized experimental design with five replications was used. The
experimental unit was represented by a 15 L container with 12 seedlings. After
checking homoscedasticity and normality, the results were analyzed using ANOVA
and the Tukey test.
Results
and discussion
Seedlings could grow up to salt concentrations of 200 mmol L-1
Na2SO4
(figure 1).
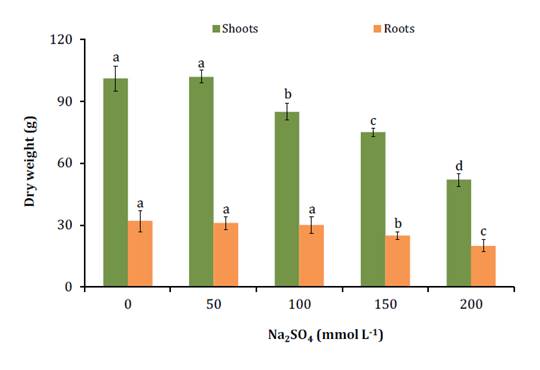
Las barras
verticales representan la desviación estándar de la media (n=5). Para cada
órgano, letras diferentes indican diferencias significativas según el test de
Tukey al 5%.
Figure
1. Dry matter of aerial part and roots of Neltuma
ruscifolia seedlings grown hydroponically in increasing concentrations of
Na2SO4.
Figura 1. Materia
seca de parte aérea y raíces de plántulas de Neltuma ruscifolia cultivadas
hidropónicamente en concentraciones crecientes de Na2SO4.
Higher salt concentrations resulted in chlorosis and seedling
death before the end of the trial. Aerial growth was more sensitive than root
growth. Aerial growth was reduced at 100 mmol L-1 Na2SO4, while root growth
was reduced at 150 mmol L-1 Na2SO4. These results
differ from those reported for the species in NaCl, in which growth threshold
was 400 mmol L-1 NaCl (18).
Neltuma ruscifolia excluded Na from the
aerial part up to 100 mmol L-1 Na2SO4,
compartmentalizing in the roots (table 1).
However, SO4 accumulated in leaves in
all saline treatments.
Table 1. Mineral
composition (mg g DW-1)
in leaves and roots of N. ruscifolia seedlings grown in increasing
concentrations of Na2SO4.
Tabla 1. Composición
mineral (mg g MS-1)
en hojas y raíces de plántulas de N. ruscifolia cultivadas en
concentraciones crecientes de Na2SO4.
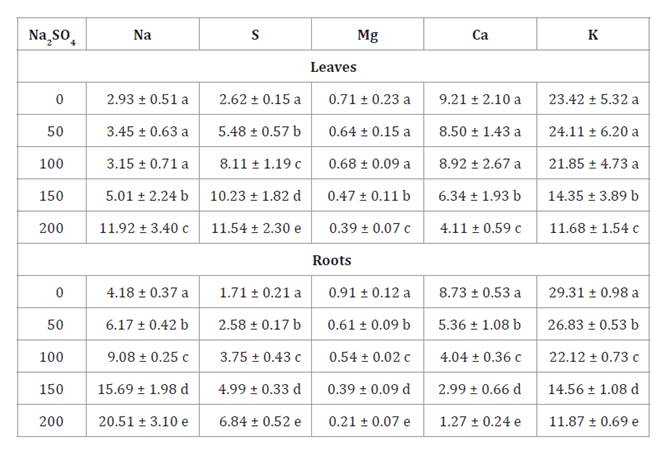
Different letters indicate
significant differences among treatments according to the Tukey test at 5%.
Letras diferentes indican diferencias significativas
entre tratamientos según el test de Tukey al 5%.
Plant tolerance to
salt stress depends on the species and the type of salt. In Brassica rapa (31) and Aeluropus
littoralis (4), Na2SO4
was more toxic than NaCl. The opposite was observed in Oryza
sativa (15) and Chenopodium
quinoa (25). In Allium cepa,
both salts had the same inhibitory effect on growth (1). Reginato
et al. (2014) reported that Strombocarpa strombulifera (ex Prosopis
strombulifera), a shrub species distributed from the Arizona desert to
Argentine Patagonia, behaved as a halophyte to NaCl but was quite sensitive to
Na2SO4.
