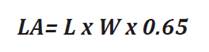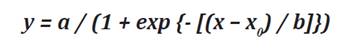Revista de la Facultad de Ciencias
Agrarias. Universidad Nacional de Cuyo. Tomo 57(2). ISSN (en línea) 1853-8665.
Año 2025.
Original article
Green
Synthesis and Foliar Application of Copper Nanoparticles in Sunflower (Helianthus
annuus L.) to Improve Physiological Parameters and Yield
Síntesis
verde y aplicación foliar de nanopartículas de cobre en híbridos de girasol (Helianthus
annuus L.) para mejorar parámetros fisiológicos y el rendimiento
Sergio Andrés
Granados Ortiz1, 2,
3,
Flavia Fátima
Visentini1,
Fernando Felipe
Muñoz5,
Juan Pablo Malano2,
Marcos Gabriel
Derita3,
Adrián Alejandro
Pérez Rubin1,
1Área de Biocoloides y Nanotecnología. Instituto de Tecnología de
Alimentos. Facultad de Ingeniería Química. Universidad Nacional del Litoral. 1°
de Mayo 3250 (CP 3000). Santa Fe. República Argentina.
2Universidad Nacional del Litoral. Facultad de Ciencias Agrarias.
Cátedra de Fisiología Vegetal. Kreder 2805. C. P. 3080. Esperanza. Argentina.
3Instituto de
Ciencias Agropecuarias del Litoral (ICiAgro Litoral). Universidad Nacional del
Litoral (UNL). Consejo Nacional de Investigaciones Científicas y Técnicas
(CONICET). Facultad de Ciencias Agrarias (FCA). Laboratorio de Investigaciones
en Fisiología y Biología Molecular Vegetal (LIFiBVe). Kreder 2805, S3080HOF.
Esperanza, provincia de Santa Fe. República Argentina.
4Universidad Nacional del Litoral. Facultad de Ciencias Agrarias.
Cátedra de Morfología Vegetal. Kreder 2805. C. P. 3080. Esperanza. Argentina.
5Universidad Nacional de Mar del Plata. CONICET. Instituto de
Investigaciones.
*gabrielcnbj@yahoo.com.ar
Abstract
Nanotechnology
holds significant interest across various domains, including agriculture. The
green synthesis of nanoparticles offers environmentally friendly solutions.
This study aimed to synthesize copper nanoparticles (NPs) using Aloe vera extracts
and evaluate their foliar application on two sunflower hybrids, Chané (Ch) and
Calchaquí (Ca). The two types of Aloe Vera extracts used to produce
nanoparticles were characterized by UV-vis spectral analysis and dynamic light
scattering (DLS). The Np particles synthesized with Aloe vera Home (Np1)
measured 242.8 nm (62.6%) and 74.87 nm (37.4%), while Aloe vera Commercial
(Np2) resulted in sizes of 339.6 nm (90.7%) and 66.07 nm (9.3%). Two different
doses of Np (150 ppm and 300 ppm) were applied to sunflower plants. We measured
germination power (GP), plant height (PH), leaf number (LN), leaf area (LA),
dry weight accumulation and achene yield. Chané’s parameters improved at both
nanoparticle doses, while Calchaquí only improved with the 300 ppm treatment.
This research highlights the potential use of green nanotechnology to improve
growth and yield in sunflower.
Keywords: plant physiology,
oilcrops, agrotechnology, reducing agent, crop science
Resumen
La nanotecnología
es un área de gran interés en diferentes campos de la ciencia, entre ellos la
agricultura. La síntesis verde de nanopartículas ofrece soluciones sostenibles
para el medioambiente. El objetivo del presente trabajo fue sintetizar nanopartículas
de cobre (NPs) utilizando como agente reductor el Aloe Vera y evaluar el impacto de su aplicación foliar en dos
híbridos de girasol, Chané (Ch) y Calchaquí (Ca). Se utilizaron dos tipos de
extractos de Aloe Vera como agente reductor, las cuales se caracterizaron
mediante análisis espectral UV-vis y dispersión dinámica de luz (DLS). Las Nps
sintetizadas con Aloe Vera Home (Np1) presentaron tamaños de partícula
de 242,8 nm (62,6%) y 74,87 nm (37,4%), mientras que las obtenidas con Aloe
Vera comercial (Np2) dieron como resultados tamaños de partícula de 339,6
nm (90,7%) y 66,07 nm (9,3%). Se midieron parámetros fisiológicos de la planta
como fue el poder germinativo (PG), la altura de la planta (PH), el número de
hojas (NL) y el área foliar (LA). Se aplicaron dos dosis diferentes de Nps (150
ppm y 300 ppm) a las plantas y se cuantifico la acumulación de materia seca en
tallo, peciolo, hoja y capitulo. El híbrido Chané presentó una mejora respuesta
con ambas dosis de nanopartículas, mientras que Calchaquí mostró una mejora en
sus parámetros solo con el tratamiento de 300 ppm. Esta investigación destaca
el uso potencial de la nanotecnología verde en girasol para mejorar el
crecimiento y el rendimiento.
Palabras claves: fisiología vegetal,
cultivos oleaginosos, tecnología agraria, agente reductor, ciencias de los
cultivos
Originales: Recepción: 22/08/2024 - Aceptación: 04/08/2025
Introduction
Recently,
nanotechnology has emerged as a novel field with far-reaching applications
across diverse sectors, including agriculture. Nanoparticles, with unique
physicochemical properties, have garnered significant attention in crop
management strategies (35, 36). Among these
nanoparticles, copper nanoparticles stand out for their multifaceted
properties, such as high surface-area-to-volume ratio, excellent conductivity,
and intrinsic antimicrobial attributes (8, 28, 33). Their application
in agriculture, particularly increasing plant growth and defense mechanisms,
has sparked immense interest (17).
The exploration of
natural sources for nanoparticle green synthesis constitutes a focal point in
this expanding field (3, 15). Aloe vera,
popular for its medicinal and bioactive properties, is a compelling candidate
for synthesizing copper nanoparticles, aligning with sustainable practices and
offering biocompatible and eco-friendly nanomaterials for agriculture (31).
