Revista de la Facultad de Ciencias
Agrarias. Universidad Nacional de Cuyo. En prensa. ISSN (en línea) 1853-8665.
Original article
Biofungicide
formulation based on Bacillus velezensis EM-A8 for control of maize
foliar diseases
Formulación
de biofungicida a base de Bacillus velezensis EM-A8 para el control de
enfermedades foliares en maíz
Daiana García1,
María Fiamma Grossi Vanacore2,
Aluminé Fessia1,
Andrea Nesci1
1 Universidad Nacional de Río Cuarto. Facultad de Ciencias
Exactas, Físico-Químicas y Naturales. Departamento de Microbiología e
Inmunología. Laboratorio de Ecología Microbiana. Consejo Nacional de
Investigaciones Científicas y Técnicas (CONICET). Ruta 36 km 601. Río Cuarto
5800. Córdoba. Argentina.
2
Universidad Nacional de Río Cuarto. Facultad de Ciencias Exactas,
Físico-Químicas y Naturales. Departamento de Microbiología e Inmunología. PHD
Student Laboratorio de Ecología Microbiana.
* msartori@exa.unrc.edu.ar
Abstract
The aim was to
evaluate inoculum production of Bacillus velezensis EM-A8, a native
bacterium of maize phyllosphere, antagonist to foliar pathogens Exserohilum
turcicum and Puccinia sorghi. Six economic media were tested: 1)
Nutrient Broth (8 g.L-1);
2) Whole soybean flour (40 g.L-1)
+ sucrose (20 g.L-1);
3) Whole soybean flour (10 g.L-1)
+ molasses (20 g.L-1),
4) Whole soybean flour (40 g.L-1)
+ molasses (10 g.L-1);
5) Yeast extract (10 g.L-1)
+ molasses (5 g.L-1)
and 6) Yeast extract (10 g.L-1)
+ sucrose (5 g.L-1).
Growth was determined spectrophotometrically at 620 nm and viability was
estimated. M2 showed the shortest generation time (g 1.22 h). M3 and M5
were selected for efficiency and aW was
modified with glycerol at 0.97. Inoculums were stored under refrigeration (5°C)
and room temperature (20-25°C) for 8 months. At 5°C the viability of the
antagonist at 3.3-4.5 log CFU ml-1 was significantly lower
than at room temperature (6-6.8 log CFU ml-1),
where M5 showed the highest stability. The bioformulation of B. velezensis EM-A8
in M5 at aW 0.97 and stored at room
temperature will allow successful control of maize foliar diseases.
Keywords: biological control,
formulation, growth medium, Northern leaf blight
Resumen
El objetivo fue
evaluar la producción de inóculo de Bacillus velezensis EM-A8, bacteria
nativa de la filosfera de maíz, antagonista de los patógenos foliares Exserohilum
turcicum y Puccinia sorghi. Se probaron seis medios económicos: M1)
Caldo Nutritivo (8 g.L-1);
M2) Harina integral de soja (40 g.L-1)
+ sacarosa (20 g.L-1);
M3) Harina integral de soja (10 g.L-1)
+ melaza (20 g.L-1),
M4) Harina integral de soja (40 g.L-1)
+ melaza (10 g.L-1);
M5) Extracto de levadura (10 g.L-1)
+ melaza (5 g.L-1); M6) Extracto de
levadura (10 g.L-1) + sacarosa (5 g.L-1). El crecimiento
se determinó espectrofotométricamente a 620 nm y se estimó la viabilidad. M2
mostró el menor tiempo de generación (g 1.22 h). Por eficiencia se
seleccionaron M3 y M5. Se modificó aW a
0,97 con glicerol. Los inóculos se almacenaron en refrigeración (5°C) y a
temperatura ambiente (20-25°C), durante 8 meses. A 5°C la viabilidad del
antagonista se mantuvo en 3.3-4.5 log UFC ml-1, significativamente menor que a temperatura ambiente (6-6.8 log
UFC ml-1), donde M5 mostró
la mayor estabilidad. El bioformulado de B. velezensis EM-A8 en M5, aW
0,97 y almacenado a temperatura ambiente, nos permitirá avanzar
en el control exitoso de enfermedades foliares en maíz.