It has been
reported that Neltuma species respond to salinity through anatomical
modifications that allow minimizing detrimental effects. Bravo et
al. (2016) reported that stress increases the number of root cortex cell
strata and decreases the diameter of xylem vessels in N. ruscifolia. The
increased number of cortex cells suggests a greater capacity for toxic ion
storage. Decreasing diameter of xylem vessels increase cavitation resistance at
low water potentials in saline soils. In Strombocarpa strombulifera,
salt stress produces suberization and early endodermis lignification,
contributing to ionic entrance control in the root (26).
The increase in Na
concentrations in the aerial part and roots was accompanied by a reduction in
Mg, Ca, and K concentrations (table 1). These results agree with Reginato et al. (2019) in S. strombulifera,
in which Na2SO4
produced a significant decrease in foliar Ca and Mg
concentrations, correlating with growth inhibition and senescence. According to
Ahmadizadeh
et al. (2016), the accumulation of Na and SO42-
causes ionic imbalance and compromises uptake of other essential
nutrients, such as K and Ca. The antagonism between Ca and Na ions has been
attributed to the nonselective cation channels in cell membranes (NSCC),
allowing the entry of both cations without discriminating one from the other (27,
32).
All salt treatments increased foliar cysteine concentrations (figure
2). Thus, in the 200 mmol L-1 Na2SO4
treatment, cysteine concentrations were six times higher than in
the control.
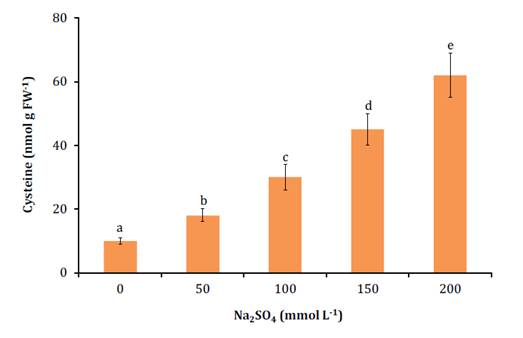
Vertical bars
represent mean standard deviation (n=5).
Different
letters indicate significant differences according to the Tukey test at 5%.
Las
barras verticales representan la desviación estándar de la media (n=5).
Letras diferentes
indican diferencias significativas según el test de Tukey al 5%.
Figure
2. Cysteine concentration in leaves of N. ruscifolia
seedlings grown in increasing concentrations of Na2SO4.
Figura 2. Concentración
de cisteína en hojas de plántulas de N. ruscifolia cultivadas en
concentraciones crecientes de Na2SO4.
Sulfate is stored
in the vacuoles of root and xylem parenchyma cells or transported via xylem to
the aerial part (Takahashi
et al., 2011). Once in leaves, sulfate is again stored in vacuoles or
reduced to sulfite in chloroplasts. Sulfite can be involved in cysteine
synthesis in a reaction catalyzed by cysteine synthase. This mechanism
incorporates sulfite into organic compounds, avoiding its inhibitory effect on
mitochondrial respiration. Cysteine is a highly reactive thiol and helps
maintain redox homeostasis in plants subjected to different abiotic stresses (39). Reginato
et al. (2019) also reported increased foliar cysteine concentrations in S.
strobulifera under Na2SO4
stress.
Salinity inhibited
photosynthesis and stomatal conductance at 100 mmol L-1 Na2SO4
(figure
3A, B),
causing internal CO2 concentration (figure 3C) and transpiration
(figure
3D)
to decrease.
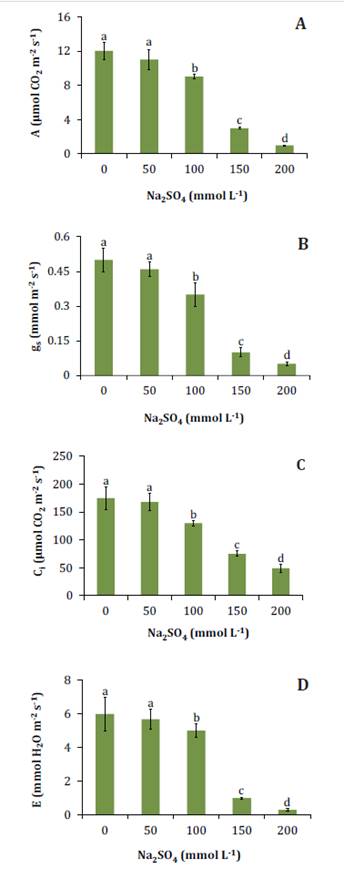
Las
barras verticales representan la desviación estándar de la media (n=5). Letras
diferentes indican diferencias significativas según el test de Tukey al 5%.
