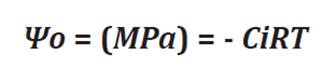Revista de la Facultad de Ciencias
Agrarias. Universidad Nacional de Cuyo. Tomo 57(1). ISSN (en línea) 1853-8665.
Año 2025.
Original article
Challenges
in germination of Neltuma caldenia in semi-arid regions: optimization of
germination protocols, influence of saline stress and seed quality
Desafíos en la germinación
del Neltuma caldenia en regiones semiáridas: optimización de protocolos
de germinación, influencia del estrés salino y evaluación de la calidad de las
semillas
1 Universidad Nacional de Río Negro. Sede Atlántica. Av. Don Bosco
y Leloir. Viedma. (C. P. 8500). Argentina.
2 CIT-Río Negro - CONICET. RP N° 1 y Rotonda Cooperación, Viedma
(C.P. 8500), Argentina.
3 Universidad Nacional de La Plata. Facultad de Ciencias Agrarias
y Forestales. Laboratorio de Investigaciones en Madera (LIMAD). Calle 60 y 119.
La Plata. C. P. 1900. Argentina.
4 Sistema
Nacional de Investigación. Asociación de Interés Público (SIN AIP). Ciudad del
Saber. Clayton. Panamá.
* pboeri@unrn.edu.ar
Abstract
Global climate
change presents challenges to arid and semi-arid ecosystems, impacting native
species such as Neltuma caldenia, endemic to Argentina. This underscores
the importance of understanding germination processes for both conservation
programs and the restoration of degraded areas. We aimed to evaluate the
germination rate of N. caldenia seeds from the south Espinal, using
various scarification methods (chemical, mechanical and physical), and
temperatures (25-30°C). Additionally, we investigate the effects of accelerated
aging (0-96 h at 45°C and 100 relative humidity) and different saline solution
concentrations during germination (0-0.6 M NaCl). Our results show that all
scarification treatments effectively break seed dormancy while temperature
significantly affects germination rates. Prolonged storage (0 to 96h) decreased
seed viability. Moderate NaCl levels (0-0.2 M) did not affect germination, but
higher concentrations inhibited it completely, with a threshold of -1.81 MPa
osmotic potential. Understanding the impact of environmental stressors on seed
germination can inform the development of effective conservation strategies
among these climate change pressures.
Keywords: Fabaceae, Prosopis,
caldén,
dormancy, scarification, optimal temperature
Resumen
El cambio climático
global presenta desafíos para los ecosistemas áridos y semiáridos, impactando a
especies nativas como Neltuma caldenia, endémica de Argentina. Esto
resalta la importancia de comprender los procesos de germinación tanto para
programas de conservación como para la restauración de áreas degradadas.
Nuestro objetivo fue evaluar la tasa de germinación de semillas de N.
caldenia del sur del Espinal, utilizando varios métodos de escarificación
(químico, mecánico y físico) y temperaturas (25-30°C). Además, investigamos los
efectos del envejecimiento acelerado (0-96 h a 45°C y 100% de humedad relativa)
y de diferentes concentraciones de solución salina durante la germinación
(0-0,6 M NaCl). Nuestros resultados muestran que todos los tratamientos de
escarificación rompen eficazmente la latencia de las semillas, mientras que la
temperatura afecta significativamente las tasas de germinación. El
almacenamiento prolongado (0 a 96h) disminuyó la viabilidad de las semillas.
Niveles moderados de NaCl (0-0,2 M) no afectaron la germinación, pero
concentraciones más altas la inhibieron completamente, con un umbral de -1,81
MPa de potencial osmótico. Comprender el impacto de los factores de estrés
ambiental en la germinación de semillas puede informar el desarrollo de
estrategias de conservación efectivas ante estas presiones del cambio
climático.
Palabras clave: Fabaceae, Prosopis,
caldén,
latencia, escarificación, temperatura óptima
Originales: Recepción: 13/05/2024
- Aceptación: 13/11/2024
Introduction
Global climate
change (GCC), biodiversity loss, and environmental degradation present profound
challenges for ecosystems, especially in arid and semi-arid regions (24). These changes
exceed the physiological thresholds of many native plant species, including
those in the Neltuma genus (formerly Prosopis), posing severe
risks to their survival. Species persistence in such regions relies on traits
like seed dormancy, germination temperature, and water stress tolerance.
Understanding germination and early survival of plants under these conditions
is critical for future conservation. The ‘Decade on Ecosystem Restoration’ of
the United Nations (2021-2030) emphasizes the urgent need to address these
environmental challenges. This is especially relevant for species like Neltuma
caldenia (Burkart) C.E. Hughes & G.P. Lewisthe, known as ‘caldén’,
which is endemic to the Espinal region of Argentina and impacted by GCC and
deforestation.
One main constraint for seed germination in arid and semi-arid
regions is seed dormancy. In these ecosystems, over 80% of native shrub species
have seeds that will not germinate unless dormancy is broken. Dormancy is an
adaptive strategy that prevents a viable seed from germinating under favorable
conditions until specific triggers are met. This trait may arise from the
structures surrounding the embryo, which inhibit germination even when
conditions are suitable for non-dormant seeds (36).
The presence of seeds with various dormancy levels enables temporal
distribution of offspring, offering protection in unpredictable and variable
environments, a particularly relevant aspect in arid and semi-arid regions (36).
Studies have reported that N. caldenia seeds exhibit physical dormancy
imposed by the seed coat (31, 36).
Germination speed is crucial for species establishment and may vary based on
the scarification treatment used. Utello et al.
(2023) identified several scarification strategies to break dormancy
in central Espinal N. caldenia seeds, with mechanical and chemical
scarification showing the most success. However, physical scarification with
boiling water yielded low germination rates, around 20%. Emergence rates for N.
caldenia vary among studies, depending on scarification method and seed
origin (21, 31, 36).
Nonetheless, information specific to the La Pampa province, in the southern
Espinal region of Argentina, where N. caldenia forests predominate,
remains scarce. Dormancy type and degree can vary significantly across species
within a genus and among populations within a species, influenced by maternal
effects during seed formation (11).
