Revista de la Facultad de Ciencias
Agrarias. Universidad Nacional de Cuyo. Tomo 57(2). ISSN (en línea) 1853-8665.
Año 2025.
Original article
Essential
oils and extracts from Argentinian northwest plants as potential biofungicides
for olive and grapevine pathogens: in vitro studies
Aceites
esenciales y extractos de plantas del noroeste argentino como potenciales
biofungicidas de patógenos de olivo y vid: estudios in vitro
Ivana Ormeño2,
María Teresa Ajmat2,
1 Universidad Nacional de Chilecito. CONICET. Departamento de
Ciencias Básicas y Tecnológicas. 9 de Julio 22, Chilecito F5360CKB. La Rioja.
Argentina.
2 Universidad Nacional de Chilecito. Instituto de Ambiente de
Montaña y Regiones Áridas.
* nbarbieri@undec.edu.ar
Abstract
This work studies
the effect of 12 botanical products from Argentinian northwest plants on spores
and mycelium of Verticillium dahliae and Phaeoacremonium parasiticum,
two pathogens of agronomic importance for the region. The fungi were exposed to
essential oils (EOs) or ethanolic extracts (EEs), determining the percentage of
germinated spores and mycelial growth. All tested EOs and EEs showed varying
degrees of antifungal activity, dependent on plant species, extract type,
pathogen, and targeted fungal structures. V. dahliae germination was
completely inhibited by Zuccagnia punctata and Clinopodium gilliesii EOs.
In experiments with EEs, Z. punctata EE was the most effective in
suppressing spore germination of both fungi. The C. gilliesii EE also
controlled V. dahliae germination. The EEs of Z. punctata, C.
gilliesii and Lippia turbinata were the most active against mycelial
growth. These three EEs had a fungistatic effect on P. parasiticum while
Z. punctata and L. turbinata EEs showed a fungicidal effect on V.
dahliae. The products obtained from Z. punctata, C. gilliesii and
L. turbinata have potential as biocontrollers against V. dahliae and
P. parasiticum. This is encouraging since no effective treatments are
available for the diseases involving these pathogens.
Keywords: Verticillium
dahliae Kleb, Phaeoacremonium
parasiticum (Ajello, Georg &
C.J.K.Wang) W.Gams, Crous & M.J.Wingf., botanical antifungals,
mycelial inhibition, conidial susceptibility
Resumen
Este trabajo
estudia el efecto de 12 productos de plantas del noroeste argentino sobre las
esporas y micelio de Verticillium dahliae y Phaeoacremonium
parasiticum, dos patógenos de importancia agronómica. Los hongos fueron
expuestos a los aceites esenciales (AE) o extractos etanólicos (EE), y se
determinó el porcentaje de germinación y crecimiento micelial. Todos los AE y
EE mostraron actividad antifúngica, la cual dependió de la especie vegetal, del
extracto, del patógeno y de las estructuras fúngicas objetivo. La germinación
de V. dahliae fue inhibida con los AE de Zuccagnia punctata y Clinopodium
gilliesii. El EE de Z. punctata fue el más efectivo para suprimir la
germinación de ambos hongos. El EE de C. gilliesii también fue capaz de
controlar la germinación de V. dahliae. Mientras que los EE de Z.
punctata, C. gilliesii y Lippia turbinata fueron los más
activos sobre el micelio. Estos tres EE fueron fungistáticos sobre P.
parasiticum mientras que los EE de Z. punctata y L. turbinata fueron
fungicidas sobre V. dahliae. Los productos obtenidos de Z. punctata,
C. gilliesii y L. turbinata son potenciales biocontroladores de V.
dahliae y P. parasiticum. Esto es alentador ya que no se dispone de
tratamientos eficaces para las enfermedades en las cuales participan estos
patógenos.
Palabras clave: Verticillium
dahliae Kleb, Phaeoacremonium
parasiticum (Ajello, Georg &
C.J.K.Wang) W.Gams, Crous & M.J.Wingf., antifúngicos
botánicos, inhibición micelial, susceptibilidad conidial
Originales: Recepción: 07/05/2024 - Aceptación: 23/12/2024
Introduction
Olive and grapevine
cultivation in La Rioja province (northwest Argentina) is economically
significant. Fungal diseases affect productivity causing considerable losses (7,
12). Vascular wilt disease in olives caused by Verticillium
dahliae Kleb has acquired great importance worldwide producing tree
mortality, fruit yield reduction, and organoleptic defects in virgin olive oil
extracted from infected plants (18, 19). Olive
verticillium wilt is one major concern for olive growers in the semi-arid
regions of Argentina. Rattalino (2023) has recently shown that V. dahliae
is widely spread in La Rioja olive-growing regions, estimating 24% disease
incidence.
Grapevine trunk
diseases are the principal fungal diseases affecting viticulture worldwide (17). Among these
pathologies, hoja de malvón (related to Esca) and young vine decline
(Petri disease) are among the most devastating and challenging diseases in many
wine regions of Argentina. They are caused by multiple wood fungal pathogens,
with Phaeoacremonium parasiticum being mostly prevalent (9,
10).