Figure
3. Net photosynthesis (A), stomatal conductance (B),
internal CO2 concentration
(C), and transpiration (D) in N. ruscifolia seedlings grown in
increasing concentrations of Na2SO4.
Figura
3. Fotosíntesis neta (A), conductancia
estomática (B), concentración interna de CO2 (C) y transpiración (D) en plántulas de N.
ruscifolia cultivadas en concentraciones crecientes de Na2SO4.
Photosynthesis can
be inhibited by salinity due to stomatal and nonstomatal limitations.
The latter include
inhibition of Rubisco activity, decreased photosynthetic pigment concentration,
and alterations at photochemical level (17). In the present
case, salt stress simultaneously reduced net photosynthesis, stomatal
conductance, and internal CO2 concentration. Therefore,
in this study, photosynthesis inhibition can be attributed to stomatal closure.
In agreement with these results, in Neltuma alba the photochemical stage
of photosynthesis was only inhibited by concentrations over 400 mM NaCl (20). In S.
strombulifera, photosynthesis response to salt stress depends on the type
of salt. Thus, whereas NaCl did not affect maximum quantum yield of photosystem
II, Na2SO4
produced a significant reduction of this variable, indicating
photoinhibition (29).
Concentrations over
50 mmol L-1 Na2SO4
produced a significant increase in foliar ABA (figure 4A). In the 200 mmol
L-1 Na2SO4
treatment, ABA concentration was 100% higher than the control.
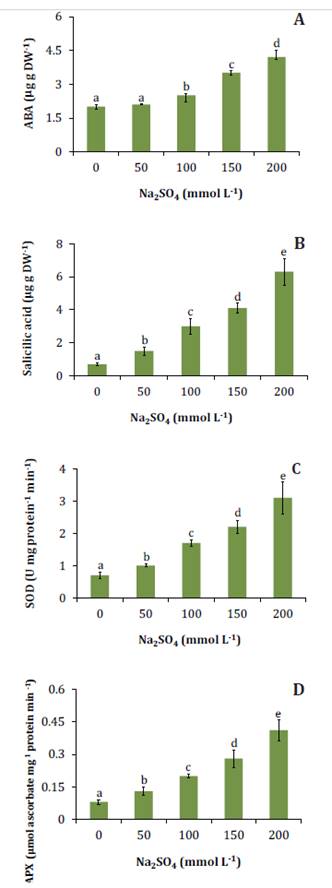
Las
barras verticales representan la desviación estándar de la media (n=5). Letras
diferentes indican diferencias significativas según el test de Tukey al 5%.
Figure
4. Abscisic acid (A), salicylic acid (B), superoxide
dismutase activity (C), and ascorbate peroxidase activity (D) in N.
ruscifolia seedlings grown in increasing concentrations of Na2SO4.
Figura
4. Concentración de ácido abscísico
(A), concentración de ácido salicílico (B), actividad superóxido dismutasa (C)
y actividad peroxidasa (D) en plántulas de N. ruscifolia cultivadas en
concentraciones crecientes de Na2SO4.
Phytohormones play a key role in plant physiological responses
under stress conditions. In saline stress, ABA gains particular
ecophysiological importance as it causes stomatal closure and reduces
transpiration (35).
Under water and salt stress, ABA works as a signal from the root to the aerial
part, increasing water economy through stomatal closure and reduced leaf
expansion (38).
In S. strombulifera, Na2SO4
significantly increases foliar ABA concentrations (10),
suggesting that ABA accumulation in leaves would be protective against
dehydration.
All salt concentrations significantly increased foliar SA
concentrations (figure
4B).
Discrepancies exist regarding the role of SA in regulating plant tolerance to
salt stress. In Vigna angularis seedlings, SA mitigated the inhibitory
effect of salt stress on photosynthesis and growth. This response was
associated with increased tissue antioxidant activity (2). In contrast, SA
was not involved in NaCl tolerance of the halophytes Lycium humile and S.
strombulifera, nor was it identified as a stress signal (10,
24).
It has been
suggested that SA induce antioxidant enzymes or alter the expression of their
genes (21). In agreement with that observation,
all salt concentrations increased the activities of SOD and APX enzymes (figure 4 C, D). However,
according to Miura
et al. (2013), low SA concentrations improve antioxidant capacity, whereas
high concentrations produce oxidative stress and cell death. In agreement with
these results, the high tolerance of N. ruscifolia to NaCl was
attributed in part to its high antioxidant capacity due to the activities of
SOD and peroxidase enzymes and polyphenols (19). Reginato
et al. (2021) also reported significant increases in SOD and APX activities
in leaves of S. strombulifera seedlings subjected to salt stress with Na2SO4.