Thus, studying this trait in local seed provenances is important for
understanding germination patterns.
Once dormancy is
overcome, temperature becomes a critical factor in the seed germination
process, highlighting ideal establishment times. Optimal germination
temperatures for other Neltuma species from arid regions range from 20
to 40°C, varying by species and origin (4). However, this has
not yet been evaluated for N. caldenia. Seed banks are vital for
preserving genetic material for future conservation and restoration, yet
storage conditions also influence germination. Assessing seed vigor, critical
for this purpose, requires evaluating seed viability under simulated long-term
storage conditions, such as accelerated aging (AA) tests (1). AA testing,
though common in agronomic seeds, is less common for native species, despite
its relevance for predicting viability thresholds in germplasm conservation (14). Fontana
et al. (2016) were the first to apply the AA technique to the Neltuma genus,
suggesting that seed vigor may be influenced by geographic origin and
environmental conditions.
Salinity tolerance
during germination is crucial for plant establishment in N. caldenia-inhabited
environments. Soil salinity can hinder germination, particularly during dry
years when saline conditions may increase due to GCC (22). Many native and
endemic species in arid and semi-arid regions employ strategies to tolerate or
avoid environmental filtering at different development stages. Salt stress
tolerance has been confirmed in the genus, supporting its potential for
restoring soils degraded by salinity (22, 33, 34). While N.
caldenia has not been studied for salt tolerance, its presence in
central-west La Pampa, where saline soils and salt flats are common, suggests
it may possess similar tolerance mechanisms (8,
13).
In this study, we
aim to evaluate the germination rate of N. caldenia seeds from southern
Espinal in La Pampa province using various scarification methods and identify
optimal germination temperatures. Additionally, we also examine the effects of
accelerated aging and salinity on germination quality under laboratory conditions.
Materials
and methods
Study
area
The sampling zone
is situated in the Caldenal district, within the Phytogeographic Province of
Espinal, characterized by a temperate and dry climate with predominantly summer
rainfall (6, 23). The collection of N. caldenia pods
took place in La Pampa province (37°24’10.91’’ S; 63°40’22.93’’ W), where the
average annual precipitation is 500 mm and the average temperature is 15°C (17). Permission to
access and use native flora was obtained from provincial authorities in
compliance with the Convention on Biological Diversity and the Nagoya Protocol.
Seed
collection, conditioning and storage
Mature pods from 10
to 20 plants were manually harvested between February and April of 2018 and
2019, following FAO Forest Seed Handling Guide Guidelines (35). Seeds were
extracted in the laboratory, using tweezer, selecting only those exhibiting no
visible signs of deterioration. Selected seeds were then stored in paper
envelopes at room temperature (20±2°C) for 2 to 14 months until the final
experiment.
Germination
of N. caldenia
Various
scarification methods were tested and the optimal germination temperature was
determined. Before each trial, seeds were disinfected following the protocol
for N. alpataco (4). Briefly, seeds
were soaked in 70% (v/v) ethanol for 15 minutes, followed by a 20 minutes
immersion in 30% (v/v) NaClO solutions (48 g of active chlorine/L) and followed
by three rinses with distilled water.
Dormancy
breaking
Physical (PS),
mechanical (MS), and chemical scarification (CS) methods were assessed
following guidelines in the FAO Forest Seed Handling Guide (35) and the ISTA
International Rules for Seeds Testing (18). PS involved
soaking the seeds in water at 100°C until reaching room temperature. For MS, a
small incision was made in the seed coat with tweezers avoiding damage to the
embryo. CS required immersing the seeds in sulfuric acid (98%) for 10, 20, 30,
and 40 min (CS10, CS20, CS30, and CS40, respectively), followed by rinsing with
distilled water, as proposed for other Norpatagonian Neltuma spp (4). Based on the
obtained results, mechanical scarification was selected for the remaining
experiments to be conducted, as it is a more environmentally sustainable
methodology and avoids the potential interference of acid with other
treatments.
Optimal
germination temperature
Seeds were
evaluated at temperatures ranging from 25°C to 45°C with a germination chamber,
based on previous findings identifying optimal temperatures for Neltuma spp (4). The optimal
temperature was considered as the one yielding the highest germination
percentage in the shortest time.
Determination
of seed vigor through accelerated aging (AA) test
The methodology
from Fontana
et al. (2016) for Neltuma alba was employed to assess seed vigor.
Seeds were exposed to 45°C and 100% relative humidity in a germination chamber
for durations of 0 (control), 24, 48, 72, and 96 h. Glass jars containing 100
ml of distilled water with a mesh above the water level were used to support
the seeds during this procedure. Then, seeds were mechanically scarified and
disinfected for germination.
Effect
of salinity on germination
Seeds, previously scarified and disinfected, were arranged for
germination in Petri dishes containing moistened cotton and filter paper with
NaCl solutions at the following concentrations: 0 (control - T0), 0.05 (T1),
0.1 (T2), 0.2 (T3), 0.4 (T4), and 0.6 M (T5). These were converted to osmotic
potential (Ψo) using the van’t Hoff relationship (27):
where
Ψo = the osmotic
potential in MPa
C = the concentration
in mol/L
i = the dissociation
constant of NaCl (i.e. 1.8)
R = the gas constant
(0.0083 L/atm/mol/K)
T = the temperature
in Kelvins.
The obtained
osmotic potentials were: 0 (T0), -0.22 (T1), -0.45 (T2), -0.90 (T3), -1.81
(T4), -2.71 (T5) MPa. As temperature can influence osmotic potential it is
recommended to perform these tests at the optimal temperature for each species (9); in the case of N.
caldenia it was 30°C. After 14 days of treatment, the inhibitory effect of
salts on the development of surviving seedlings was evaluated by measuring the
length of the radicle and hypocotyl (cm). Subsequently, seedling vigor index
(SVI), percentage phytotoxicity for roots and hypocotyls (RPT and HPT,
respectively) and the tolerance index for roots and hypocotyls (RTI and HTI,
respectively) parameters were calculated (26).