The green synthesis
of copper nanoparticles using Aloe Vera provides various phytochemicals
with significant electrochemical reducing power. Aloe vera contains
active components like polysaccharides, flavonoids, phenolic compounds, and
anthraquinones (21, 25, 31) with functional
groups like hydroxyl (-OH) and carbonyl (-C=O), that possess reducing and
stabilizing power. Polysaccharides, particularly mannose-rich polymers and
acetylated mannans, reduce copper ions to copper nanoparticles (9,
23, 29). Hydroxyl groups also lead to the reduction of copper ions and
subsequent formation of copper nanoparticles (4,
27). Additionally, synergistic effects among various bioactive
compounds in these extracts help stabilization and control the synthesis of
copper nanoparticles.
Aloe Vera as green reducing agent may influence
the synthesis of copper nanoparticles (31).
Homemade extracts (self-grown plants) may exhibit composition variations due to
cultivar differences, growth conditions, extraction methods, and storage,
potentially affecting concentrations of bioactive compounds. Alternatively,
commercial products are subjected to standardized processing methods,
potentially containing stabilizers or additives (21)
that influence concentration and quality compared to homemade extracts (18).
These variations in chemical composition and concentrations of bioactive
compounds in Aloe Vera extracts might lead to differences in their
reducing potential and, consequently, affect the synthesis of copper
nanoparticles. Such differences can result in varied nanoparticle sizes,
shapes, and stability, impacting their potential applications (34).
Parallely, the
study of nanoparticle-induced responses in crop plants represents a promising
alternative in agricultural research (17). Sunflower (Helianthus
annuus L.) is an emblematic oilseed crop known for its adaptability to
various environments and the capacity to provide oil, seeds, and biomass (1,
7, 39). Beyond their economic significance, sunflowers play a key role
in phytoremediation and agricultural ecosystems (9,
20). Understanding the influence of Aloe Vera-based copper
nanoparticles on growth, development, and nutrient dynamics of sunflower
hybrids may optimize crop management strategies and contribute to sustainable
agricultural practices.
This research aims
to describe the mechanisms underlying nanoparticle-plant interactions, and how Aloe
Vera-based copper nanoparticles affect growth, biomass accumulation and
partitioning in two sunflower hybrids. This will expand our understanding of
nanoparticle-mediated plant responses, for potential tailored
nanoparticle-based strategies to optimize sunflower productivity and
sustainability.
Materials
and Methods
Synthesis
and Characterization of Aloe Vera-based Copper Nanoparticles
Synthesis
Copper
nanoparticles (Np) were synthesized using two Aloe Vera extracts as
reducing agents. The first Aloe Vera extract (AVH) was obtained and
characterized at the Biocolloids and Nanotechnology Laboratory of the Facultad
de Ingeniería Química (FIQ), Instituto de Tecnología de Alimentos (ITA),
Universidad Nacional del Litoral (UNL) in Santa Fe (Argentina). The second
extract (AVC) was commercial (Jual Aloe Calchaquí SRL.). Np synthesis used two
solutions containing copper sulfate pentahydrate (CuSO4.5H2O) (Anedra-Research
AG) at [0.1 M]. The AVH and AVC were added to this solutions in a 8:2 ratio of
copper salt and reducing agent. The obtained solutions were shaken
(Fisatom-Model 753A) for 15 minutes at moderate and constant speed. Then, both
solutions were placed in a thermal bath (Dalvo-Model BTMP) for 4 h at 85°C.
Subsequently, they were left for 2 h at room temperature (25°C). The resulting
solutions were oven-dried (Dalvo-Model FHR/I) at 90°C for 24 h, obtaining a
green powder. This powder was weighed with a high-precision digital balance
(Ohaus-Model PA 214), reconstituted with the same amount of evaporated water
and stirred until a homogeneous solution was obtained. Then, solutions were
centrifuged (Neofuge 18R Heal Force) at 3000 rpm or 1.016 g for 20 min at 20°C.
Finally, supernatants were collected and stored, obtaining liquid Np1 (CuSO4.5H2O
with Aloe Vera Home) and Np2 (CuSO4.5H2O with Aloe Vera
commercial) (13).
Characterization
The Np were
spectrally characterized using UV-vis spectroscopy (Perkin Elmer Lambda 20) to
determine surface plasmon resonance (SPR) characteristic of metallic
nanoparticles (35). Additionally, the
percentage conversion was calculated using the normalized spectrum equation (12), and particle size
was determined using the dynamic light scattering (DLS) technique (ZetaNano ZS
Malvern UK) (37).
Plant
Culture and Growth Conditions
Plant
Material and Growth Conditions
This study was conducted under field conditions with summer
rainfall and controlled irrigation at Donnet field, Facultad de Ciencias
Agrarias (FCA-UNL) in Esperanza (31°26’34.4” S 60°56’26.3” W, Santa Fe,
Argentina). Soil was a Mollisol subgroup typical Argiudol of the Esperanza
Series, with 29% sand, 66% silt, and 5% clay in the Ap horizon (0 to 0.27 m
deep) (7).
A total of 440 sunflower (Helianthus annuus L.) seeds were sown,
comprising 25 plants of the Chané (Ch) hybrid and 25 plants of Calchaquí (Ca)
hybrid. The seeds underwent pre-germination treatment with
dynasty-metalaxil-imida (DMI) to prevent fungal growth. Seeds were supplied by
Dr. Daniel Álvarez (Estación Experimental Agropecuaria, Instituto Nacional de
Tecnología Agropecuaria, EEA-INTA-Manfredi).
Experimental
Design
Two
plots were prepared, each undergoing two mechanical weed control sessions. Plot
“A” was 6.26 m long and 4.30 m wide, with Ch hybrid sown in the western part
and Ca hybrid in the eastern part. Similarly, plot “B” measured 6.47 m in
length and 4.50 m in width. Ch was planted in the eastern section while Ca was
planted in the western section of this plot. A total of 16 rows were created, 8
rows per plot, with 4 rows for each hybrid in both plots. Externally, plots
were surrounded by three rows of plants with the same density to reduce edge
effect. Sowing density was 3-4 seeds per linear meter, plus 15% for potential
seed loss.