Unfortunately,
effective treatments against these mycoses are not available, and their
management remains difficult. To date, recommendations focus on timely
monitoring of these diseases and integrated management strategies including
biological control as a potential tool (17, 19).
Plant essential oils (EOs), extracts and related molecules have
demonstrated inhibitory efficacy against pathogenic fungi (3,
26). They represent eco-friendly control alternatives for
integrated disease management, contributing to sustainable agricultural
production. The antifungal activity (AA) of some EOs and a few plant extracts
is reported against V. dahliae (6, 8, 11, 14, 24). However,
insufficient studies focus on biological control of P. parasiticum using
plant products. This study focused on plant species with previous AA against
dermatophytes or molds: Zuccagnia punctata, Clinopodium gilliesii, Lippia
turbinata, Lippia integrifolia, Argemone subfusiformis, Erythrostemon gilliesii,
and Senecio subulatus var. salsus (1). We explore their
AA against olive and grapevine pathogenic fungi, hypothesizing that plant
products from these species could control plant pathogenic fungi in regional
crops. We evaluated the effect of 12 botanical products (secondary metabolites)
obtained from the mentioned plants on spore viability and mycelial growth of V.
dahliae and P. parasiticum.
Material
and methods
Plant
Material
Z. punctata Cav., C.
gilliesii Kuntze, L. turbinata Griseb., L. integrifolia Hieron.,
A. subfusiformis Ownbey., E. gilliesii (Hook.) Klotzsch and S.
subulatus var. salsus (Griseb.) were collected in
2018. Georeferenced specimens were deposited in the herbarium of the
Universidad Nacional de Chilecito (UNDEC). Supplementary Table 1 provides data on
collection sites, yield and voucher specimens.
Obtaining
Essential Oils (EOs) and Ethanolic Extracts (EEs)
Air-dried canopies
were used. EOs were obtained by hydrodistillation in a Clevenger-type apparatus
and stored at -20°C until further use. To obtain EEs, the plant material was
macerated in ethanol 96° for 24 h, filtered and the solvent evaporated. Then, waxes
were removed by precipitation from an ethanol-water solution. Later, EEs were
dissolved in 50% ethanol, shaken using a 40 kHz ultrasonic cleaning bath (1 h)
and centrifuged (5000 rpm, 10 min). Finally, the separated supernatants were
evaporated and samples were stored until use (2).
Phytopathogenic
Fungi
We used a native
non-defoliating strain of V. dahliae Kleb. previously
isolated from an infected olive plant in La Rioja (21). The P. parasiticum
strain was obtained from the Phytopathology Laboratory of INTA Mendoza,
Argentina. First, stock cultures (stored at -80°C) were activated in potato
dextrose agar (PDA, Britania, Argentina) and grown in microcultures (PDA block
on a microscopic slide) to check morphological traits (Supplementary
Figure 1). Secondly, fungi were maintained in PDA for antifungal assays.
Inhibition
of Spore Germination
The phytopathogens V.
dahliae and P. parasiticum were cultured for 7 and 14 days,
respectively, allowing spore development. To obtain spores, 2 mL sterile
distilled water were added, and mycelia was gently scraped with a Drigalsky
spatula. The suspension was recovered and adjusted to 1 x 103 spores/mL using a
Neubauer counting chamber. For the assays, a 100 uL spore suspension was
incubated with 100 uL of different concentrations of EO or EE (1-3 mg/mL) for
1h at 24°C. For plant products with 100% inhibitory activity at 1 mg/mL, lower
concentrations (range of 0.2-1 mg/mL) were also evaluated. Following
incubation, an aliquot was taken and seeded in PDA. After 48 h for V.
dahliae and 72 h for P. parasiticum at 24°C incubation, spores were
counted and the percentage of inhibited spores (number of non-germinated
spores/total number of spores × 100) was determined (16). Growth control
for each tested phytopathogen (distilled water), solvent control (DMSO or
ethanol 96°) and EO and EE sterility controls were included. According to own
experimental data, Benomyl (fungicide) constituted the positive control in
concentrations ranging from 0.1 to 0.4 mg/mL for V. dahliae and 7 to 10
mg/mL for P. parasiticum. The minimum inhibitory concentration (MIC) was
defined as the lowest EO or EE concentration producing 100% inhibition of spore
germination.
Synergism
(Checkerboard Test)
The MIC values obtained previously served as a reference and
combinations ranging from 0.125xMIC to 1xMIC of EOs, EEs and Benomyl were
formulated. Inhibition on spore germination was determined using the
methodology described above. To evaluate combination effects, the fractional
inhibitory concentration (FIC) index was calculated as FIC index = FICA + FICB,
where FICA and FICB are the minimum concentrations inhibiting fungal growth
(MIC) for samples A and B, respectively. FICA = (Combination MICA) / (MICA alone),
FICB = (Combination MICB)/ (MICB alone). According to the FIC index, results
indicated synergism (≤ 0.5), addition (> 0.5 and ≤ 1.0), indifference (>
1.0 and ≤ 2.0), or antagonism (> 2.0) (25).