Germination
conditions
Seeds from all experiments were placed on moistened cotton and
filter paper in Petri dishes and then incubated in germination chambers at
30°C, except for the optimal germination temperature assay and the accelerated
aging test. All germination experiments were conducted in darkness, as has been
done by other authors with N. caldenia and other species of the genus (4).
The trials were randomized and included 10 replications of 10 seeds each
(N=100), with controls. Germination was defined as the emergence of a radicle
at least 2 mm long (37),
and daily germination counts were recorded for up to a week or until no further
germination occurred.
Seed
viability
The viability of
seeds that appeared healthy but failed to germinate for all experiments was
verified using tetrazolium testing (5). A solution of
2,3,5-triphenyl tetrazolium chloride 1% (p/v) in a phosphate buffer at pH 7.4
was prepared. The seeds were immersed in the solution for 24 hours at room
temperature and then cut in half to observe viability. Stains were analyzed
based on the color patterns described by Craviotto et al. (2011).
Germination
evaluation
The germination
parameters evaluated after one week of daily observations in the various
germination assays (scarifications, optimal temperature, accelerated aging, and
saline stress) were:
Germination Capacity (GC) (3),
which is the total germination percentage at the end of the experiment and it
is calculated as shown in table 1,
in equation
E1, where G is the number of germinated seeds at the end of
the experiment and n is the total number of seeds in the test.
Table 1. Equations
used to calculate the germination parameters evaluated in this study.
Tabla
1. Ecuaciones utilizadas para calcular
los parámetros germinativos evaluados en este estudio.
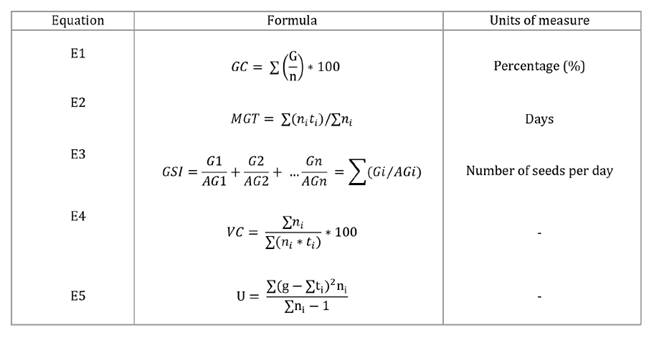
The table presents the equations
for Germination Capacity (GC), Mean Germination Time (MTG), germination speed
index (GSI), Velocity Coefficient (VC), and Uniformity Factor (U), along with
their respective units of measurement.
La tabla presenta las ecuaciones para la Capacidad Germinativa
(GC), el Tiempo Medio de Germinación (MTG), el índice de velocidad germinativa
(GSI), el Coeficiente de Velocidad (VC) y el Factor de Uniformidad (U), junto
con sus respectivas unidades de medida.
Mean Germination
Time (MTG) with the formula proposed by Martínez-Gonzáles et al. (2022), (E2):
where
T = germination time
ti = number of days of
assay
ni = number of seeds
germinated on day i
Germination Speed
Index (GSI) or Maguire’s Index (19), which is
expressed as the number of germinated seeds per day (E3):
where
G1 = the number of
seeds that germinated on day 1 (not cumulative)
G2 = the number of
seeds that germinated on day 2 (not cumulative)
Gn = the number of
seeds that germinated on day n (not cumulative, end of the experiment)
AG1 = the cumulative
number of germinated seeds on day 1
AG2 = the cumulative
number of germinated seeds on day 2
AGn = the cumulative
number of germinated seeds on day n (end of the experiment).
Germinative energy (GE)(4)
is the percentage of daily cumulative germination at the highest germination
rate.
Specifically, for
defining an efficient scarification protocol in terms of velocity and
uniformity, the following parameters were also assessed:
Velocity
Coefficient (VC) (31) is an index based
on the number of germinated seeds inversely related to the time and the number
of seeds germinated per day. It is a measure of the distribution of germination
over time in relation to the number of germinated seeds and is expressed in the
equation E4:
where
VC = velocity
coefficient
n = number of seeds
germinated on day i
t = number of days
since sowing.
Uniformity Factor
(U) (31) is proposed as a measure of the
variance in germination time or the germination over time (E5):
where
U = uniformity factor
g = mean germination
time
ti = number of days
after sowing
ni = number of seeds
germinated on day i.
Additionally, the
time to reach the maximum accumulated germination (Tmax) was considered, which
indicates the day from which no further germinations occurred.
Statistical
treatment of data
The study employed
a completely randomized experimental design. Data analysis was conducted using
the open-source statistical analysis software InfoStat (30). Treatment
differences were assessed using ANOVA with Tukey’s test, or the non-parametric
Kruskal-Wallis test when assumptions were not met (i.e. optimal
germination temperature, accelerated aging test and effect of salinity on
germination). Results were presented as mean ± standard error (SE) of the
replications. The Pearson Correlation Coefficient assessed the relationship
between germination parameters in the dormancy interruption assay (see section
Dormancy interruption) following reference ranges by Schober
et al. (2018): very low correlation for r2 less than 0.00, low for
0.10-0.39, moderate for 0.40-0.69, strong for 0.70-0.89, and very strong for
0.90-1.
Results
and discussion
Seed
germination protocol optimization
Dormancy
breaking
In the control
group of N. caldenia seeds, 56% did not germinate but were viable based
on the tetrazolium test, indicating dormancy. Conversely, 42% germinated, and
2% did not germinate and were non-viable. Scarification enabled germination of
all viable seeds, regardless of treatment, confirming physical dormancy imposed
by the seed coat, as reported by Utello et al. (2023).
The evaluated
germination parameters revealed a strong to very strong positive correlation
between GC and GSI parameters (r2=0.99
and 0.80, respectively). The correlation indicates that treatments improved
both the germination percentage and speed (table 2). This improved
germination uniformity, with the CS10 and MS treatments exhibiting less
dispersion, is consistent with previous findings (31). Furthermore, due
to rapid germination, the obtained Germination Energy (GE) values were similar
to those of CG and thus were excluded from further analysis.
Table 2. Germinative
parameters evaluated in scarification of N. caldenia seeds.