Conclusions
N. ruscifolia growth threshold is 200
mmol L-1 Na2SO4. Growth inhibition
can be attributed to affected ionic homeostasis and inhibition of
photosynthesis due to stomatal closure. Nevertheless, the species shows
adaptive responses to this salt. Thus, stomatal closure associated with
increased foliar ABA concentrations contributes to water economy. Cysteine
synthesis reduces sulfate toxicity when absorbed by the roots. On the other
hand, salt stress induces SA accumulation in leaves and increases antioxidant
activity, preventing oxidative stress. These characteristics demonstrate the
high potential of the species for afforestation schemes on sodic saline soils.
Acknowledgments
The authors thank the Consejo de Investigaciones Científicas y
Tecnológicas de la Universidad Nacional de Santiago del Estero (CICyT-UNSE) for
funding.
1.
Aghajanzadeh, T. A.; Reich, M.; Hawkesford, M. J.; Burow, M. 2019. Sulfur
metabolism in Allium cepa is hardly affected by chloride and sulfate
salinity. Archives of Agronomy and Soil Science. 65: 45-95.
https://doi.org/10.1080/03650340.2018.1540037
2.
Ahanger, M. A.; Aziz, U.; Alsahli, A. A.; Alyemeni, M. N.; Ahmad, P. 2019.
Influence of exogenous salicylic acid and nitric oxide on growth,
photosynthesis, and ascorbate-glutathione cycle in salt-stressed Vigna
angularis. Biomolecules. 26: 42. https://doi.org/10.3390/ biom10010042
3.
Ahmadizadeh, M.; Vispo, N. A.; Calapit-Palao, C. D. O.; Pangaan, I. D.; Viña,
C. D.; Singh, R. K. 2016. Reproductive stage salinity tolerance in rice: a
complex trait to phenotype. Indian Journal of Plant Physiology. 21: 528-536.
https://doi.org/10.1007/s40502-016-0268-6
4.
Barhoumi, Z. 2018. Physiological response of the facultative halophyte, Aeluropus
littoralis, to different salt types and levels. Plant Biosystems. 153:
298-305. https://doi.org/10.1080 /11263504.2018.1478901
5.
Boletta, P.; Ravelo, A.; Planchuelo, A.; Grilli, M. 2006. Assessing
deforestation in the Argentine Chaco. Forest Ecology and Management. 228:
108-114. https://doi.org/10.1016/j. foreco.2006.02.045
6.
Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of
microgram quantities of protein utilizing the principle of protein-dye binding.
Analytical Biochemistry. 72: 248-54. https://doi.org/10.1006/abio.1976.9999
7.
Bravo, S. J.; Pece, M.; del Corro, F.; Ojeda Brozovich, F.; Lepiscopo, M. 2016.
Anatomical changes in roots and hypocotyls of Prosopis ruscifolia (Fabaceae)
seedlings exposed to saline stress. Revista de Biología Tropical. 64:
1007-1017.
8.
Chrysargyris, A.; Xylia, P.; Botsaris, G.; Tzortzakis, N. 2017. Antioxidant and
antibacterial activities, mineral and essential oil composition of spearmint (Mentha
spicata L.) affected by the potassium levels. Industrial Crops and
Products. 103: 202-212. https://doi.org/10.1016/j.
indcrop.2017.04.010
9. Dalzotto, D.; Sharry, S.; Piñuel, L.;
Boeri, P. (en prensa). Challenges in germination of Neltuma caldenia in
semi-arid regions: optimization of germination protocols, influence of saline
stress and seed quality. Revista de la Facultad de Ciencias Agrarias.
Universidad Nacional de Cuyo. Mendoza. Argentina.
10.
Devinar, G.; Llanes, A.; Masciarelli, O.; Luna, V. 2013. Different relative
humidity conditions combined with chloride and sulfate salinity treatments
modify abscisic acid and salicylic acid levels in the halophyte Prosopis
strombulifera. Plant Growth Regulation. 70: 247-256. https://
doi.org//10.1007/s10725-013-9796-5
11.
Durgbanshi, A.; Arbona, V.; Pozo, O.; Miersch, O.; Sancho, J. V.;
Gómez-Cadenas, A. 2005. Simultaneous determination of multiple phytohormones in
plant extracts by liquid chromatography-electrospray tandem mass spectrometry.