Inhibition
of Mycelial Growth
EEs were added at different concentrations (0.25-3 mg/mL) on
molten PDA. Petri dishes with PDA plus EE were inoculated with a 5-mm diameter
mycelial disc obtained from the edge of 7-and 14-day-old cultures of V.
dahliae and P. parasiticum, respectively. Growth control for each
tested phytopathogen (PDA plate with the mycelial disc), solvent control (PDA
plate plus 96° ethanol with the mycelial disc), and positive control (PDA plate
plus Benomyl with the mycelial disc) were included. Inoculated plates were
incubated at 24°C and growth of V. dahliae and P. parasiticum was
evaluated at 7 days by measuring mycelial diameter of each colony. Percentage
of growth inhibition was calculated by equation 1:
where
D = colony diameter
of growth controls
d = diameter in EE
or Benomyl treatments
The MIC was equal
to the lowest EE concentration at which mycelial growth was completely
inhibited (24). When EEs
inhibitory effects were fungicidal or fungistatic, PDA plates with mycelium
discs and different EE treatments would be incubated for 2-5 additional days
(mycelial inhibition at 9 days for V. dahliae and 12 days for P.
parasiticum). When no mycelium re-growth occurred during additional
incubation, EE was considered fungicidal. Otherwise, it was considered
fungistatic.
Total
Phenolic and Flavonoid Content (PC and FC) in the EEs
Total PC was
determined by the Folin-Ciocalteu spectrophotometric method. Different volumes
of EE solutions were mixed with Folin-Ciocalteu reagent and sodium carbonate.
After incubation, absorbance was measured at 765 nm. The PC was determined
using a Gallic acid calibration curve and results were expressed as mg Gallic
acid equivalents/g of dry extract (mg GAE/g) (2). FC was estimated
by a spectrophotometric assay based on aluminum chloride complexes. Serial
dilutions from EEs were mixed with aluminum chloride, and incubated for 1 h.
Absorbance was measured at 420 nm. FC was calculated using a Quercetin
calibration curve and expressed as mg quercetin equivalents/g of dry extract
(mg QE/g) (2).
Statistical
Analysis
Each treatment had
two replicates, and experiments were conducted at least three times using a
randomized design. Results are expressed as mean ± standard deviation/standard
error. Statistical significance of the data was determined by ANOVA followed by
Tukey’s test (MINITAB software version 15 for Windows, SPSS Inc., Chicago, IL),
p ≥0.05. Pearson’s correlation coefficient was calculated between AA and
phenols or flavonoid content of EEs using InfoStat software (5).
Results
Effect
of EOs and EEs on V. dahliae and P. parasiticum Spore Germination
The AA of five EOs
and seven EEs obtained from plants in northwest Argentina was evaluated against
spore germination of V. dahliae and P. parasiticum. Inhibition of
conidia germination varied among treatments and increased with increasing EO or
EE concentration.
Only the EOs from Z. punctata and C. gilliesii exhibited
100% inhibitory activity on V. dahliae spores, with MIC values of 3
mg/mL each (figures
1a and b). At the highest concentration tested, the EOs from L.
turbinata and L. integrifolia showed remarkable activity against V.
dahliae spores, with inhibition values of 96.4 and 96% respectively (figure 1c and d).
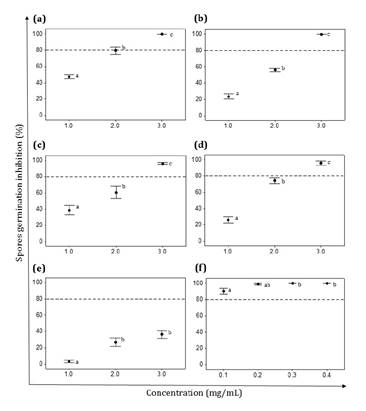
(a) Z. punctata,
(b) C. gilliesii, (c) L. turbinata, (d) L. integrifolia,
(e) S. subulatus var. salsus,
(f) Benomyl (Fungicide).
Data
are expressed as mean ± standard error, n=6-8. Different letters indicate
significant differences among concentrations of the same EO. (p˂ 0.05).
(a)
Z. punctata, (b) C. gilliesii, (c) L. turbinata, (d) L.
integrifolia, (e) S. subulatus var. salsus,
(f) Benomil (fungicida).
Los
datos se expresan como la media ± error estándar, n=6-8. Letras distintas
indican diferencias significativas entre concentraciones del mismo AE. (p˂
0,05).
Figure
1. Effect of essential oils (EOs) on V. dahliae spore
germination.
Figura
1. Efecto de los aceites esenciales
(AE) sobre la germinación de las esporas de V. dahliae.
Concerning spore
germination of P. parasiticum, no EO had a 100% inhibitory effect. C.
gilliesii EO inhibited 85.8% of spores at 3 mg/mL, while the remaining oils
showed low activity, with 30-54 % inhibition at the highest concentration
evaluated (Supplementary
Figure 2).
On the other hand,
in assays with EEs, only Z. punctata EE effectively controlled spore
germination of both pathogenic fungi (figure 2a and 3a). The effective
concentration (MIC) of this extract on V. dahliae was 0.4 mg/mL, similar
to the MIC obtained with the synthetic antifungal Benomyl (MIC=0.3 mg/mL) (figure 2a and h).