Tabla
2. Parámetros germinativos evaluados
en las escarificaciones de las semillas de N. caldenia.
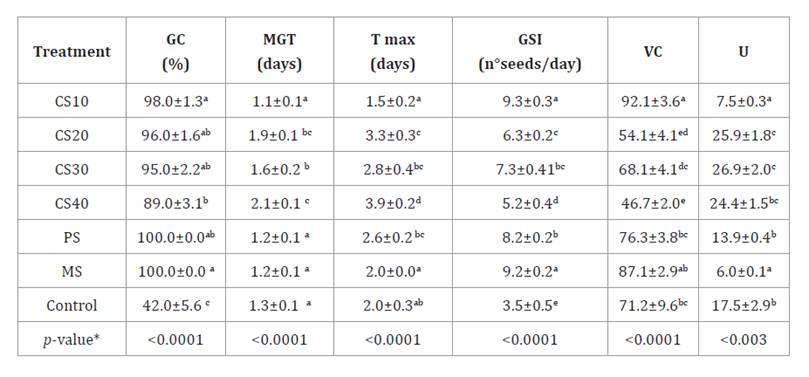
Germinative Capacity (GC), Mean
Germination Time (MGT), Time of maximum germination (Tmax), Germinative Speed
Index (GSI), Velocity Coefficient (VC) and Uniformity Factor (U) for chemical
scarification treatments with sulfuric acid for 10 (CS10), 20 (CS20), 30 (CS30)
and 40 (CS40) min, physical scarification (PS), mechanical scarification (MS)
and the control. Results were expressed as the mean ± standard error (SE) of
the repetitions. *different letters are not significantly different (p-value
> 0.05).
Capacidad Germinativa (GC),
Tiempo Medio de Germinación (MGT), Tiempo máximo de germinación (Tmax), Índice de
Velocidad Germinativa (GSI), el Coeficiente de Velocidad (VC) y el Factor de
Uniformidad (U) para los tratamientos de escarificación química con ácido
sulfúrico durante 10 (CS10), 20 (CS20), 30 (CS30) y 40 (CS40) min,
escarificación física (PS), escarificación mecánica (MS) y el control. Los
resultados se expresaron como la media ± error estándar (EE) de las
repeticiones. *letras distintas son significativamente diferentes (p-valor
> 0,05).
Scarification with sulfuric acid for up to 30 minutes (CS30) resulted
in a GC greater than 95%, but longer exposure negatively affected germination.
However, the GC values obtained were considerably higher than those reported in
seeds from other provenance, which ranged between 75% and 30% (31,
36). Although acid treatments (CS20, CS30, CS40) did not
significantly affect the GC, they impacted GSI, MGT and Tmax. A negative or low
correlation was observed among these parameters (r2= -0.83 to 0.53).
This suggests that while a high GC was maintained, the temporal efficiency of
the process was reduced.
Prolonged acid
immersion decreased GSI and increased MGT and Tmax. This led to a more
dispersed germination pattern, indicated by a decrease in VC (<92) and an
increase in U (>7.5). Utello et al. (2023) used sulfuric acid
scarification for 15 minutes on N. caldenia seeds from another province
in the central Espinal region. Their results exhibited similar germination
speed and duration values to our study at the longest exposure times (CS30 and
CS40). The same trend was observed in the control seeds analyzed by Utello et
al. (2023), with a Tmax five times higher than that of the control seeds
in our study, despite a similar GC. This difference may stem from seed
morphology, storage conditions, chemical composition, or seed coat thickness,
influenced by regional environmental conditions (4,
21). Longer acid exposure than 10 minutes led to oxidative stress
and reduced radicle elongation, consistent with Utello et al. (2023). The decline in
germination rates may result from acid infiltration into seed tissues, raising
temperatures and potentially harming the embryo (36).
Both mechanical and
physical scarification treatments effectively broke dormancy in N. caldenia seeds,
with no significant difference in MGT. MS was more efficient for germination
speed, resulting in more uniform germination with increased VC, comparable to
CS10. Utello
et al. (2023) reported a 90% improvement in N. caldenia germination
using a mechanical method. However, their physical scarification yielded
germination rates approximately four times lower than those in our study, with
a GSI 2.5 times higher. Zeberio and Pérez (2020) observed no
germination when applying a combination of mechanical and physical
scarification to N. caldenia seeds from the northern Monte region. In
contrast, our study yielded significantly higher germination rates by applying
similar scarification methods separately.
Timing, speed,
homogeneity, and synchrony of germination are essential for understanding seed
vigor and stress performance. Homogeneous germination supports synchronized
seedling establishment, which benefits agriculture and restoration. While
varied timing aids survival in wild populations, synchronized germination in
managed environments promotes consistent and resilient growth (15). In this regard,
the shorter duration chemical scarification method (CS10) and mechanical
scarification were statistically more efficient than the other treatments.
Furthermore, mechanical treatments for seed germination represent an effective
and sustainable approach.
Optimal
germination temperature
Table
3, summarizes the evaluations of germination parameters at
different temperatures. Germination rates remained near 100% up to 40°C but
decreased at 45°C, where no seeds germinated.
Table 3.
Optimal germination temperature.
Tabla 3.
Temperatura óptima de germinación.
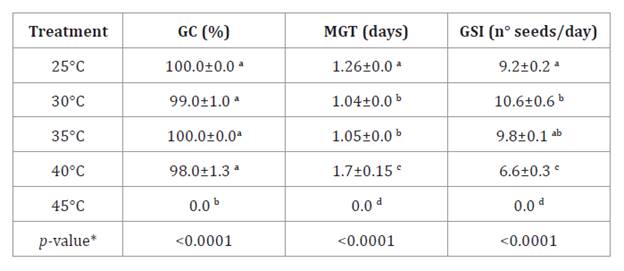
Values of Germinative Capacity
(GC), Mean Germination Time (MGT) and Germinative Speed Index (GSI) obtained
for N. caldenia germinations from 25 to 45°C. The results were expressed
as the mean ± standard error (SE). * different letters are not significantly
different (p-value > 0.05).