Palabras claves: control biológico,
formulación, medio de crecimiento, tizón foliar del norte
Originales:
Recepción: 29/05/2024 - Aceptación: 03/12/2024
Introduction
Currently, there is
a strong need for environment, human and animal health-friendly alternatives to
chemical compounds in agriculture. For this, previous studies in our lab
selected epiphytic bacteria of maize phyllosphere with antagonist capacity
against the foliar diseases Northern leaf blight produced by Exserohilum
turcicum and Common rust produced by Puccinia sorghi (15,
17). Foliar applications on maize plants controlled both diseases (17). Additionally, we
determined mechanisms of action and tolerance to environmental stress (18) and in vitro biofilm
formation of the biocontrol agent (9).
Biological control
is defined as the reduction of a pathogenic agent that causes a disease,
through the action of live microorganisms. Practically, this control uses
antagonist agents generally isolated from fruit or plant surfaces. These
microorganisms, when stimulated in situ or artificially reintroduced
into environments with the pathogens to be controlled, reduce or suppress
disease development (2). A successful biocontrol
product depends on the formulation process of a product composed of a
biocontrol agent and ingredients to improve survival and product effectiveness (23). The formulation
process can affect biocontrol efficiency, extend life, ease handling, raise
compatibility with agricultural equipment and practices, and lower production
costs. Formulations with high density of microorganisms and greater survival
during storage are key for effective biocontrol development. For a biological
product to be competitively commercialised, its shelf life should last from 6
months to a year (11, 23). Storage and
packaging conditions affect product viability. Temperature modulates bacterial
survival during storage (23).
The development of
an economic culture medium allowing a large amount of a microbial agent at a
low price must maintain control efficacy (23,
26). Low-cost medium components must provide enough energy for
biosynthesis and cell maintenance (20). Numerous
amendments have been utilised in experimental and commercial formulations of Bacillus
and other biocontrol agents. Generally, amendments can be grouped as either
carriers (fillers, extenders) or those improving chemical, physical, or
nutritional properties (19). On the other
hand, waste products from soy-based food processing are considered excellent
substrates for industrial production of beneficial bacteria and metabolites.
Soy waste provides high percentages of proteins (40%), carbohydrates (35%),
vitamins and minerals. Soy by-products such as defatted flours are used for
industrial production of beneficial bacteria and their metabolites including Bacillus
subtilis (13, 23).
Also, molasses is a
by-product of sugar cane and sugar beet. Given its low cost and contents of
sugars (sucrose in 50%), nitrogen, B vitamins and minerals like iron,
phosphorus, potassium, zinc, sodium, copper and magnesium, molasses constitutes
one of the most used carbon sources for industrial production of microorganisms
(23,
27). Medium growths with molasses and other nitrogen sources
provide the nutrients and energy necessary for a rapid cellular increase and
maintenance (14, 26). Various nitrogen
sources such as peptone, significantly affect the production of antifungal
substances in B. subtilis (21, 23). Microorganisms
for biocontrol are subjected to low aw causing
water stress and synthesis of compatible solutes like polyhydroxyalcohols,
carbohydrates or amino acids (23).
Formulation of
microbial products can be liquid (aqueous suspensions and flowables) or dry
(wettable powders, dust and granules) (19). Lyophilization
can maintain viability over twenty years and under no special temperature
conditions (10, 25). However, post-lyophilization
viability varies depending on numerous factors. This study aimed to obtain an
efficient and low-cost formulation of Bacillus velezensis EM-A8, able to
maintain high cell viability during storage, and evaluating different
conditions. This in vitro study would provide a reliable basis for
further industrial scaling.
Materials
and methods
Biocontrol
agent
Antagonist bacteria
were isolated from maize leaves with disease lesions from fields of different
sites in Córdoba province, Argentina. Antagonistic ability was evaluated in
vitro and potential control agents were selected (15). These isolates
were identified and deposited in the culture collection of the Department of
Microbiology and Immunology, Universidad Nacional de Río Cuarto. B.
velezensis EM-A8 Genbank accession number KY694464.1 was selected as the
more effective strain in biological control of leaf blight caused by E.
turcicum and common rust caused by P. sorghi (16,
17, 18).