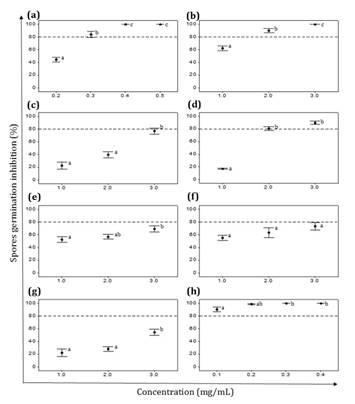
(a) Z. punctata,
(b) C. gilliesii, (c) L. turbinata, (d) L. integrifolia,
(e) A. subfusiformis, (f) E. gilliesii, (g) S. subulatus var. salsus,
(h) Benomyl (Fungicide). Data are expressed as mean ± standard error,
n=6- 8. Different letters indicate significant differences among concentrations
of the same EE. (p˂ 0.05).
(a) Z.
punctata, (b) C. gilliesii, (c) L. turbinata, (d) L.
integrifolia, (e) A. subfusiformis, (f) E. gilliesii, (g) S.
subulatus var. salsus, (h) Benomyl
(Fungicida). Los datos se expresan como la media ± error estándar, n=6-8.
Letras distintas indican diferencias significativas entre concentraciones del
mismo EE. (p˂ 0,05).
Figure
2.
Effect of ethanolic extracts (EEs) on V. dahliae spore germination.
Figura
2. Efecto de los extractos etanólicos
(EEs) sobre la germinación de esporas de V. dahliae.
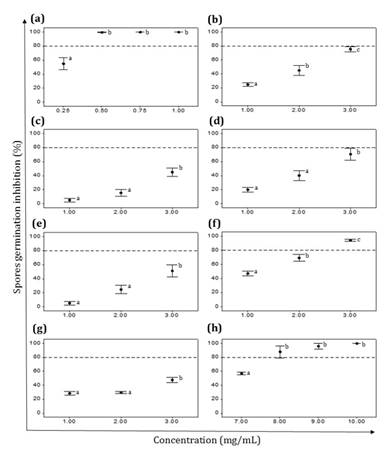
(a) Z. punctata,
(b) C. gilliesii, (c) L. turbinata, (d) L. integrifolia,
(e) A. subfusiformis, (f) E. gilliesii, (g) S. subulatus var.
salsus,
(h) Benomyl (Fungicide). Data are expressed as mean ± standard error, n=6-8.
Different letters indicate significant differences among concentrations of the
same EE. (p˂ 0.05).
(a) Z.
punctata, (b) C. gilliesii, (c) L. turbinata, (d) L.
integrifolia, (e) A. subfusiformis, (f) E. gilliesii, (g) S.
subulatus var. salsus, (h) Benomyl
(Fungicida). Los datos se expresan como la media ± error estándar, n=6-8.
Letras distintas indican diferencias significativas entre concentraciones del
mismo EE. (p˂ 0,05).
Figure
3. Effect of ethanolic extracts (EEs) on P.
parasiticum spore germination.
Figura
3. Efecto de los extractos etanólicos (EEs) sobre la
germinación de esporas de P. parasiticum.
Spore germination of V. dahliae was also completely
inhibited at 3 mg/ml of C. gilliesii EE (MIC), while other EEs showed
inhibitions ranging between 54% and 89% (figure 2). P.
parasiticum spore germination was controlled at 0.75 mg/mL of Z.
punctata EE (MIC), a much lower value than the obtained with the antifungal
Benomyl (MIC=10 mg/mL) (figure
3a and h). In addition, significant inhibition of P. parasiticum spore
germination (94%) was obtained at 3 mg/mL of E. gilliesii EE (figure 3f).
Evaluation
of Synergistic Antifungal Effect
The results
demonstrated no synergistic effect against V. dahliae and P.
parasiticum spores for any of the evaluated combinations. Antifungal
interaction was additive or indifferent (Supplementary Table 2).
Effect
of EEs on Mycelial Growth of V. dahliae and P. parasiticum
Since no EOs could
completely inhibit P. parasiticum spore germination, and their activity
on V. dahliae spore germination was weaker than the extracts, assays
considering mycelial growth inhibition were performed with EEs only.
Mycelial growth
inhibition increased with EEs concentration. All seven EEs tested showed growth
inhibition of over 25% for both phytopathogens (figure 4 and figure 5).
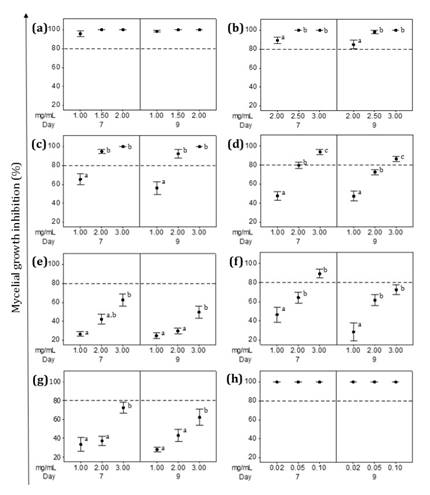
(a) Z. punctata,
(b) C. gilliesii, (c) L. turbinata, (d) L. integrifolia,
(e) A. subfusiformis, (f) E. gilliesii, (g) S. subulatus var.
salsus,
(h) Benomyl (Fungicide). The percentage of inhibition was determined at 7 and 9
days of incubation at 24°C. Data are expressed as mean ± standard error, n=6-
8. Different letters indicate significant differences among the concentrations
tested (p< 0.05).