Soil
Preparation, Germination, and Transplanting
Germination
was carried out as previously described (5,
6, 7, 19). Briefly, seeds were washed with a 30% commercial bleach
solution for 20 min, followed by three washes with distilled water and drying
with inert paper. Subsequently, in vitro germination was conducted using
20 sterilized Petri dishes conditioned with inert paper and saturated with
distilled water. Each Petri dish contained 22 seeds of each hybrid germinated
under controlled conditions of saturated humidity and 27.2°C in a germination
oven (Bioelec-Model RE-41.1). Temperature was monitored using a Data Logger
(Cavadevices SATM), recording measurements every 15 minutes. After 72 h in the
germination oven, seeds were transplanted, considering visible radicle without
necrotic tissue.
Morphological
and Physiological Parameters
We
measured germination energy (GE), germination power (PG), plant height (PH),
leaf number (LN), and leaf area (LA) of the 15th and 18th
leaves. Parameters a, b, and X0 were obtained by fitting Leaf
expansion curves to a sigmoidal equation.
Harvest
was carried out when plants reached physiological maturity, corresponding to
stage R9 on the Scheiter and Miller scale (32).
Dry weight accumulation was measured for the two copper nanoparticles (Np) at
three doses (control, C; 0ppm; D1, 150 ppm of Np AVC and AVH; and D2, 300 ppm
of AVC and AVH, respectively). Dry weight was partitioned into Heads, Stems,
Petioles, and Leaves, then stored and oven-dried (DALVO-Model XHRF 6189) at
60°C until constant weight. Hybrids were harvested at 2141.7°C d-1 (5,
12).
Leaf
Growth Analysis
Leaf
Area (LA) was estimated as described in Eq.1 (6,
24) from length and width measurements as follows (2):
Relative
rate of leaf expansion was calculated as described in Eq.2 (6,
24) as the slope of the regression curve between LA natural
logarithm and thermal time.
Leaf
expansion dynamics were analyzed by sigmoid curves with three parameters (a,
b, and x0):
Final
LA was determined as the upper asymptote (a).
Maximum
expansion velocity value (Vmax) was calculated as follows (6,
24):
![]()
Absolute leaf expansion rates (AER) were calculated as the
slopes of the linear regression between leaf area and thermal time between two
consecutive measurements for the entire experimental time (6,
24).
Leaf relative expansion
rates (RER) were calculated as the ratios between the differences in leaf area
logarithms and thermal time interval between two successive measurements (hn-1
and hn), (Eq.4) (5,
24):
Nanoparticle
Foliar Application
The Np were applied
to leaves 15 and 18 in each hybrid (Ch and Ca) using a trigger spray
applicator, at two different thermal moments (1507.4°C d-1 and 1645.9°C d-1), with
temperatures of 25.6 and 24.1°C, respectively. Both applications were carried
out in the morning, ensuring open stomata and no wind. A volume of 12.5 mL of
Np solution was applied to each leaf at each thermal time, totaling 25 mL per
plant. Np were applied at two different doses in both hybrids: D1, 150 ppm of
Np per plant and D2, 300 ppm of Np per plant (14).
Statistical
Analysis
Data were analyzed
by ANOVA and Fisher’s least significant difference (LSD) test for 5 %
significance level. ANOVA assumptions were verified by Shapiro-Wilks and Levene
tests (7). Statistical analyses were run using
InfoStat Professional software (Universidad Nacional de Córdoba) (7).
Results
and Discussion
Characterization
of Copper Nanoparticles (Np)
Spectral
Characterization and Particle Size
Other studies
report that the green synthesis method also enabled the observation of the SPR
phenomenon at a wavelength of 398 nm for copper nanoparticles (35). Additionally,
other peaks were observed at wavelengths ranging from 277 to 305 nm, similar to
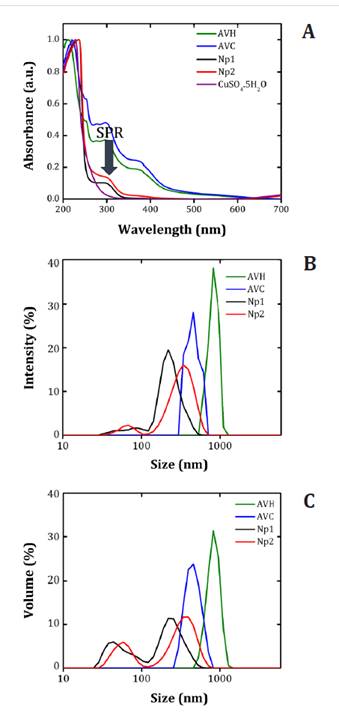
those observed in the present work (26). Figure 1A, shows comparable
peaks in copper nanoparticles synthesized using different reducing agents (Aloe
Vera Home and Aloe Vera Commercial), potentially attributed to the
small nanoparticles. Understanding particle size is crucial as it directly
influences physical and chemical properties of nanoparticles (37). Employing the DLS
technique (figure
1B
and figure1C), Yugandhar
et. al. (2018) observed
synthesized copper nanoparticles of 61.1 nm. Furthermore, Sánchez
Gómez et al. (2018) documented a size distribution of 50 nm for their synthesized
copper nanoparticles. These findings can be compared to the second population
observed in Np2. Notably, both nanoparticle sets synthesized using Aloe Vera
exhibited sizes as those reported in the previously mentioned studies.
(Green
line) Aloe Vera Home (AVH); (Blue line) Commercial Aloe Vera (AVC);
(Black line) UV-Visible spectra of copper nanoparticles (Np1); (Red line)
UV-Visible spectra of copper nanoparticles (Np2); and (Purple line)
pentahydrated copper sulfate (CuSO4.5H2O).
(Línea
verde) Aloe Vera Home (AVH); (Línea azul) Aloe Vera Comercial
(AVC); (Línea negra) espectros de UV-Visible de nanopartículas de cobre (Np1);
(Línea roja) espectros de UV-Visible de nanopartículas de cobre (Np2); y (Línea
púrpura) sulfato de cobre pentahidratado (CuSO4.5H2O).