Inoculum
preparation
B. velezensis EM-A8 inoculum was
prepared from cultures grown on nutrient broth (NB) for 24 h at 140 rev min-1
and 25°C up to the exponential phase. Serial dilutions were
performed and plated on nutrient agar (NA) to evaluate cell viability and count
of colony-forming units per ml (CFU ml-1).
Growth
media and culture conditions
The following
low-cost media were used to increase viable cells of B. velezensis EM-A8:
1) Nutrient Broth
(8 g L-1) (control)
(M1).
2) Whole soy flour
(40 g L-1) + sucrose
(20 g L-1) (26) (M2).
3) Whole soy flour
(10 g L-1) +
molasses (20 g L-1)
(14) (M3).
4) Whole soy flour
(40 g L-1) +
molasses (10 g L-1)
(26) (M4).
5) Yeast extract
(10 g L-1) +
molasses (5 g L-1)
(26) (M5).
6) Yeast extract
(10 g L-1) + sucrose
(5 g L-1) (6) (M6).
All media were
prepared in 250 ml flasks with 50 ml of each tested medium with 0.97 water
activity (aw)
adjusted by glycerol addition (7) and autoclaved.
Flasks were inoculated with 100 μl of fresh inoculum suspension of B.
velezensis EM-A8, with an initial inoculum of 3 x 108 CFU ml-1 and incubated at 25°C
under orbital agitation at 140 rev min-1 for 32 h.
Growth in each treatment was determined spectrophotometrically
at 620 nm. Cell viability was estimated using the surface-plated method at 5,
9, 24 and 32 hours. For this, serial decimal dilutions until 10-9 were performed in NB. An
aliquot of 0.1 ml of each dilution was spread on the surface of NA. Plates were
incubated in darkness at 25°C for 24 h. Total number of viable bacteria was
expressed as mean colony-forming units developed per ml of medium (CFU ml-1). The experiments
were replicated three times for each treatment and the assay was repeated
twice. Growth parameters g (generation time) and k (constant
growth rate) were calculated by linear regression of the exponential growth
phase.
Viability
of B. velezensis EM-A8 at two different storage conditions
The viability
evaluation of two storage conditions was carried out for two media (M3 and M5).
For this, 250 ml of each media inoculated with B. velezensis EM-A8 were
stored at room temperature (20-25°C) and in cool storage (5°C) for 8 months
(240 days). Antagonist viability was determined by plate count at different
times. Sample dilutions were made in NB and spread-plated onto NA. Plates were
incubated at 25°C for 24 h and the viable count was expressed as colony-forming
units per ml (CFU ml-1).
The experiments were conducted in triplicates.
Viability
of B. velezensis EM-A8 under lactose-added
The effect of
adding a post-incubation protector such as lactose was evaluated for the same
media. For this assay, M1 was used as control and M3 and M5 were supplemented
with 5% lactose post-incubation of B. velezensis EM-A8. Media
were stored at room temperature and cool storage. Bacterial viability was
monitored after 5 months of storage (150 days) and estimated using a
surface-plated method, as previously mentioned.
Lyophilized
formulation
Since the addition
of glycerol does not allow freeze-drying, flasks with M5 and not modified aW, were inoculated
with a suspension of 0.1 ml of B. velezensis EM-A8, incubated in an
orbital shaker at 140 rev min-1 and 25°C for 24 h the
number of colony forming units per millilitre (CFU ml-1)
was calculated. In order to protect cells from temperature, 5% lactose was
added before lyophilization (1). The conical
flasks containing inoculum were frozen directly at -20°C for 12 h and then at
-80°C for 4 h. These were then connected to a freeze-dryer operating at a
chamber pressure of 50.05 mbar and -45°C for 48 h.