Valores de Capacidad Germinativa
(GC), Tiempo Medio de Germinación (MGT) e Índice de Velocidad Germinativa (GSI)
las germinaciones de N. caldenia de 25 a 45°C. Los resultados se
expresaron como la media ± error estándar (EE). * letras distintas son
significativamente diferentes (p-valor > 0,05).
The tetrazolium staining indicated that these seeds were
non-viable. GSI values showed significantly faster germination at 30 and 35°C.
Thus, the optimal temperature range for N. caldenia in the south-central
region of Espinal was between 30 and 35°C. Similar ranges have been reported
for related species. Boeri et al. (2019)
found an optimum germination temperature of 30°C for N. alpataco, while Villagra
et al. (2017) suggested 35°C for both N. alpataco and N. argentina.
In this sense, the optimal germination temperature varies according to species
and geographic distribution.
In the study
region, the highest precipitation occurs during the warm semester (October to
March), accounting for 69% of the annual total. During these months, average
maximum temperatures range between 28 and 36°C (29). In this sense,
the optimal germination temperature of N. caldenia coincides with the
period of highest precipitation in the region. However, climate change has led
to a significant increase in temperature amplitude, which may alter the optimal
conditions for germination and seedling survival.
Seed
vigor
Accelerated aging
(AA) of Neltuma caldenia seeds significantly reduced germination rates
over time (table
4).
Table 4. Accelerated
Aging (AA) test.
Tabla
4.
Prueba del envejecimiento acelerado (AA).
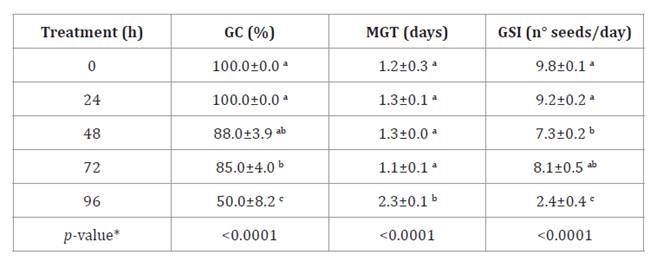
Germinative Capacity (GC), Mean
Germination Time (MGT) and Germinative Speed Index (GSI) obtained for the AA
test of N. caldenia seeds for 0, 24, 48, 72 and 96 h. Results were
expressed as the mean ± standard error (SE) of the repetitions. *different
letters are not significantly different (p-value > 0.05).
Capacidad Germinativa (GC),
Tiempo Medio de Germinación (MGT) e Índice de Velocidad Germinativa (GSI)
obtenidos para el ensayo de AA de las semillas de N. caldenia durante 0,
24, 48, 72 y 96 h. Los resultados se expresaron como la media ± error estándar
(EE) de las repeticiones. * letras distintas son significativamente diferentes
(p-valor > 0,05).
The highest GC
occurred within the first 24 hours of AA. This was followed by a 15% decline
between 48 and 72 hours compared to the control. MGT remained stable for up to
72 hours. However, at 96 hours, GC decreased by 50%, accompanied by a twofold
increase in MGT. Fontana
et al. (2016) applied this method to N. alba seeds from northern
Argentina and observed 50% lethality within 48 hours of storage. This suggests
that N. caldenia seeds may demonstrate greater resilience to
high-temperature and humidity conditions, potentially due to higher vigor. Seed
vigor can vary by species and is influenced by environmental factors such as
light, temperature, soil moisture, and nutrients (15). GSI decreased
significantly with longer AA durations, resulting in germination rates 2.17
times lower than the control (table 4). This decline indicates potential physiological and
biochemical changes, such as reduced plasma membrane integrity, molecular
alterations in nucleic acids, decreased enzymatic activities during seed
senescence, and delayed germination (25).
Effect
of salinity on germination
The inhibitory effects
of salinity on germination, due to ionic toxicity and osmotic stress impeding
water uptake by the embryo, are well documented in various Neltuma species.
Table
5,
summarizes these effects on N. caldenia germination. Germination capacity
remained unaffected at osmotic potentials up to -0.90 MPa (T1-T3).
Table 5. Values
of Germinative Capacity (GC), Mean Germination Time (MGT) and Germinative Speed
Index (GSI) obtained for seeds subjected to treatments T0, T1, T2, T3, T4 and
T5 with different osmotic potentials (Ψo) induced by NaCl.
Tabla
5. Valores de Capacidad Germinativa (GC), Tiempo Medio
de Germinación (MGT) e Índice de Velocidad Germinativa (GSI) obtenidos para las
semillas sometidas a los tratamientos T0, T1, T2, T3, T4 y T5 con diferentes
potenciales osmóticos (Ψo) inducidos por NaCl.
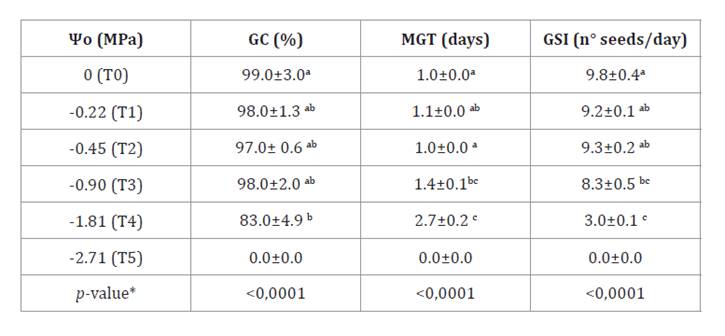
The results were expressed as the
mean ± standard error (SE) of the repetitions. * different letters are not
significantly different (p-value > 0.05).
Los resultados se expresaron como
la media ± error estándar (EE) de las repeticiones. * letras distintas son
significativamente diferentes (p-valor > 0,05).
However, it
decreased significantly under higher osmotic stress, with total inhibition at
the most severe level (T5) and no viable seeds according to the tetrazolium
test. The germination response of N. caldenia under saline conditions
resembles that of salt-tolerant plants, halophytes, showing resistance up to a
critical concentration followed by a sharp decline.
Similar patterns were observed in N. alpataco, with
reduced germination at comparable osmotic potentials (32).