Figure
1. UV-visible spectra (A), Particle size distribution
(PSD) based on Intensity (B) and Volume percentage (C) of the systems.
Figura
1. Espectros de UV-Visible (A), Distribución
del tamaño de las partículas (PSD) en función de la intensidad (B) y del
porcentaje de volumen (C) de los sistemas.
The different
compositions of Aloe Vera extracts, whether Home or Commercial, might
affect the reduction mechanisms or stabilize the nanoparticles differently
during synthesis, potentially influencing particle size, as detected with DLS
technique.
Plant
Analysis
Physiological
Parameters Before Nanoparticles Applicacion
Germinative Energy (GE) and Germinative Power (GP)
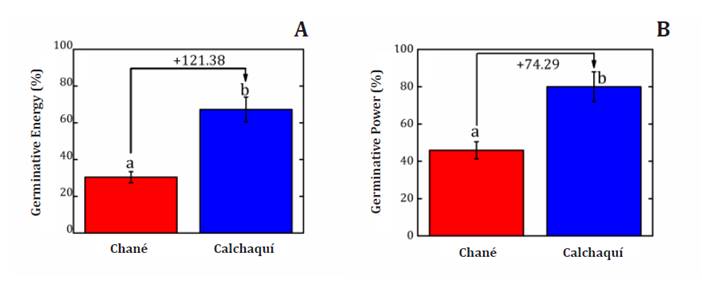
Germination energy in Ch was 94.0%, similar to that in Pisum
sativum L. seeds with Treatment 1 (control) at 3 days (16).
This implies that the Ca sunflower hybrid possesses a lower GE at 67.3% (figure
2A), while the Ch hybrid shows an even lower GE at 30.4%. Sánchez
Gómez et al. (2018) analyzed GE at 7 days for Huaxyacac
seeds cv. Cunningham (Leucaena leucocephala (Lam.) de Wit. treated with
IA24 (water immersion at 24°C for 12 h), finding GE of 31.7%. In comparison, Ch
sunflower hybrid seeds display a higher value (45.9%), while Ca seeds achieve
80.0% (figure
2b).
(A)
Germination Energy at 72 h (EG) and (B) Germination Power (PG) at 168 h
evaluated in two sunflower hybrids (Helianthus annuus L.) Chané (Ch) and
Calchaquí (Ca).
(A) Energía
Germinativa a las 72 h (EG) y (B) Poder Germinativo (PG) a las 168 h en dos híbridos
de girasol (Helianthus annuus L.) Chané (Ch) y Calchaquí (Ca).
Figure
2. Germinative energy (%) and Germinative power (%) in
two sunflower hybrids.
Figura 2. Energía
germinativa (%) y Poder Germinativo (%) en dos híbridos de girasol.
Plant
Height (PH) and Leaf Number (LN)
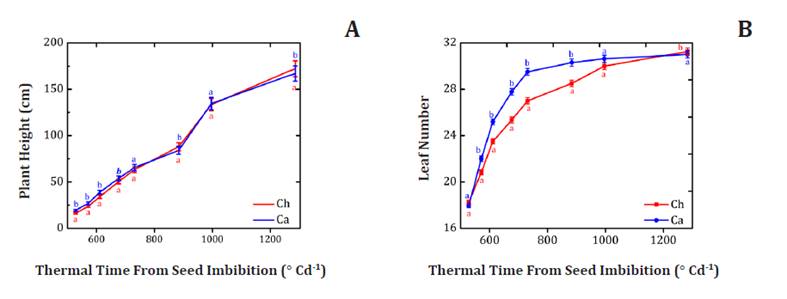
Figure 3A shows
statistically significant differences in PH, except at 996.7 and 1283.7°C d-1, probably since
PH under controlled growth conditions is genotype dependent (24).
A research
conducted by Ortis
et al. (2005) involving 20 sunflower inbred lines found the KLM 295 hybrid
exhibited similar behavior in PH as Ch and Ca hybrids, measuring 170 cm.
Similarly, two sunflower hybrids PARSUN-1 and SMH-9707 (10), were shorter than
Ch and Ca hybrids (136.61 cm and 137.63 cm, respectively). As previously
described, this could be genotype-dependent. However, differences in PH can
also be explained by internode elongation as a response to sowing density (1).
Figure 3B
shows LN of Ch and Ca at eight different thermal times, starting from 528.3°C d-1. At this moment,
both hybrids had 18 visible leaves. Similarly, at 571.3°C d-1,
Ch exhibited 21 leaves while Ca had 22 leaves. Furthermore, at 611.7, 676.6,
731.5, and 884.1°C d-1,
Ch showed 23, 25, 27, and 29 true leaves, respectively. In contrast, during
these days, Ca had 25, 27, 29, and 31 leaves, indicating an average difference
of 2 extra leaves for Ca. Lastly, both hybrids had equal number of leaves (30
and 31) at 996.7 and 1283.7°C d-1 of plant development. As
with PH, this difference in LN may be genetic (24).
Figure
3. (A) Average plant height (cm) and (B) Leaf Number of
two sunflower hybrids (Helianthus annuus L.), Chané (Ch), and Calchaquí
(Ca).
Figura 3. (A)
Altura media de planta (cm) y (B) número de hojas de dos híbridos de girasol (Helianthus
annuus L.), Chané (Ch) y Calchaquí (Ca).
Leaf
Expansion Dynamics of Leaves 15 and 18
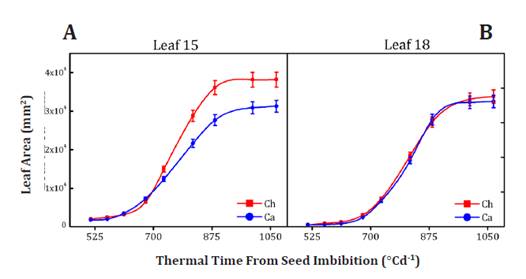
Figure 4A, describes leaf
expansion dynamics of the 15th and 18th leaves of Ch and Ca
hybrids. Leaf 15 in Ch grew faster than Ca. Comparing these results with figure
3B, we concluded that Ch had fewer leaves but a bigger 15th
leaf at all recorded thermal times. Figure 4A, shows that at
758.20°C d-1, the Ch hybrid
reached 50% of its final leaf area, while the Ca hybrid reached this value at
762.89°C d-1.