A week later,
viability of lyophilized M5 was determined. For this, 0.2 g and 1 g were
rehydrated with 9 ml of NB, incubated for 1 h at room temperature and
homogenised with a vortex mixer. Serial dilutions were spread-plated on the
surface of NA plates. These plates were incubated at 25°C for 24 h and viable
populations were counted. Survival levels were expressed as number of CFU ml-1
(15). The experiments
were conducted in triplicates.
Statistical
analysis
ANOVA test was made
for growth parameters and viable counts using InfoStat version 2012 (8). Means were
compared according to the DGC test (p > 0.05).
Results
B.
velezensis EM-A8 growth on low-cost media
Growth data at
different media and time incubation were analysed by ANOVA. All media provided
high B. velezensis EM-A8 growth. After 9 h of incubation, no significant
differences (p<0.05) were observed between media and time. The lowest count
(4 log CFU ml-1)
was determined at 5 h. M5 presented a significantly higher count (f: 2,22; gl:
5; p-value 0,1192).
Significant
differences were observed for B. velezensis EM-A8 level between culture
media at 24 h (f: 40.3; gl:5: p-value <0.0001) and
32 h of incubation (f: 12.95; gl: 5; p-value 0.0002). Growth was significantly
higher in M2, M3, M4 and M5 with similar counts (10.4-10.7 log CFU ml-1), while the
lowest count corresponded to control media (Ml) and M6 (9.3-9.7 log CFU ml-1) (figure 1).
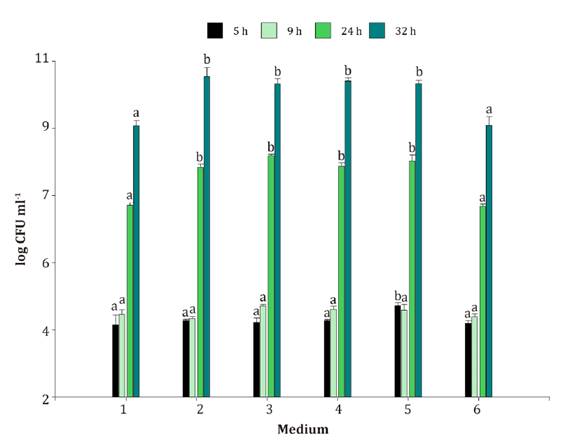
Datos
con la misma letra no son significativamente diferentes para cada medio a
diferentes tiempos de incubación según el test DGC (P<0,05).
Figure
1. Incubation of B. velezensis EM-A8 in
different low-cost media for 32 h.
Figura
1. Incubación de B. velezensis EM-A8
en diferentes medios de bajo costo durante 32 h.
Growth parameters
significantly differed among mediums (table 1). B. velezensis
EM-A8 had the shortest generation time (g) in M2, followed by M3,
M4, M5, M6 and M1 (control). Besides, the growth rate constant (k) was
greater for M2, M3 and M4 with an average value of 0.55 and no significant
differences among the three media (p<0.05). However, M1, M5 and M6 showed
significant differences for k.
Table 1. Influence
of growth media on growth parameters of B. velezensis EM-A8.
Tabla
1. Influencia de los medios de
crecimiento en los parámetros de crecimiento de B. velezensis EM-A8.
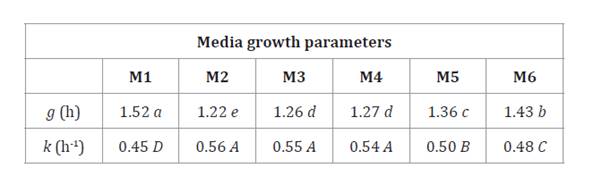
g: generation time. k: constant growth rate. Data with the same letter are not
significantly different according to the DGC test (P<0.05).
g: tiempo de generación. k: constante de la velocidad de crecimiento. Los
datos en una misma letra no son significativamente diferentes según la prueba
DGC (P<0,05).
Culture media selection discarded M2 for its high soy flour
content (40 g L-1)
and considering that M3 showed comparable growth with less soy flour content
(10 g L-1). We also
selected M5 for growth parameters obtained in a very different media
composition (yeast extract and molasses).