(a) Z.
punctata, (b) C. gilliesii, (c) L. turbinata, (d) L.
integrifolia, (e) A. subfusiformis, (f) E. gilliesii, (g) S.
subulatus var. salsus, (h) Benomyl
(Fungicida). El porcentaje de inhibición se determinó a los 7 y 9 días de
incubación a 24°C. Los datos se expresan como media ± error estándar, n=6-8.
Letras diferentes indican diferencias significativas entre las concentraciones
ensayadas. (p< 0,05).
Figure
4.
Effect of ethanolic extracts (EEs) on V. dahliae mycelial growth.
Figura
4. Efecto de los extractos etanólicos (EE) sobre el
crecimiento micelial de V. dahliae.
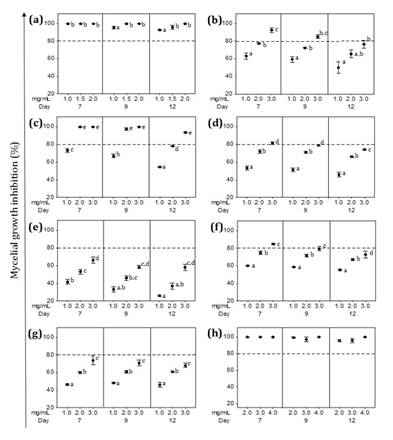
(a) Z. punctata,
(b) C. gilliesii, (c) L. turbinata, (d) L. integrifolia,
(e) A. subfusiformis, (f) E. gilliesii, (g) S. subulatus var.
salsus,
(h) Benomyl (Fungicide). The percentage of inhibition was determined at 7, 9
and 12 days of incubation at 24°C. Data are expressed as mean ± standard error,
n=6-8. Different letters indicate significant differences among concentrations.
(p< 0.05).
(a) Z.
punctata, (b) C. gilliesii, (c) L. turbinata, (d) L.
integrifolia, (e) A. subfusiformis, (f) E. gilliesii, (g) S.
subulatus var. salsus, (h) Benomyl
(Fungicida). El porcentaje de inhibición se determinó a los 7, 9 y 12 días de
incubación a 24°C. Los datos se expresan como media ± error estándar, n=6-8.
Letras diferentes indican diferencias significativas entre concentraciones.
(p< 0,05).
Figure
5.
Effect of ethanolic extracts (EEs) on P. parasiticum mycelial growth.
Figura
5. Efecto de los extractos etanólicos (EE) sobre el
crecimiento micelial de P. parasiticum.
Considering EEs
inhibitory effect on V. dahliae, three treatments (EEs from Z.
punctata, C. gilliesii and L. turbinata) completely inhibited
mycelial growth (figure
4a-c).
Z. punctata EE was the most effective, obtaining the lowest MIC value
(MIC=1.5 mg/mL for Z. punctata EE, MIC=2.5 mg/mL for C. gilliesii EE
and MIC=3 mg/mL for L. turbinata EE; (figure 4a-c). The EEs of L.
integrifolia and E. gilliesii reached inhibition values of 93.7% and
89.5% against V. dahliae at 3 mg/mL (figure 4d and f). The two
remaining EE treatments (A. subfusiformis and S. subulatus)
achieved 60-70% inhibition figure 4e and g. Given that no mycelium re-growth
occurred during additional incubation time (day 9), Z. punctata, L.
turbinata, L. integrifolia, A. subfusiformis and S. subulatus EEs
resulted fungicidal against V. dahliae (figure 4). In the
case of C. gilliesii and E. gilliesii, mycelial recovery was
observed at 9 days. The C. gilliesii EE MIC value changed from 2.5 to 3
mg/mL while inhibition percentage of E. gilliesii EE at 3 mg/mL
decreased significantly (figure
4b and f). Thus, AA of these EEs on V. dahliae was considered
fungistatic.
P. parasiticum mycelial growth was
completely inhibited by Z. punctata and L. turbinata EEs (MIC=1
mg/mL for Z. punctata EE and MIC=2 mg/mL for L. turbinata EE) (figure 5a and c). In addition, the
EEs of C. gilliesii, L. integrifolia, and E. gilliesii showed
strong inhibitory effects on P. parasiticum mycelial growth reaching
92.6, 81.4 and 84.9% at 3 mg/mL, respectively (figure 5b, d and f). The EE
treatments A. subfusiformis and S. subulatus also inhibited
60-70% of mycelial growth (figure
5e and g). The EE treatments produced reversible inhibition of P.
parasiticum mycelium growth. During additional incubation time, the MIC
value of Z. punctata and L. turbinata EEs increased to 2 mg/mL
and 3 mg/mL, respectively. A significant reduction in inhibition percentage of
the other EEs was also observed on day 12 (figure 5). Therefore, these
EEs were fungistatic against P. parasiticum.