Figure 4B,
describes leaf expansion dynamics of the 18th leaf in both hybrids.
When compared, both genotypes showed similar results in parameters “a” and “x0”,
34,307.21 and 808.31 for Ch, and 33,410.21 and 809.19 for Ca. Additionally,
leaf expansion ceased at 979.08°C d-1 and 963.88°C d-1 in Ch and Ca,
respectively. In conclusion, leaf growth dynamics of the 18th
leaf were the same between hybrids and comparable to Céccoli
et al. (2012).
Figure 4. (A) Leaf area (LA) of the 15th leaf in Chané (Ch) and Calchaquí
(Ca) hybrids; (B) Leaf area (LA) of the 18th leaf in Chané (Ch) and Calchaquí
(Ca) hybrids.
Figura 4. (A) Área foliar (LA) de la hoja 15 en Ch y Ca; (B) Área (LA) de
la hoja 18 en los híbridos Ch y Ca.
Leaf Growth Analyses
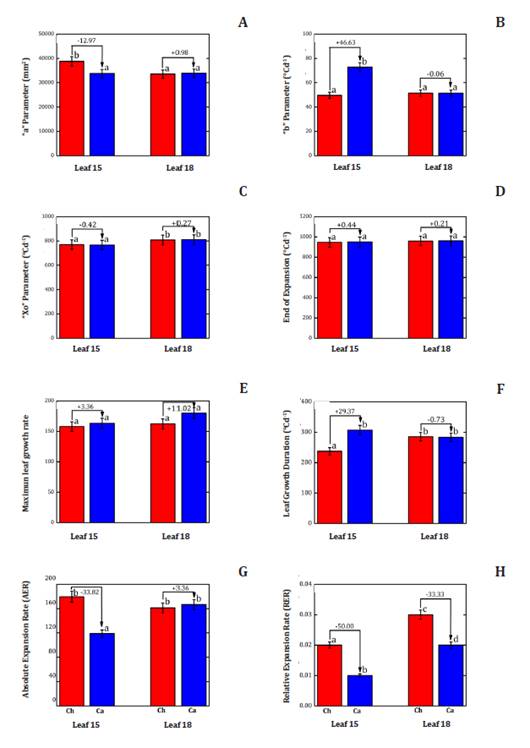
Figure 5, compares
different lea physiological parameters between leaves 15th and 18th,
in Ch and Ca. Parameter “a” had statically lower area in leaf 15 of Ca
(-12.97%; figure
5A),
but no differences were seen for leaves 18 (figure 5A). Regarding
parameter “b” in the 15th leaf, Ca had a
significantly higher curvature in the sigmoid curve compared to Ch (figure 5B) while, the18th
leaf showed no significant differences (figure 5B).
(A)
Parameter “a” (mm²), (B) parameter “b”, (C) parameter “x0”, (D) End of leaf
expansion (°C d-1),
(E) parameter Vmax, (F) Duration of leaf expansion (°C d-1), (G) Absolute rate of leaf expansion
(AER, mm². °Cd-1),
and (H) Relative rate of leaf expansion (RER °Cd-1) in two sunflower hybrids (Helianthus
annuus L.), Chané (Ch) and Calchaquí (Ca) for leaf 15 and 18.
(A)
Parámetro “a” (mm²), (B) parámetro “b”, (C) parámetro “x0”, (D) Fin de la
expansión foliar (°C d-1), (E) parámetro Vmax, (F) Duración de
la expansión foliar (°C d-1), (G) Tasa absoluta de expansión
foliar (AER mm².°Cd-1),
y (H) Tasa relativa de expansión foliar (RER °Cd-1) en dos híbridos de girasol (Helianthus
annuus L.), Chané (Ch) y Calchaquí (Ca) para la hoja 15 y la hoja 18,
respectivamente.
Figure
5. Leaf Growth Analyses.
Figura
5. Análisis del crecimiento de las
hojas.
No significant
differences were found for “x0” in the 15th leaf of any hybrid. Ca
showed -0.42% (figure
5C),
and Ch reached 50% leaf expansion in less thermal time compared to Ca (figure 5C). No statistically
significant differences were found for leaf expansion cessation (figure 5D).
Leaf growth
duration and Vmax increase were not statistically significant (figure 5F). Absolute leaf
expansion rate (AER) was -33.82% in the 15th leaf of Ca with respect
to Ch, with significant differences (figure 5G). The 18th
leaf showed no significant differences (figure 5G). Finally, Leaf
Relative Expansion Rate (RER) was -50.0% lower in Ca with respect to Ch in
the15th leaf (figure 5H), and -33.33%
considering the 18th leaf (figure 5H).
Physiological
and Productive Parameters After Foliar Application of Copper Nanoparticles
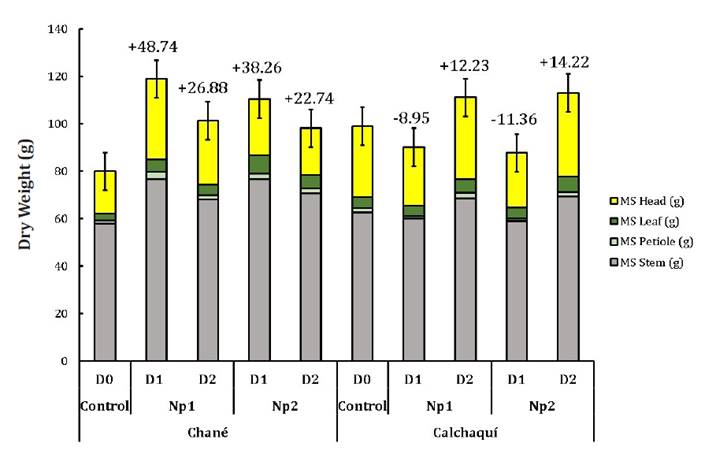
Finally, Plant DW
accumulation in Ch hybrid with Np1D1 and Np2D1 increased by 48.74% and 38.26%,
respectively, compared to control plants. These Nps resulted in more benefits
for this hybrid than for Ca, which decreased by 8.95% and 11.36% with Np1D1 and
Np2D1, respectively (figure
6).