However, studies on Strombocarpa strombulifera and N. alba report
a higher saline tolerance, with GC above 80% at -1.2 and -2.2 MPa, respectively
(22).
Additionally, N. chilensis showed 56% germination at -2.7 MPa,
highlighting species-specific adaptations to salinity within the genus (34).
This variation underscores the diverse salinity responses within Neltuma,
illustrating the complex nature of salinity adaptation.
MGT and GSI were
unaffected at lower salinity levels (T1, T2). However, they decreased significantly
at higher salinity (T3, T4), likely due to delayed seed imbibition from low
water potential, as observed in N. alba (22). Westphal
et al. (2015) reported a similar germination delay in N. chilensis under
saline conditions (NaCl 450-600 mM), requiring 5 days longer than controls to
reach maximum germination. Similarly, N. caldenia seeds needed 4 days to
reach maximum germination at high NaCl levels (T4), twice the duration of the
control.
To quantify the impact of the osmotic pressure on seedling
growth, both radicle and shoot lengths were measured. Increased salinity
significantly reduced root and shoot lengths (figure 1).
Root length showed no significant reduction at -0.22 and -0.45 MPa but declined
beyond T3, reaching 6.7 times less than control length at -1.81 MPa (figure
1A).
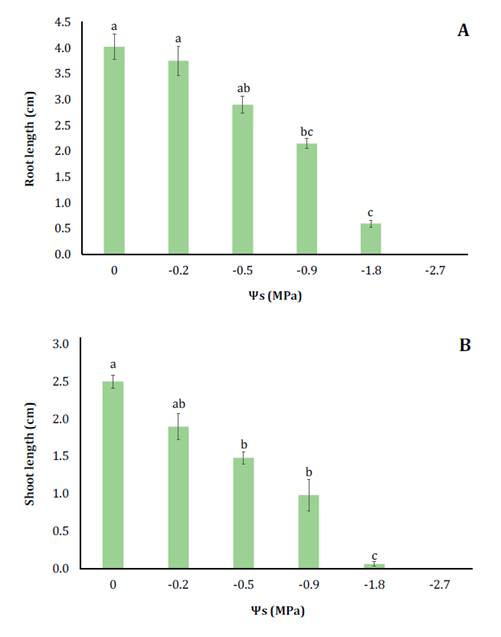
Los
resultados se expresaron como la media y las barras indican el error estándar
(EE) de las repeticiones. Medias con letra común no son significativamente
diferentes (p-valor > 0,05).
Figure
1. Influence of osmotic treatments induced by NaCl (0
to -2.7 MPa) on the development of roots (A) and hypocotyls (B), recorded in cm
of N. caldenia.
Figura 1.
Influencia de los tratamientos osmóticos inducidos por NaCl (0 a -2,7 MPa) en
el desarrollo de las raíces (A) y de los hipocótilos (B) de N. caldenia,
registrado en cm.
Shoot length declined from the lowest NaCl concentration,
approaching minimal values at -1.81 MPa (figure 1B).
These results indicate that NaCl inhibits shoot growth more than root growth,
potentially due to endogenous abscisic acid (ABA), a phytohormone that reduces
shoot growth and moderates root elongation under osmotic stress (2).
Similar findings in S. strombulifera showed increased ABA levels and
reduced shoot growth under high humidity and NaCl (12).
The reduction in
shoot and root growth led to a decline in SVI (table 6), which remained
statistically similar to the control up to an osmotic pressure of -0.22 MPa.
From treatment T1 onwards, SVI progressively decreased, with vigor dropping
below half at -1.81 MPa. Root and shoot tolerance indices showed similar
patterns, exceeding 50% up to -0.45 MPa, while salinity at T3 and T4 induced
more severe phytotoxic effects on shoots. Total toxicity was observed for roots
and shoots at T5.
Table 6. Seedling
Vigor Index (SVI), Root Phytotoxicity (RPT), Hypocotyl Phytotoxicity (HPT),
Root Tolerance Index (RTI) and Hypocotyl Tolerance Index (HTI) obtained for
seedlings subjected to treatments T0, T1, T2, T3, T4 and T5 with different
osmotic potentials (Ψo) induced by NaCl.
Tabla
6. Índice de Vigor de Plántula (SVI),
Fitotoxicidad de la raíz (RPT) y de hipocótilo (HPT), Índice de Tolerancia de
la raíz (RTI) e índice de Tolerancia del hipocótilo (HTI) obtenidos para las
plántulas sometidas a los tratamientos T0, T1, T2, T3, T4 y T5 con diferentes
potenciales osmóticos (Ψo) inducidos por NaCl.
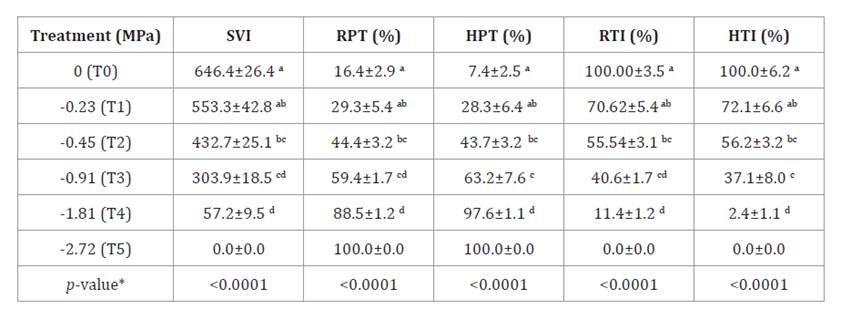
The results were expressed as the
mean ± standard error (SE) of the repetitions.
*means with common letter are not
significantly different (p-value > 0.05).
Los resultados se expresaron como
la media ± error estándar (EE) de las repeticiones.
*medias con letra común no son
significativamente diferentes (p-valor > 0,05).
Given that N. caldenia inhabits saline soils and salt
flats during wet seasons, it may possess adaptive mechanisms to salinity, as
our findings indicated. However, tolerance at the germination stage does not
ensure similar tolerance in seedling growth (8,
13). While seeds tolerated up to -0.90 MPa during germination,
14-day-old seedlings were more sensitive, showing toxic effects at -0.45 MPa.