Viability
of B. velezensis EM-A8 in storage
Figure 2, shows B.
velezensis EM-A8 viability in M1, M3 and M5 under two different storage
conditions. After 240 days of storage, viability was significantly lower in the
three-growth media stored under refrigeration (5°C), showing a count average of
3.3-4.5 log CFU ml-1,
while viability in growth media stored at room temperature (20°C) was 6-6.8 log
CFU ml-1. Growth
media 5 showed smaller variations in B. velezensis EM-A8 counts
throughout storage time at room temperature.
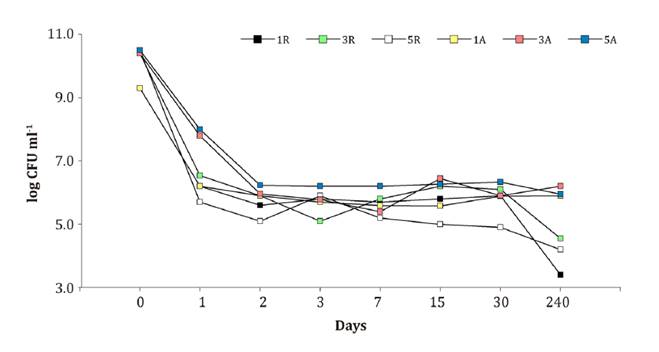
1R (M1
con refrigeración), 3R (M3 con refrigeración), 5R (M5 con refrigeración), 1A
(M1 temperatura ambiente), 3A (M3 temperatura ambiente), 5A (M5 temperatura
ambiente).
Figure
2. B. velezensis EM-A8
viability (CFU ml-1)
during storage with refrigeration (R) and room temperature (A) for 240 days.
Media 1, 3 y 5.
Figura
2. Viabilidad de B. velezensis EM-A8
(UFC ml-1)
durante el almacenamiento con refrigeración (R) y temperatura ambiente (A)
durante 240 días. Medios 1. 3 y 5.
Viability
of B. velezensis EM-A8 stored with added lactose
Lactose effect of B. velezensis EM-A8 viability during
storage was determined in M5 at 150 days of incubation. Initial strain
population was approximately 10 log (figure 3). However, after
incubation, the population decreased to 4-4,5 log CFU ml-1 under refrigerated
conditions with and without added lactose; and at room temperature at the end
of the experiment, decreasing from 10 to 6 log CFU ml-1 with and without lactose
addition.
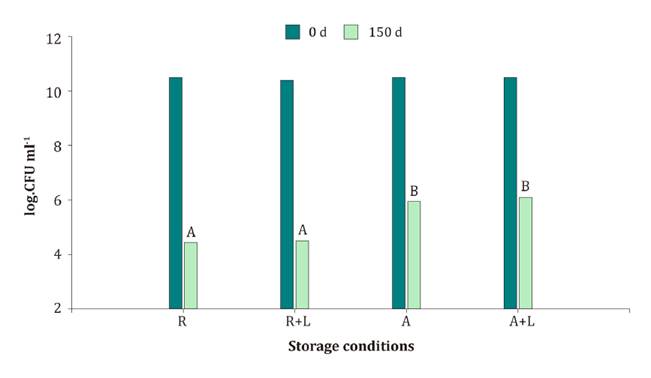
Cada
barra representa el promedio de tres replicaciones independientes y letras
diferentes indican diferencias significativas (P>0,05) según la prueba DGC.
Figure
3. Viability of B. velezensis EM-A8 (CFU ml-1)
during storage under refrigeration (R) and room temperature (A), with and
without lactose, at zero and 150 days.
Figura
3. Viabilidad de B. velezensis EM-A8
(UFC ml-1)
durante el almacenamiento en refrigeración (R) y temperatura ambiente (A), con
adición de lactosa y sin lactosa, a cero y a los 150 días después.
Lyophilized
formulation
The initial inoculum of B. velezensis EM-A8, in M3 and M5
showed 5.6 x 109 CFU ml-1 before freezing. After
lyophilization, each gram of rehydrated powder had 2 x 106 CFU ml-1,
indicating a significant loss of B. velezensis EM-A8 viability.