Figure
6. Dry weight accumulation of two sunflower hybrids (Helianthus
annuus L.), Chané (Ch), and Calchaquí (Cal), partitioned into head, stem,
petiole and leaf blade, after application of copper nanoparticles at harvest.
Figura
6. Peso de materia seca de los dos
híbridos de girasol (Helianthus annuus L.), Chané (Ch) y Calchaquí
(Cal), particionados en capitulo, tallo, pecíolo y lámina, luego de la
aplicación de nanopartículas de cobre. La cosecha se realizó en madurez
fisiológica.
We conclude that
dry weight accumulation using Np1 and Np2 at two doses suggests an interaction
between the treatments and genotypes used (1,
7, 10, 11).
Foliar application
of copper nanoparticles at 300 ppm is beneficial for plant development under
saline stress, preventing biomass loss, while enhancing the levels of various
bioactive compounds (17). These reported
results can be compared with the present research, indicating positive effects
of copper nanoparticles on leaf growth dynamics and dry weight accumulation in
sunflower.
Significant
increases in dry weight accumulation of head, stem, petiole, and leaf in Ch
hybrid with Np1D1, Np2D1, Np1D2, and Np2D2 may indicate a better response to
those specific doses or Aloe Vera genotypes. Conversely, the Ca hybrid
showed varied responses, indicating diverse sensitivity upon Aloe Vera extracts
(36,
38).
Differences in
extract composition may lead to variations in synthesis or delivery of
nanoparticles, altering their efficacy. The applied doses might have triggered
diverse metabolic pathways, resulting in distinct responses between hybrids (1,
7, 10, 11).
Understanding these intricate relationships between nanoparticles,
Aloe Vera extracts, and plant physiology requires further investigation
to optimize nanoparticle application for enhanced agricultural production.
Conclusions
This study assessed
physiological responses in two sunflower (Helianthus annuus L.) hybrids,
Chané (Ch) and Calchaquí (Ca), after foliar application of two types and doses
of copper nanoparticles.
Different particle
sizes of copper nanoparticles were observed employing Aloe Vera homemade
extracts (Np1). The DLS technique allowed detecting two peaks at 242.8 nm and
74.87 nm, constituting 62.6% and 37.4% of the particles, respectively.
A comparison
between sunflower hybrids showed that Calchaquí (Ca) had a higher Germinative
Energy (GE) and Germinative Power (GP) by +121.38% and +74.29% respectively,
than Chané. Leaf number was higher in the Calchaquí hybrid at all thermal
times, except for the last measurement (1283.7°C d-1).
The Chane hybrid had higher expansion and relative expansion rates on leaf 15.
Leaf 18 had similar parameter values in both hybrids.
Finally, Np1 (CuSO4.5H2O
with Aloe Vera Home), at 150 ppm (D1) for Chané (Ch), increased “Plant
DW” accumulation by 48.74%. The study lays groundwork for further optimization
of nanoparticle application to different sunflower hybrids.
1. Aguilar, L.;
Escalante, J.; Fucikovsky, L.; Tijerina, L.; Engelman, E. 2005. Leaf Area, Net
Assimilation Rate, Yield and Plant Density in Sunflower. Terra Latinoam. 23:
303-310.
2. Aguirrezábal, L.
A. N.; Lavaud, Y.; Dosio, G. A. A.; Izquierdo, N. G.; Andrade, F. H.; González,
L. M. 2003. Intercepted Solar Radiation during Seed Filling Determines
Sunflower Weight per Seed and Oil Concentration. Crop Sci. 43: 152-161.
https://doi.org/10.2135/cropsci2003.1520
3. Alishah, H.; Pourseyedi, S.; Ebrahimipour, S. Y.; Mahani, S.
E.; Rafiei, N. 2017. Green synthesis of starch-mediated CuO nanoparticles:
preparation, characterization, antimicrobial activities and in vitro MTT assay
against MCF-7 cell line. Rend. Lincei. 28: 65-71. https://doi. org/10.1007/s12210-016-0574-y
4. Aminuzzaman, M.;
Kei, L. M.; Liang, W. H. 2017. Green synthesis of copper oxide (CuO)
nanoparticles using banana peel extract and their photocatalytic activities.
AIP Conf. Proc. 1828. https:// doi.org/10.1063/1.4979387
5. Céccoli, G.;
Eugenia Senn, M.; Bustos, D.; Ismael Ortega, L.; Córdoba, A.; Vegetti, A.;
Taleisnik, E. 2012. Genetic variability for responses to short- and long-term
salt stress in vegetative sunflower plants. J. Plant Nutr. Soil Sci. 175:
882-890. https://doi.org/10.1002/jpln.201200303
6. Céccoli, G.;
Bustos, D.; Ortega, L. I.; Senn, M. E.; Vegetti, A.; Taleisnik, E. 2015.
Plasticity in sunflower leaf and cell growth under high salinity. Plant Biol.
17: 41-51. https://doi.org/10.1111/ plb.12205
7. Céccoli, G.;
Granados Ortiz, S. A.; Buttarelli, M. S.; Pisarello, M. L.; Muñoz, F. F.;
Daurelio, L. D.; Bouzo, C. A.; Panigo, E. S.; Perez, A. A. 2022. Salinity
tolerance determination in four sunflower (Helianthus annuus L.) hybrids
using yield parameters and principal components analysis model. Ann. Agric.