Phenolic
and Flavonoid Contents (PC and FC) in EEs
Both PC and FC of
the studied EEs were significantly different (Supplementary Table 3). The EE of Z.
punctata showed the highest PC, followed by C. gilliesii EE, L.
turbinata EE, L. integrifolia EE = A. subfusiformis EE = E.
gilliesii EE, and S. subulatus EE. Regarding FC, Z. punctata EE
presented the highest value (327.6 mg QE/g) and the other EEs ranged between 13
and 73 mg QE/g.
Pearson’s correlation coefficient between PC and spore
germination inhibition was r=0.63 (p < 0.0001) for V. dahliae and
r=0.39 (p < 0.0001) for P. parasiticum. Additionally, a significant
correlation was observed between PC and mycelial growth inhibition, with values
of r=0.79 (p < 0.0001) for V. dahliae and r=0.78 (p < 0.0001) for P.
parasiticum. The FC and inhibition of spore germination showed correlation
coefficients of r=0.73 (p < 0.0001) for V. dahliae and r=0.72 (p <
0.0001) for P. parasiticum, while for FC and mycelial growth inhibition,
r=0.60 (p < 0.0001) was observed for V. dahliae and r=0.67 (p <
0.0001) for P. parasiticum.
Discussion
V. dahliae and P.
parasiticum are important phytopathogens in La Rioja province, involved in
Verticillium wilt of olive and grapevine trunk diseases, respectively (10,
20). Given the lack of control treatments, searching for antifungal
agents is strategic (17, 19). We tested EOs and
EEs from seven Argentinian northwest plants as natural alternatives against V.
dahliae and P. parasiticum.
Three mg/mL of our EOs had remarkable activity against V.
dahliae spore germination (100% inhibitory activity for Z. punctata and
C. gilliesii EOs, and 96% inhibitory activity for L. turbinata and
L. integrifolia EOs). Similarly, other EOs (orégano, thyme, laurel, and
lavender) block V. dahliae conidia germination at concentrations ranging
from 0.2 to 3 mg/mL (11, 14).
On the other hand,
only the C. gilliesii EO was able to significantly inhibit P.
parasiticum conidia germination, suggesting V. dahliae spores are
more susceptible to the tested EOs than P. parasiticum. In addition,
although previous reports demonstrated the activity of C. gilliesii and L.
turbinata EOs against other phytopathogenic fungi (15,
22, 27), this is the first report on AA of Z. punctata and L.
integrifolia EOs against this type of pathogens.
Based on EEs
activity on conidia germination, only Z. punctata EE was able to control
both V. dahliae (MIC= 0.4 mg/mL) and P. parasiticum (MIC=0.75
mg/mL). These results coincide with previous research showing the Z.
punctata EE effectiveness against soybean pathogenic and brown rot fungi
spore development at concentrations between 0.25-0.5 mg/mL (4,
23). Our results also showed that C. gilliesii EE controlled
germination of V. dahliae spores, apparently never studied before
against phytopathogenic fungi.
Although no
synergistic antifungal effect was found for the mixtures of EO and EE tested,
antagonistic absence and additive effects of the combinations of Z. punctata
EO/Z. punctata EE and C. gilliesii EO/Z. punctata EE,
constitute encouraging outcomes. This suggests that the botanical effective
antifungal concentration (MIC) could be halved when combined.
On the other hand,
the most effective inhibitors of mycelial growth of both phytopathogens were Z.
punctata, C. gilliesii, and L. turbinata EEs. All three
extracts behaved as fungistatic on P. parasiticum, while Z. punctata and
L. turbinata EEs killed V. dahliae mycelium (fungicidal effect),
evidencing that V. dahliae vegetative growth was more susceptible to our
EEs than P. parasiticum.
In vitro studies with Z.
punctata EE at 1.6 mg/mL could not completely inhibit hyphal growth of Fusarium
species associated with Ear Rot in cereals (13). In contrast, our
findings showed that the AA of Z. punctata EE, ranging from 1-1.5 mg/mL
could completely inhibit mycelial growth of V. dahliae and P.
parasiticum. Results also showed that Z. punctata EE MIC values were
2-3 times lower on the spores than on the mycelium of both phytopathogens,
consistent with previous findings (13). Considering C.
gilliesii EE, MIC was similar for conidia germination and mycelial growth
of V. dahliae. Surprisingly, a complete reduction of V. dahliae and
P. parasiticum mycelial growth was observed with L. turbinata EE.
However, it did not provide complete control over conidia germination,
suggesting a differential effect of extract components on each fungal
structure.
Considering all the
evaluated EEs, Z. punctata EE was the most effective at suppressing
spore germination and mycelial growth. Previous research has corroborated the
AA of Z. punctata EE against other phytopathogenic fungi, attributing
this property to polyphenolic compounds, especially chalcone type (4,
13, 23). Considering the difference in the AA observed among the
different EEs evaluated, phenols and flavonoid content were quantified, showing
that Z. punctata EE had the highest content of phenols and flavonoids
likely responsible for its potent AA.
Finally, we found
that total phenols had the best correlation with mycelial growth inhibition,
while flavonoid levels best correlated with inhibition of spore germination.