Furthermore, the volume of liquid formulation to obtain one gram of lyophilized
powder was excessive and impractical.
Discussion
The biological
control of plant pathogens is an effective and eco-friendly method to manage
diseases, preserving nature and human health from chemical pesticides (1). Biofungicides
curb and control fungal plant pathogens by inoculating microbiota in or onto
the plant. Several complex interactions among pathogen-biocontrol agents,
biocontrol agent-plant, and environment play a significant role in disease
control (3). In this sense, formulation
determines biofungicide efficacy. Our study showed that commercial products and
by-products in different media provide high growth and shelf life of B.
velezensis EM-A8. The chosen sources of nitrogen and carbon (yeast
extract, whole soy flour, sucrose, molasses) are widely described for producing
beneficial microorganisms, including B. velezensis EM-A8 (6,
23). After one day of incubation in the studied media, cell count
was 9 log. For M2, M3, M4 and M5, cell count increased by one logarithm after
32 hours, showing that B. velezensis EM-A8 population increased in one
day of incubation and all media.
On the other hand,
growth rate of B. velezensis EM-A8 was significantly different between
M5 and M6, being higher with molasses as sugar source. Molasses concentration
did not negatively affect B. velezensis EM-A8 growth. M3 and M4 show
similar growth, even with twice the concentration of molasses in M3. However,
some authors observed that high concentrations of molasses (20 and 40 g L-1) did not support B.
subtilis CPA-8 growth (26). Similar results
were found by Costa
et al. (2001) for P. agglomerans CPA-2, where increased molasses
concentration (40 g L-1)
did not improve production, probably given by high toxic concentrations (6).
Regarding whole soy
flour concentration, B. velezensis EM-A8 showed similar growth in M2 and
M4 with 40 g L-1 and M3 with 10 g L-1
suggesting no influence over growth. The high concentrations of B.
velezensis EM-A8 obtained in the whole soy flour media compared to yeast
extract media showed that this carbon source promotes bacterial growth
influenced by the capacity of microorganisms to use available nitrogen, similar
to what Costa
et al. (2002) and Yáñez-Mendizábal et al. (2012) found. However,
media with whole soy flour became opaque, with lumps and debris from soy seed,
complicating viability controls. On the contrary, selected media with yeast
extract as nitrogen source were translucent and without lumps.
Storage temperature
and media composition significantly affected B. velezensis EM-A8
viability, which markedly decreased in both storage conditions. After two days,
the count was 4 log less than initial count. However, in both growth media (3
and 5), as well as in the control, viability remained stable throughout 8
months of storage. In all media B. velezensis EM-A8 cell viability
showed significant difference in cold storage. In M3 and M5, viability
decreased to 4 log while in the control, it decreased to 3 log. In contrast, Yáñez-Mendizabal
et al. (2012) showed that all formulations of B. subtilis CPA-8 during
6 months of storage in cold and at room temperature had the same behaviour,
viable cells maintained or slightly decreased around 0.2 - 0.3 log.
Contrarily, other
authors demonstrated greater stability and higher microbial survival in
cold-stored samples compared to those stored at room temperature (4). Moreover, it was
observed that in-storage protective lactose failed to reverse viability loss of
B. velezensis EM-A8, both in refrigeration and at room temperature.
Lactose additions did not reverse viability loss, unlike previously seen (23). On the other
hand, unlike other investigations (6, 10, 25), lyophilization
was discarded as conservation and storage method given the amount of powder
needed for later foliar application.
The viability loss of the microorganisms after the formulation
and during the storage and distribution is a regular problem (23). All three liquid
formulations stored at room temperature maintained stable viability in 6 log
for 8 months. Practically, this finding determines such a convenient storage
condition as room temperature is preferable to refrigerated storage.
Formulation, production and stabilization of the biocontrol agent determines
the final efficacy of a Bacillus-based product (19). An ideal
formulation is not toxic to the host plant, easy to handle, has long shelf
life, is compatible with other agrochemicals, cost-effective and stable.