Journal of Agricultural and Food Chemistry. 53: 8437-8442.
https://doi.org/10.1021/jf050884b
12.
Galizzi, F.; Angueira, C.; Prieto, D. 1999. Suelos de la planta piloto de
drenaje del INTA, Santiago del Estero. Revista Quebracho. 7: 52-60.
13.
Giannopolitis, N.; Ries, S. K. 1977. Superoxide dismutase. I. Occurrence in
higher plants. Plant Physiology. 59: 309-314.
https://doi.org/10.1104/pp.59.2.309
14.
Giménez, A. M.; Moglia, J. G. 2003. Árboles del Chaco Argentino. Guía para el
reconocimiento dendrológico. Editorial El Liberal. Argentina. 307p.
15.
Irakoze, W.; Vanpee, B.; Rufyikiri, G.; Dailly, H.; Nijimbere, S.; Lutts, S.
2019. Comparative effects of chloride and sulfate salinities on two contrasting
rice cultivars (Oryza sativa L.) at the seedling stage. Journal of Plant
Nutrition. 42: 1-15. https://doi.org/10.1080/01904167.2 019.1584222
16.
Li, J.; Pu, L.; Han, M.; Zhu, M.; Zhang, R.; Xiang, Y. 2014. Soil salinization
research in China: Advances and prospects. Journal of Geographical Science. 24:
943-960.
17.
Meloni, D. A. 2017. Fisiología Vegetal: respuestas de especies leñosas al
estrés salino. Editorial Universidad Nacional de Santiago del Estero,
Argentina. 165 p.
18.
Meloni, D. A.; Gulotta, M. R.; Martínez, C. A. 2008a. Prosopis ruscifolia Griseb.
(vinal) tolera concentraciones salinas equivalentes al
agua de mar y excluye iones tóxicos de la parte aérea. Revista Quebracho. 16:
32-40.
19.
Meloni, D. A.; Gulotta, M. R.; Oliva Cano, M. A. 2008b. El estrés salino
incrementa la actividad de enzimas antioxidantes y la concentración de
polifenoles en vinal (Prosopis ruscifolia G.). Revista Quebracho. 15:
27-31.
20.
Meloni, D. A.; Gulotta, M. R.; Silva, D. M.; Arraiza, M. P. 2019. Effects of
salt stress on germination, seedling growth, osmotic adjustment, and
chlorophyll fluorescence in Prosopis alba G. Revista de la Facultad de
Ciencias Agrarias. Universidad Nacional de Cuyo. Mendoza. Argentina. 51(1):
69-78.
21.
Miura, K.; Okamoto, H.; Okuma, E.; Shiba, H.; Kamada, H.; Hasegawa, P. M.;
Murata, Y. 2013. SIZ1 deficiency causes reduced stomatal aperture and enhanced
drought tolerance via controlling salicylic acid-induced accumulation of
reactive oxygen species in Arabidopsis. Plant Journal. 73: 91-104.
https://doi.org/10.1111/tpj.12014
22.
Munns, R.; Gilliham, M. 2015. Salinity tolerance of crops-what is the cost? New
Phytology. 208: 668-673. https://doi.org/10.1111/nph.13519
23.
Nakano, Y.; Asada, K. 1981. Hydrogen peroxide is
scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell
Physiology. 22: 867-880. https://doi.org/10.1093/ oxfordjournals.pcp.a076232
24.
Palchetti, M. V.; Llanes, A.; Reginato, M.; Barboza, G.; Luna, V.; Cantero, J.
J. 2020. Germination responses of Lycium humile, an extreme halophytic
Solanaceae: understanding its distribution in saline mudflats of the southern
Puna. Acta Botanica Brasilica. 34: 540-548. https://doi.org/10.1590/0102-33062020abb0034
25.
Peterson, A.; Murphy, K. 2015. Tolerance of lowland quinoa cultivars to sodium
chloride and sodium sulfate salinity. Crop Science. 55: 331-338.
https://doi.org/10.2135/cropsci2014.04.0271
26.
Piraino, S.; Roig, F. A. 2024. Landform heterogeneity drives multi-stemmed Neltuma
flexuosa growth dynamics. Implication for the Central Monte Desert forest
management. Revista de la Facultad de Ciencias Agrarias. Universidad Nacional
de Cuyo. Mendoza. Argentina. 56(1): 26-34. DOI:
https://doi.org/10.48162/rev.39.120
27.