Thus, the AA of studied EEs on conidial germination could be mainly attributed
to flavonoid content, while phenols would be responsible for inhibitory effects
on mycelial growth.
Conclusions
This work searched for antifungals of plant origin against
pathogenic fungi involved in grapevine trunk diseases and Verticillium wilt of
olive. We explored the in vitro antifungal properties of five EOs and
seven EEs obtained from Argentinian northwest plants. All tested EOs and EEs
showed varying AA degrees against both phytopathogenic fungi. This activity
depended on plant species, extract type (EO or EE), pathogen identity, and
targeted fungal structures. According to our findings, the products obtained
from Z. punctata, C gilliesii and L. turbinata were the
most effective against V. dahliae and P. parasiticum, suggesting
their potential as biofungicides for integrated disease control. This is
particularly encouraging considering absent effective treatments against these
two pathogens. Further research should determine antifungal effectiveness of
these botanical products in plants and identify their specific antifungal compounds.
Acknowledgments
This research was
funded by Secretaría de Políticas Universitarias and Universidad Nacional de
Chilecito, Argentina (grants PAFCyT I+D 35/18 and FICyT-2022).
We thank Translator
A. López López for improving English in the manuscript and M.J. Loyola from
UNDEC Herbarium for assisting with plant taxonomic identification.
M.S is a fellow and N.B is a researcher, both at CONICET,
Argentina.
1. Alonso, J.;
Desmarchelier, C. 2015. Plantas medicinales autóctonas de la Argentina. Corpus
Libros Médicos y Científicos. Buenos Aires. 748 p.
2. Barbieri, N.;
Gilabert, M.; Benavente, A. 2023. Phytochemical analysis and biological
potential of Argentinian plant essential oils and extracts. Braz J Med Plants
25: 17-28.
3. Boiteux, J.;
Fernández, M. de los Á.; Espino, M.; Silva, M. F.; Pizzuolo, P. H.; Lucero, G.
S. 2023. In vitro and in vivo efficacy of Larrea divaricata extract
for the management of Phytophthora palmivora in olive trees. Revista de
la Facultad de Ciencias Agrarias. Universidad Nacional de Cuyo. Mendoza.
Argentina. 55(2): 97-107. DOI: https://doi.org/10.48162/rev.39.112.
4. Di Liberto, M.
G.; Stegmayer, M. I.; Fernández, L. N.; Quiroga, A. D.; Svetaz, L. A.; Derita,
M. G. 2023. Control of brown rot produced by Monilinia fructicola in
peaches using a full-spectrum extract of Zuccagnia punctata Cav.
Horticulturae. 9(10): 1141. DOI: 10.3390/horticulturae9101141
5. Di Rienzo, J.
A.; Casanoves, F.; Balzarini, M. G.; Gonzalez, L.; Tablada, M.; Robledo, C. W.
InfoStat versión 2020. Centro de Transferencia InfoStat, FCA. UNC. Córdoba.
Argentina. http://www.infostat.com.ar
6. Đorđević, M.;
Dolovac, N.; Ivanović, M.; Damnjanović, J.; Zečević, B. 2013. Effectiveness of
essential oils in control of Verticillium dahliae in vitro. Zaštita
bilja. 64(3): 162-168.
7. EPSA. 2023.
Estrategia provincial para el sector agroalimentario. Provincia de La Rioja.
Ministerio de Producción y Ambiente.
www.argentina.gob.ar/sites/default/files/2023/05/la_rioja_2023.pdf
8. Erdogan, O.;
Çelik, A.; Zeybek, A. 2016. In vitro antifungal activity of mint, thyme,
lavender extracts and essential oils on Verticillium dahliae Kleb.
Fresenius Environ Bull. 25: 4856-4862.
9. Escoriaza, G.;
Sansberro, P.; Lampasona, S. G.; Gatica, M.; Piccoli, P. 2013. In vitro cultures
of Vitis vinifera L. cv Chardonnay synthesize the phytoalexin nerolidol
upon infection by Phaeoacremonium parasiticum. Phytopathol Mediterr.
52(2): 289-297.
10. Escoriaza, G.;
García Lampasona, S.; Gomez Talquenca, S.; Piccoli, P. 2019. In vitro plants
of Vitis vinifera respond to infection with the fungus Phaeoacremonium
parasiticum by synthesizing the phytoalexin nerolidol. PCTOC. 138(3):
459-466. DOI: 10.1007/s11240-019-01641-3
11. Giamperi, L.;
Fraternale, D.; Ricci, D. 2002. The in vitro action of essential oils on
different organisms. J Essent Oil Res. 14(4): 312-318. DOI:
10.1080/10412905.2002.9699865
12. INV. 2023.
Informe anual de superficie. 2022. Instituto Nacional de Vitivinicultura.
www.argentina.gob.ar/inv/vinos/estadisticas/superficie/anuarios
13. Jimenez, C. M.;
Sampietro, D. A.; Sgariglia, M. A.; Soberón, J. R.; Vattuone, M. A. 2014.