Formulations should work under different environmental conditions, providing
reliable control of plant diseases (12). Moreover, the
ability of biocontrol agents to control foliar diseases is largely affected by
environmental fluctuation (12). B. velezensis EM-A8
is native to the phyllosphere with the advantage of being adapted to such
conditions.
Agroecological
management strategies would not only help in optimal recycling of nutrients and
organic matter turnover, closed energy flows, and water and soil conservation
but also would help in balancing pest-natural enemy populations (24). Diverse
biocontrol agents have successfully contributed to the sustainable management
of phytopathogens and various foliar diseases can be effectively controlled by
spray application of bacterial and fungal antagonists. Bacillus strains
are gaining enormous attention due to their ability to effectively cause
disease suppression (1).
Conclusions
The
liquid formulation developed from B. velezensis EM-A8 in growth medium
with yeast extract and molasses, modified aW with glycerol at 0.97 and
stored at room temperature is a promising product and an important step towards
the successful control of foliar diseases in maize.
Acknowledgements
This
study was financed by Agencia Nacional de Promoción Científica y Tecnológica
(ANPCYT), FONCYT-PICT 4220/18 y PIP 2022-2024 161CO
1.
Babbal, A.; Khasa, Y. 2017. Microbes as biocontrol agents. In: Probiotics and
Plant Health. Kumas, V. (Eds). Springer. 507-551.
2.
Barkai-Golan, R. 2001. Postharvest diseases of fruits and vegetables.
Development and control. Elsevier, Amsterdam, The Netherlands.
3.
Bonaterra, A.; Badosa, E.; Daranas, N.; Francés, J.; Roselló, G.; Montesinos,
E. 2022. Bacteria as biological control agents of plant diseases.
Microorganisms. 10: 1759.
4.
Boza, Y.; Barbin, D.; Scamparini, A. 2004. Effect of spray drying on the quality
of encapsulated cells of Beijerinckia sp. Process Biochemistry. 39:
1275-1284.
5.
Costa, E.; Teixidó, N.; Usall, J.; Atarés, E.; Viñas, I. 2001. Production of
the biocontrol agent Pantoea agglomerans strain CPA-2 using commercial
products and byproducts. Applied Microbiology and Biotechnology. 56: 367-371.
6.
Costa, E.; Teixidó, N.; Usall, J.; Atarés, E.; Viñas, I. 2002. The effect of
nitrogen and carbon sources on growth of the biocontrol agent Pantoea
agglomerans strain CPA-2. Letters in Applied Microbiology. 35: 117-120.
7.
Dallyl, H.; Fox, A. 1980. Spoilage of material of reduced water activity by
xerophilic fungi. Technical Series 15. London: Academic Press. 219-239.
8.
Di Rienzo, J.; Casanoves, F.; Balzarini, M.; Gonzalez, L.; Tablada, M.; Robledo,
C. 2012. InfoStat versión. Argentina: GrupoInfoStat, FCA, Universidad Nacional
de Córdoba. http://www.infostat. com.ar
9.
Fessia, A.; Sartori, M.; García, D.; Fernández, L.; Ponzio, R.; Barros, G.;
Nesci, A. 2022. In vitro studies of biofilm-forming Bacillus strains,
biocontrol agents isolated from the maize phyllosphere. Biofilm 4, 100097.
10.
Fonseca, F.; Cenard, S.; Passot, S. 2015. Freeze-drying of lactic acid
bacteria. In: Cryopreservation and Freeze-Drying Protocols. Methods in
Molecular Biology, vol 1257. Wolkers, W.; Oldenhof, H. (eds) Springer, New
York,
11.
Kinay, P.; Yildiz, M. 2008. The shelf life and effectiveness of granular
formulations of Metschnikowia pulcherrima and Pichia guilliermondii yeast
isolates that control postharvest decay of citrus fruit. Biological Control.
45: 433-440.
12.
Kumar, S.; Thakur, M.; Rani, A. 2014. Trichoderma: Mass production,
formulation, quality control, delivery and its scope in commercialization in
India for the management of plant diseases. African Journal of Agricultural
Research. 9(53): 3838-3852.