Sci. 67: 211-219. https://doi.org/10.1016/j.
aoas.2022.12.005
8. Chowdhury, R.;
Khan, A.; Rashid, M. H. 2020. Green synthesis of CuO nanoparticles using:
Lantana camara flower extract and their potential catalytic activity towards
the aza-Michael reaction. RSC Adv. 10: 14374-14385.
https://doi.org/10.1039/d0ra01479f
9. Darroudi, M.;
Ahmad, M. B.; Abdullah, A. H.; Ibrahim, N. A.; Shameli, K. 2010. Effect of
accelerator in green synthesis of silver nanoparticles. Int. J. Mol. Sci. 11:
3898-3905. https://doi. org/10.3390/ijms11103898
10.
Fayyaz-Ul-Hassan; Qadir, G.; Cheema, M. A. 2005. Growth and development of
sunflower in response to seasonal variations. Pakistan J. Bot. 37: 859-864.
https://doi.org/10.2298/ hel0542159f
11. Flagella, Z.;
Giuliani, M. M.; Rotunno, T.; Di Caterina, R.; De Caro, A. 2004. Effect of
saline water on oil yield and quality of a high oleic sunflower (Helianthus
annuus L.) hybrid. Eur. J. Agron. 21: 267-272.
https://doi.org/10.1016/j.eja.2003.09.001
12. Gonzalez, M.
A.; Bernardo, V.; Garita, S.; Plaza Cazón, J.; Arango, C.; Hernández, M. P.;
Ruscitti, M. 2024. Morphophysiological and biochemical responses of Schedonorus
arundinaceus to Zinc (II) excess: insights from biomarkers and elemental
accumulation. Revista de la Facultad de Ciencias Agrarias. Universidad Nacional
de Cuyo. Mendoza. Argentina. 56(2): 34-47. DOI:
https://doi.org/10.48162/rev.39.135
13. Gunalan, S.;
Sivaraj, R.; Venckatesh, R. 2012. Aloe barbadensis Miller mediated green
synthesis of mono-disperse copper oxide nanoparticles: Optical properties.
Spectrochim. Acta - Part A Mol. Biomol. Spectrosc. 97: 1140-1144.
https://doi.org/10.1016/j.saa.2012.07.096
14.
Hernández-Fuentes, A. D.; López-Vargas, E. R.; Pinedo-Espinoza, J. M.;
Campos-Montiel, R. G.; Valdés-Reyna, J.; Juárez-Maldonado, A. 2017. Postharvest
behavior of bioactive compounds in tomato fruits treated with Cu nanoparticles
and NaCl stress. Appl. Sci. 7: 1-14. https:// doi.org/10.3390/app7100980
15. Jayarambabu,
N.; Akshaykranth, A.; Venkatappa Rao, T.; Venkateswara Rao, K.; Rakesh Kumar,
R. 2020. Green synthesis of Cu nanoparticles using Curcuma longa extract and
their application in antimicrobial activity. Mater. Lett. 259: 126813. https://doi.org/10.1016/j. matlet.2019.126813
16. Lastochkina, O.
V.; Garipova, S. R.; Pusenkova, L. I.; Garshina, D. Y. 2023. Effect of
Endophytic Bacteria Bacillus subtilis on Seedling Growth and Root Lignification
of Pisum sativum L. under Normal and Sodium Chloride Salt Conditions.
70: 1-11. https://doi.org/10.1134/ S102144372360085X
17. Lira Saldivar,
R. H.; Méndez Argüello, B.; Vera Reyes, I.; de los Santos Villarreal, G. 2018.
Agronanotechnology: A new tool for modern agriculture. Revista de la Facultad
de Ciencias Agrarias. Universidad Nacional de Cuyo. Mendoza. Argentina. 50(2):
395-411. https:// revistas.uncu.edu.ar/ojs3/index.php/RFCA/article/view/3067
18. Liu, C.; Cui,
Y.; Pi, F.; Cheng, Y.; Guo, Y.; Qian, H. 2019. Extraction, purification,
structural characteristics, biological activities and pharmacological
applications of acemannan, a polysaccharide from Aloe Vera: A review.
Molecules 24. https://doi.org/10.3390/ molecules24081554
19. Maya-Meraz, I,
O.; Díaz-Calzadillas, M. F.; Ruiz-Cisneros, M. F.; Ornelas-Paz, J. de. J.;
Rios-Velasco, C.; Berlanga-Reyes, D. I.; Pérez-Corral, D. A.; Alonso-Villegas,
R. 2024. Effects of postharvest treatments based on calcium and silicon in
hydro-cooling on the basic quality attributes of ʹBingʹ sweet cherries (Prunus
avium L.) during storage. Revista de la Facultad de Ciencias Agrarias.
Universidad Nacional de Cuyo. Mendoza. Argentina. 56(2): 114-125. DOI:
https://doi.org/10.48162/rev.39.142
20. Mesquita, A.
C.; Lima Simões, W.; Alcantara Campos, L. D.; Braga, M. B.; Alves Sobral, Y. R.
2024. Gas exchange in yellow melon (Cucumis melo) crop under controlled
water deficit (RDI) and application of a biostimulant. Revista de la Facultad
de Ciencias Agrarias. Universidad Nacional de Cuyo. Mendoza. Argentina. 56(2):
14-25. DOI: https://doi.org/10.48162/ rev.39.133
21. Midatharahalli,
M.; Shivayogeeswar, C.; Kotresh, E. N. 2019. Green synthesis of Zinc oxide
nanoparticles (ZnO NPs) and their biological activity. SN Appl. Sci. 1: 1-10.
https://doi. org/10.1007/s42452-018-0095-7
22. Ortis, L.; Nestares, G.; Frutos, E.; Machado, N. 2005.
Combining Ability Analysis in Sunflower (Helianthus annuus L.). Pakistan
J. Biol. Sci. 8: 710-713. https://doi.org/10.3923/ pjbs.2005.710.713
23. Padil, V. V.
T.; Černík, M. 2013. Green synthesis of copper oxide nanoparticles using gum
karaya as a biotemplate and their antibacterial application. Int. J.