This heightened sensitivity could limit seedling recruitment and survival in
variable salinity environments. Beyond tolerable salinity, reductions in
radicle and seedling growth are likely due to NaCl toxicity and impaired
nutrient absorption (7).
As soil salinity fluctuates with precipitation, it is essential to consider
both germination capacity and salinity effects on seedling growth to inform
effective conservation and restoration strategies.
Conclusions
This study demonstrates the effectiveness of scarification
techniques in promoting N. caldenia seed germination, with both
mechanical and chemical methods successfully breaking seed dormancy. The seeds
showed high vigor, with germination rates strongly affected by temperature,
although prolonged storage reduced vigor, especially after accelerated aging.
These findings underscore the need for appropriate storage practices to
preserve seed viability. Additionally, N. caldenia seeds displayed
salinity tolerance levels during germination comparable to or greater than
those of other salt-tolerant species within its genus. Optimizing germination
protocols and understanding the effects of salinity are essential steps toward
formulating robust conservation and management strategies. Optimizing
germination protocols and understanding salinity impacts are key to developing
effective conservation and management strategies. Addressing these factors
supports environmental restoration and habitat preservation, contributing to
the sustainable use of N. caldenia, a notable species of the Espinal
ecosystem under significant environmental pressure.
1.
Aguirre-Mancilla, C. L.; Godínez-Galán, R. Y.; Raya-Pérez, J. C.;
Gutiérrez-Benicio, G. M.; Ramírez-Pimentel, J. G.; Covarrubias-Prieto, J.;
Guadalupe García-Rodríguez, J. G. 2020. Protein content and quality of seeds in
central mexican maize (Zea mays) accessions. Revista de la Facultad de
Ciencias Agrarias. Universidad Nacional de Cuyo. Mendoza. Argentina. 52(1):
14-25
2. Akhiyarova, G.;
Veselov, D.; Ivanov, R.; Sharipova, G.; Ivanov, I.; Dodd, I. C. 2023. Root ABA
accumulation delays lateral root emergence in osmotically stressed barley
plants by decreasing root primordial IAA accumulation. Int. J. Plant Biol.
14(1): 77-90.
3. Allali, A.;
Rezouki, S.; Louasté, B.; Bouchelta, Y.; El Kamli, T.; Eloutassi, N.; Fadli, M.
2020. Study of the nutritional quality and germination capacity of Cicer
arietinum infested by Callosobruchus maculatus (Fab.). Plant Cell
Biotechnol Mol Biol. 21: 44-56.
4. Boeri, P.; Gazo,
M. C.; Failla, M.; Barrio, D.; Dalzotto, D.; Sharry, S. 2019. Optimum
germinative conditions of a multipurpose shrub from Patagonia: Prosopis
alpataco (Fabaceae). Darwiniana. 7(2): 199-207. DOI:
https://dx.doi.org/10.14522/darwiniana.2019.72.817
5. Bonner, F. T.;
Karrfalt, R. P. 2008. The Woody Plant Seed Manual. U.S. Department of Agriculture,
Forest Service. 1240 p.
6. Cabrera, A. 1976. Regiones fitogeográficas argentinas.
Encicl. Argent. Agric. Jard. 2: 1-85.
7. Cirka, M.; Kaya,
A. R.; Eryigit, T. 2021. Influence of temperature and salinity stress on seed
germination and seedling growth of soybean (Glycine max L.). Legume Res-
Int. J. 44(9): 1053-1059.
8. Contreras, F.
I.; Duval, V. S. 2021. Respuesta lacustre a la variabilidad climática en los
valles transversales (La Pampa, Argentina). Boletín Geográfico. 43(1): 13-31.
9. Contreras-Negrete,
G.; Pineda-García, F.; Nicasio-Arzeta, S.; De la Barrera, E.;
González-Rodríguez, A. 2021. Differences in germination response to
temperature, salinity, and water potential among Prosopis laevigata populations
are guided by the tolerance-exploitation trade-off. Flora. 285: 151963. DOI:
https://doi.org/10.1016/j.flora.2021.151963
10. Craviotto, R.
M.; Arango, P. M. R.; Gallo, C. 2011. Novedades de la prueba de viabilidad por
Tetrazolio en soja. Santa Fe. Argentina. INTA-EEA Oliveros.
11. Dágata, S. L.;
Fernández, M. E.; Passera, C. B. 2021. Environmental factors affecting
germination of Mimosa ephedroides (Fabaceae), an endemic shrub
from Monte Desert, Argentina. Revista de la Facultad de Ciencias Agrarias.
Universidad Nacional de Cuyo. Mendoza. Argentina. 53(1): 139-149. DOI:
https://doi.org/10.48162/rev.39.014
12. Devinar, G.;
Llanes, A.; Masciarelli, O.; Luna, V. 2013. Different relative humidity
conditions combined with chloride and sulfate salinity treatments modify
abscisic acid and salicylic acid levels in the halophyte Prosopis
strombulifera. Plant Growth Regul. 70(3): 247-256.
13. Duval V. S.
2017. Estudio integral de áreas protegidas: reserva provincial Parque Luro y
Parque Nacional Lihué Calel, provincia de La Pampa. Tesis Doctoral. Universidad
Nacional del Sur. Departamento de Geografía y Turismo.
14. Fenollosa, E.;
Jené, L.; Munné-Bosch, S. 2020. A rapid and sensitive method to assess seed
longevity through accelerated aging in an invasive plant species. Plant
Methods. 16(1): 64.
15. Finch-Savage,
W. E.; Bassel, G. W. 2016. Seed vigour and crop establishment: extending
performance beyond adaptation. J. Exp. Bot. 67(3): 567-591. DOI:
https://doi.org/10.1093/jxb/erv490
16. Fontana, A.;
Perez, V. R.; Luna, C. V. 2016. Pruebas de envejecimiento acelerado para
determinar vigor de semillas de Prosopis alba de tres procedencias
geográficas. Fave Sección Cienc. Agrar. 15(1): 37-50.