13.
Reis, F; Servulo, E.; De Franca, F. 2004. Lipopeptide surfactant production by Bacillus
subtilis grown on low-cost raw materials. Applied Biochemistry and
Biotechnology. 113: 899-912.
14.
Sartori, M.; Nesci, A.; Etcheverry, M. 2012. Production of Fusarium
verticillioides biocontrol agents, Bacillus amyloliquefaciens and Microbacterium
oleovorans, using different growth media: evaluation of biomass and
viability after freeze-drying. Food Additives and Contaminants (A). 29(2):
287-292.
15.
Sartori, M.; Nesci, A.; Formento, A.; Etcheverry, M. 2015. Selection of
potential biological control of Exserohilum turcicum with epiphytic
microorganisms from maize. Revista Argentina de Microbiología. 47(1): 62-71.
16.
Sartori, M.; Nesci, A.; Garcia, J.; Passone, M.; Montemarani, A.; Etcheverry M.
2017a. Efficacy of epiphytic bacteria to prevent northern leaf blight caused by
Exserohilum turcicum in maize. Revista Argentina de Microbiología.
49(1): 75-82.
17. Sartori, M.;
Nesci, A.; Montemarani, A.; Barros, G.; Garcia, J.; Etcheverry M. 2017b.
Preliminary evaluation of biocontrol agents against maize pathogens Exserohilum
turcicum and Puccinia sorghi in field assays. Agricultural Sciences.
8: 1003-1013.
18.
Sartori, M.; Bonacci, M.; Barra, P.; Fessia, A.; Etcheverry, M.; Nesci, A.;
Barros, G. 2020. Studies on possible modes of action and tolerance to
environmental stress conditions of different biocontrol agents of foliar
diseases in maize. Agricultural Sciences. 11: 552-566.
19.
Schisler, D.; Slininger, P.; Behle, R.; Jackson, M. 2004. Formulation of Bacillus
spp. for biological control of plant diseases.
Phytopathology. 94: 1267-1271.
20.
Stanbury, P.; Whitaker, A.; Hall, S. 1995. Media for industrial fermentations.
In: Principles of fermentation technology. Pergamon Press, Oxford. 93-121.
21.
Tabbene, O.; Slimene, I.; Djebali, K.; Mangoni, M.; Urdaci M.; Limam F. 2009.
Optimization of medium composition for the production of antimicrobial activity
by Bacillus subtilis B38. Biotechnology Progress. 25: 1266-1274.
22.
Teixidó, N.; Cañamás, T. P.; Abadias, M.; Usall, J; Solsona, C.; Casals, C.;
Viñas, I. 2006. Improving low water activity and desiccation tolerance of the
biocontrol agent Pantoea agglomerans CPA- 2 by osmotic treatments.
Journal of Applied Microbiology. 101: 927-937.
23.
Teixidó, N.; Usall, J.; Torres, R. 2022. Insight into a Successful development
of biocontrol agents: production, formulation, packaging and shelf life as key
aspects. Horticulturae. 8: 305.
24.
Vandemeer, J. 1995. The ecological basis of alternative agriculture. Annual
review of Ecology and Systematics. 26: 201-224.
25.
Vermelho, A.; Moreira, J.; Junior, A.; da Silva, C.; Cardoso, V.; Akamine, I.
2024. Microbial preservation and contamination control in the baking industry.
Fermentation. 10: 231. doi.org/10.3390/ fermentation10050231
26.
Yánez-Mendizábal, V.; Viñas, I.; Usall, J.; Torres, R.; Solsona, C.; Teixidó,
N. 2012. Production of the postharvest biocontrol agent Bacillus subtilis CPA-8
using low-cost commercial products and by-products. Biological control. 60:
280-289.
27. Younis,
M.; Hezayen, F.; Nour-Eldein, M.; Shabeb, M. 2010. Optimization of cultivation
medium and growth conditions for Bacillus subtilis KO strain isolated from
sugar cane molasses. American Eurasian Journal of Agricultural Environmental
Science. 7: 31-37.