Nanomedicine. 8: 889-898. https:// doi.org/10.2147/IJN.S40599
24. Pereyra-Irujo,
G. A.; Velázquez, L.; Lechner, L.; Aguirrezábal, L. A. N. 2008. Genetic
variability for leaf growth rate and duration under water deficit in sunflower:
Analysis of responses at cell, organ, and plant level. J. Exp. Bot. 59:
2221-2232. https://doi.org/10.1093/jxb/ern087
25. Prakash, S.;
Elavarasan, N.; Venkatesan, A.; Subashini, K.; Sowndharya, M.; Sujatha, V.
2018. Green synthesis of copper oxide nanoparticles and its effective
applications in Biginelli reaction, BTB photodegradation and antibacterial
activity. Adv. Powder Technol. 29: 3315-3326.
https://doi.org/10.1016/j.apt.2018.09.009
26. Rafique, M.;
Tahir, R.; Gillani, S. S. A.; Tahir, M. B.; Shakil, M.; Abdellahi, M. O.;
Rafique, M.; Tahir, R.; Gillani, S. S. A.; Tahir, M. B.; Shakil, M. 2020.
Plant-mediated green synthesis of zinc oxide nanoparticles from Syzygium
Cumini for seed germination and wastewater purification. Int. J. Environ.
Anal. Chem. 00: 1-16. https://doi.org/10.1080/03067319.2020.1715379
27. Reddy, S. B.;
Mandal, B. K. 2017. Facile green synthesis of zinc oxide nanoparticles by Eucalyptus
globulus and their photocatalytic and antioxidant activity. Adv. Powder
Technol. https:// doi.org/10.1016/j.apt.2016.11.026
28. Ren, G.; Hu,
D.; Cheng, E. W. C.; Vargas-Reus, M. A.; Reip, P.; Allaker, R. P. 2009.
Characterisation of copper oxide nanoparticles for antimicrobial applications.
Int. J. Antimicrob. Agents. 33: 587-590.
https://doi.org/10.1016/j.ijantimicag.2008.12.004
29. Roy, A.; Bulut,
O.; Some, S.; Mandal, A. K.; Yilmaz, M. D. 2019. Green synthesis of silver
nanoparticles: Biomolecule-nanoparticle organizations targeting antimicrobial
activity. RSC Adv. 9: 2673- 2702. https://doi.org/10.1039/c8ra08982e
30. Sánchez Gómez,
A.; Rosendo Ponce, A.; Vargas Romero, J. M.; Rosales Martínez, F.; Platas Rosado,
D. E.; Becerril Pérez, C. M. 2018. Energía germinativa en guaje (Leucaena
leucocephala cv. Cunningham) con diferentes métodos de escarificación de la
semilla. Agrociencia. 52: 863- 874.
31. Sangeetha, G.;
Rajeshwari, S.; Venckatesh, R. 2011. Green synthesis of zinc oxide
nanoparticles by Aloe barbadensis Miller leaf extract: Structure and
optical properties. Mater. Res. Bull. 46: 2560-2566.
https://doi.org/10.1016/j.materresbull.2011.07.046
32. Schneiter, A.
A.; Miller, J. F. 1981. Description of Sunflower Growth Stages 1. Crop Sci. 21:
901-903. https://doi.org/10.2135/cropsci1981.0011183x002100060024x
33. Siddiqui, V.
U.; Ansari, A.; Chauhan, R.; Siddiqi, W. A. 2019. Green synthesis of copper
oxide (CuO) nanoparticles by Punica granatum peel extract. Mater. Today
Proc. 36: 751-755. https:// doi.org/10.1016/j.matpr.2020.05.504
34. Veisi, H.;
Karmakar, B.; Tamoradi, T.; Hemmati, S.; Hekmati, M.; Hamelian, M. 2021.
Biosynthesis of CuO nanoparticles using aqueous extract of herbal tea (Stachys
lavandulifolia) flowers and evaluation of its catalytic activity. Sci. Rep.
11: 1-13. https://doi.org/10.1038/s41598- 021-81320-6
35. Velsankar, K.;
Aswin Kumara, R. M.; Preethi, R.; Muthulakshmi, V.; Sudhahar, S. 2020. Green
synthesis of CuO nanoparticles via Allium sativum extract and its
characterizations on antimicrobial, antioxidant, antilarvicidal activities. J.
Environ. Chem. Eng. 8: 104123. https://doi.org/10.1016/j.jece.2020.104123
36. Vidovix, T. B.;
Quesada, H. B.; Januário, E. F. D.; Bergamasco, R.; Vieira, A. M. S. 2019.
Green synthesis of copper oxide nanoparticles using Punica granatum leaf
extract applied to the removal of methylene blue. Mater. Lett. 257: 126685.
https://doi.org/10.1016/j.matlet.2019.126685
37. Visentini, F.
F.; Sponton, O. E.; Perez, A. A.; Santiago, L. G., 2017. Formation and
colloidal stability of ovalbumin-retinol nanocomplexes. Food Hydrocoll. 67:
130-138. https://doi. org/10.1016/j.foodhyd.2016.12.027
38. Yugandhar, P.;
Vasavi, T.; Jayavardhana Rao, Y.; Uma Maheswari Devi, P.; Narasimha, G.;
Savithramma, N. 2018. Cost Effective, Green Synthesis of Copper Oxide
Nanoparticles Using Fruit Extract of Syzygium alternifolium (Wt.) Walp.,
Characterization and Evaluation of Antiviral Activity. J. Clust. Sci. 29:
743-755. https://doi.org/10.1007/s10876-018-1395-1
39. Zhao, Y.; Li,
Yuyi; Wang, J.; Pang, H.; Li, Yan. 2016. Buried straw layer plus plastic
mulching reduces soil salinity and increases sunflower yield in saline soils.
Soil Tillage Res. 155: 363-370. https://doi.org/10.1016/j.still.2015.08.019
Funding
This study was funded by Agencia Nacional de Promoción de la
Investigación, el Desarrollo Tecnológico y la Innovación (Agencia I+D+i)
PICT-2021-CAT-II-00097; Consejo Nacional de Investigaciones Científicas y
Tecnológicas (CONICET) PIP 11220200100488CO; and the Universidad Nacional del
Litoral (UNL) CAI + D 85520240100144LI.