17. Friedel, M.;
Duval, V.; Benedetti, G. 2022. Cobertura y uso del suelo en el sureste de la
provincia de La Pampa (Argentina) durante el período 1980-2020. Huellas. 26(1):
29-44.
18. ISTA. 2010.
International Rules for Seeds Testing. https://www.seedtest.org/en/home.html
19. Maguire, J. D.
1962. Speed of germination-aid in selection and evaluation for seedling emergence
and vigor. Crop Sci. 2(2): 176-177.
20.
Martínez-González, L.; Pérez-Domínguez, G.; López-Padrón, I.; Reyes-Guerrero,
Y.; Núñez-Vázquez, M. de la C. 2022. Efecto de un extracto de Sargassum
fluitans sobre la germinación de semillas de tomate. Cultiv. Trop. 43(2):
e11-e11.
21. Massa, A.;
Quagliariello, G.; Martinengo, N.; Calderón, A.; Pérez, S. 2023. Growth and
slenderness index in sweet algarrobo, Neltuma flexuosa, according to the
vermicompost percentage in the substrate and seed origin. Revista de la
Facultad de Ciencias Agrarias. Universidad Nacional de Cuyo. Mendoza.
Argentina. 55(2): 12-19. DOI: https://doi.org/10.48162/ rev.39.105
22. Meloni, D. A.;
Gulotta, M. R.; Silva, D. M.; Arraiza, M. P. 2019. Effects of salt stress on
germination, seedling growth, osmotic adjustment, and chlorophyll fluorescence
in Prosopis alba G. Revista de la Facultad de Ciencias Agrarias.
Universidad Nacional de Cuyo. Mendoza. Argentina. 51(1): 69-78.
23. Oyarzabal, M.;
Clavijo, J.; Oakley, L.; Biganzoli, F.; Tognetti, P.; Barberis, I.; Maturo, H.
M.; Aragón, M.; Campanello, P. I.; Prado, D.; Oesterheld, M.; León, R. J. C.
2018. Unidades de vegetación de la Argentina. Ecol. Austral. 28(1): 40-63. DOI:
https://doi.org/10.25260/EA.18.28.1.0.399
24. Piraino, S.; Roig,
F. A. 2024. Landform heterogeneity drives multi-stemmed Neltuma flexuosa growth
dynamics. Implication for the Central Monte Desert forest management. Revista
de la Facultad de Ciencias Agrarias. Universidad Nacional de Cuyo. Mendoza.
Argentina. 56(1): 26-34. DOI: https://doi.org/10.48162/rev.39.120
25. Rastegar, Z.;
Sedghi, M.; Khomari, S. 2011. Effects of accelerated aging on soybean seed
germination indexes at laboratory conditions. Not. Sci. Biol. 3(3): 126-129.
26. Revathy, K.;
Shanker, S. R. 2023. Effect of cadmium stress on seed germination and seedling
morpho-physiological growth parameters of barnyard millet (Echinochloa
frumentacea Link). Plant Sci. Today. 10(1): 252-260. DOI:
https://doi.org/10.14719/pst.2012
27. Salisbury, F.
B. 1996. Units, symbols, and terminology for plant physiology: A reference for
presentation of research results in the plant sciences. Oxford University
Press. 249 p.
28. Schober, P.;
Boer, C.; Schwarte, L. A. 2018. Correlation Coefficients: Appropriate Use and
Interpretation. Anesth. Analg. 126(5): 1763-68. DOI:
10.1213/ANE.0000000000002864
29. Sistema
Meteorológico Nacional. 2023. Estado del clima en Argentina 2023. Buenos Aires,
Argentina: Ministerio de Defensa de Argentina. https://repositorio.smn.gob.ar/
handle/20.500.12160/2621. (Accessed September 2024).
30. Universidad
Nacional de Córdoba. Infostat - Software estadístico. https://www.
infostat.com.ar/. (Accessed September 2024).
31. Utello, M. J.; Tarico, J. C.; Demaestri, M. A.; Plevich, J.
O. 2023. Evaluation of pre-germination treatments in Prosopis caldenia seeds.
Rev. Bosque. 44(1): 37-45. DOI: https://doi.
org/10.4067/S0717-92002023000100037
32. Villagra, P. E.
1997. Germination of Prosopis argentina and P. alpataco seeds
under saline conditions. J. Arid Environ. 37(2): 261-267. DOI:
https://doi.org/10.1006/jare.1997.0275
33. Villagra, P.
E.; Passera, C. B.; Greco, S.; Sartor, C.; Aranibar, J. N.; Meglioli, P. A.;
Álvarez, J. A.; Allegretti, L. I.; Fernández, M. E.; Cony, M. A.; Kozub, P. C.;
Riveros, C. V. 2017. Uso de plantas nativas en la restauración y recuperación
productiva de ambientes salinos de las zonas áridas de la región del Monte,
Argentina. Ambient. Salinos Alcalinos Argent. Univ. Católica
Córdoba-Orientación Gráfica Ed. Córdoba Argent. 419-444.
34. Westphal, C.;
Gachón, P.; Bravo, J.; Navarrete, C.; Salas, C.; Ibáñez, C. 2015. The potential
of algarrobo (Prosopis chilensis (Mol.) Stuntz) for regeneration of
desertified soils: Assessing seed germination under saline conditions. Environ.
Manage. 56(1): 209-220.
35. Willan, R. L.
1991. Guía para la manipulación de semillas forestales con especial referencia
a los trópicos. Roma, Italia. Fao. FAO ed.
36. Zeberio, J. M.;
Perez, C. A. 2020. Tratamientos pregerminativos en especies leñosas del Monte
patagónico. For. Veracruzana. 22(1): 11-16.
37. Zhang, Y.; Tao, Q.; Zhang, R.; Ma, Y.; Xing J.; Zhou, S.;
Liu, Y.; Meng, F.; Sun, J. 2023. A seed vigour test based on radicle emergence
during germination at 5°C for four forage species. Seed Sci. Technol. 51(3):
361-369. DOI: https://doi.org/10.15258/sst.2023.51.3.08