Isolation, identification and usefulness of antifungal compounds from Zuccagnia
punctata for control of toxigenic ear rot pathogens. Nat Prod Commun. 9(10):
1934578X1400901.
14. Kadoglidou, K.;
Lagopodi, A.; Karamanoli, K.; Vokou, D.; Bardas, G.; Menexes, G.;
Constantinidou, H. I.; Kadoglidou, K.; Karamanoli, K.; Lagopodi, A.; Bardas,
G.; Vokou, D.; Menexes, G. 2011. Inhibitory and stimulatory effects of
essential oils and individual monoterpenoids on growth and sporulation of four
soil-borne fungal isolates of Aspergillus terreus, Fusarium oxysporum,
Penicillium expansum, and Verticillium dahliae. Eur. J. Plant
Pathol. 130. DOI: 10.1007/s10658-011-9754-x
15. Leal, L. E.;
Alarcón, A. A.; Ortega-Baes, P.; Cayo, F.; Alarcón, R. 2018. Effects of
essential oils from two Lippia species on growth of phytopathogenic fungi.
BLACPMA. 17(1): 30-35.
16. Liu, J.;
Hagberg, I.; Novitsky, L.; Hadj-Moussa, H.; Avis, T. J. 2014. Interaction of antimicrobial
cyclic lipopeptides from Bacillus subtilis influences their effect on
spore germination and membrane permeability in fungal plant pathogens. Fungal
Biol. 118(11): 855-861. DOI: 10.1016/j.funbio.2014.07.004
17. Mondello, V.;
Songy, A.; Battiston, E.; Pinto, C.; Coppin, C.; Trotel-Aziz, P.; Clément, C.;
Mugnai, L.; Fontaine, F. 2018. Grapevine trunk diseases: A review of fifteen
years of trials for their control with chemicals and biocontrol agents. Plant
Dis. 102(7): 1189-1217.
18. Montes-Osuna,
N.; Mercado-Blanco, J. 2020. Verticillium wilt of olive and its control: What
did we learn during the last decade? Plants. 9(6): 735. DOI:
10.3390/plants9060735
19.
Mulero-Aparicio, A.; Varo, A.; Agustí-Brisach, C.; López-Escudero, F. J.;
Trapero, A. 2020. Biological control of Verticillium wilt of olive in
the field. Crop Protection 128: 104993. DOI: 10.1016/j.cropro.2019.104993
20. Rattalino, D. 2023. Identificación molecular y variabilidad
genética de Verticillium dahliae su relación con la incidencia y
prevalencia de la verticilosis del olivo en la zona olivícola de la provincia
de La Rioja. Tesis de Doctorado en ciencias agropecuarias. Universidad Nacional
de Córdoba. Argentina. 146 p.
21. Rattalino, D.;
Otero, M. L.; Moriconi, D. N.; Rivera, P. C. 2021. Mejora de la detección del
patotipo no defoliante de Verticillium dahliae en olivo mediante PCR
anidada. AgriScientia. 38(1): 79-91. DOI: 10.31047/1668.298x.v38.n1.28985
22. Stegmayer, M.
I.; Fernández, N. L.; Álvarez, N. H.; Olivella, L.; Gutiérrez, H. F.; Favaro,
M. A.; Derita, M. G. 2021. Aceites esenciales provenientes de plantas nativas
para el control de hongos fitopatógenos que afectan a frutales. FAVE. 20(1):
317-329. DOI: 10.14409/ fa.v20i1.10273
23. Svetaz, L.;
Tapia, A.; López, S. N.; Furlán, R. L. E.; Petenatti, E.; Pioli, R.;
Schmeda-Hirschmann, G.; Zacchino, S. A. 2004. Antifungal chalcones and new
caffeic acid esters from Zuccagnia punctata acting against Soybean
Infecting Fungi. J. Agric. Food Chem. 52(11): 3297-3300. DOI: 10.1021/jf035213x
24. Varo, A.;
Mulero-Aparicio, A.; Adem, M.; Roca, L. F.; Raya-Ortega, M. C.; López-Escudero,
F. J.; Trapero, A. 2017. Screening water extracts and essential oils from
Mediterranean plants against Verticillium dahliae in olive. Crop
Protection. 92: 168-175. DOI: 10.1016/j. cropro.2016.10.018
25. Yadav, M. K.;
Chae, S. W.; Im, G. J.; Chung, J. W.; Song, J. J. 2015. Eugenol: A
phyto-compound effective against methicillin-resistant and
methicillin-sensitive Staphylococcus aureus clinical strain biofilms.
Plos One. 10(3): e0119564. DOI: 10.1371/journal.pone.0119564
26. Zaker, M. 2016.
Natural plant products as eco-friendly fungicides for plant diseases control- A
Review. The Agriculturists. 14(1): 134-141. DOI: 10.3329/agric.v14i1.29111
27. Zygadlo, J. A.; Grosso, N. R. 1995. Comparative study of the
antifungal activity of essential oils from aromatic plants growing wild in the
central region of Argentina. Flavour Fragr J. 10(2): 113-118. DOI:
10.1002/ffj.2730100210
https://drive.google.com/drive/folders/1O7n5y_WfEk5QOQJzHepnvtvOcieXZhuG?usp=sharing