Reginato, M.; Sosa, L.; Llanes, A.; Hampp, E.; Vettorazzi, N.; Reinoso, H.;
Luna, V. 2014. Growth responses and ion accumulation in the halophytic legume Prosopis
strombulifera are determined by Na2SO4 and NaCl. Plant Biology. 16: 97-106.
http://dx.doi.org/10.1111/ plb.12001
28.
Reginato, M.; Travaglia, C.; Reinoso, H.; Garello, F.; Luna, V. 2015.
Anatomical modifications in the halophyte Prosopis strombulifera caused
by salt mixture. Flora. 218: 75-85. https://doi.
org/10.1016/j.flora.2015.11.008
29.
Reginato, M.; Turcio, E. T.; Luna, V.; Papenbrock, J. 2019. Differential
effects of NaCl and Na2SO4 on the halophyte Prosopis strombulifera are
explained by different responses of photosynthesis and metabolism. Plant
Physiology and Biochemistry. 11: 306-314. https://
doi.org/10.1016/j.plaphy.2019.05.027
30.
Reginato, M.; Cenzano, A. M.; Arslan, I.; Furlán, A.; Varela, C.; Cavallin, V.;
Papenbrock, J.; Luna, V. 2021. Na2SO4 and NaCl salts differentially modulate
the antioxidant systems in the highly stress tolerant halophyte Prosopis
strombulifera. Plant Physiology and Biochemistry. 167: 748-762.
https://doi.org/10.1016/j.plaphy.2021.09.003
31. Reich, M.; Aghajanzadeh, T.; Helm,
J.; Parmar, M. J.; Hawkesford, S.; De Kok, L. J. 2017. Chloride and sulfate
salinity differently affect biomass, mineral nutrient composition and
expression of sulfate transport and assimilation genes in Brassica rapa.
Plant and Soil. 411: 319-32. https://doi.org/10.1007/s11104-016-3026-7
32.
Reich, M.; Aghajanzadeh, T.; Parmar, S.; Hawkesford, M. J.; De Kok, L. J. 2018.
Calcium ameliorates the toxicity of sulfate salinity in Brassica rapa.
Journal of Plant Physiology. 231: 1-8.
https://doi.org/10.1016/j.jplph.2018.08.014
33.
Riemenschneider, A.; Nikiforova, V.; Hoefgen, R. De Kok, L. J.; Papenbrock, J.
2005. Impact of elevated H2S on metabolite levels, activity of enzymes and
expression of genes involved in cysteine metabolism. Plant Physiology and
Biochemistry. 43: 473-483. https://doi.org/10.1016/j.
plaphy.2005.04.001
34.
Sigrist, M. R.; Stefanello, T. H.; de Souza, C. S.; Vargas, W.; Almeida, K. S.
M.; Laroca, S.; Mansano, V. F. 2018. Phenology and pollination ecology of Prosopis
rubriflora (Leguminosae, Mimosoideae), a species from the semi-arid
Brazilian Chaco. Brazilian Journal of Botany. 41: 103-115.
https://doi.org/10.1007/s40415-017-0433-9
35.
Singh, V. P.; Prasad, S. M.; Bosch, S. M.; Müller, M. 2017. Phytohormones and
the regulation of stress tolerance in plants: current status and future
directions. Frontiers in Plant Science. 8: 1871.
https://doi.org/10.3389/fpls.2017.01871
36.
Takahashi, H.; Kopriva, S.; Giordano, M.; Saito, H.; Hell, R. 2011. Sulfur
assimilation in photosynthetic organisms: molecular functions and regulations
of transporters and assimilatory enzymes. Annual Review of Plant Biology. 62:
157-184. https://doi.org/10.1146/ annurev-arplant-042110-103921
37.
van Zelm, E.; Zhang, Y.; Testerink, C. 2020. Salt tolerance mechanisms of
plants. Annual Review of Plant Biology. 71:403-433.
https://doi.org/10.1146/annurev-arplant-050718-100005
38.
Wilkinson, S.; Kudoyarova, G. R.; Veselov, D. S.; Arkhipova, T. N.; Davies, W.
J. 2012. Plant hormone interactions: innovative targets for crop breeding and
management. Journal of Experimental Botany. 63: 3499-3509.
https://doi.org/10.1093/jxb/ers148
39. Zagorchev, L.; Seal, C. E.; Kranner,
I.; Odjakova, M. A. 2013. Central role for thiols in plant tolerance to abiotic
stress. International Journal of Molecular Science. 14:7405-7432. https://doi.
org/10.3390/ijms14047405