Revista de la Facultad de Ciencias
Agrarias. Universidad Nacional de Cuyo. En prensa. ISSN (en línea) 1853-8665.
Original article
Yield
and development of winter and spring rapeseed (Brassica napus L.) at
different sowing dates in temperate environments
Desarrollo
y rendimiento de colza (Brassica napus L.) invernal y primaveral ante
distintas fechas de siembra en ambientes templados
Miguel Pereyra
Iraola1,
Mateo Zubiri1,
José Luis Bodega1,
María Luján Nagore1,
Gastón Darwich1,
1Universidad Nacional de Mar del Plata. Facultad de Ciencias Agrarias.
Ruta 226. km 73.5. Balcarce. C. P. 7620. Argentina.
* rmartinez@mdp.edu.ar
Abstract
Optimal sowing
dates should match the crop-critical period with favorable conditions. In
rapeseed, growth stages change among spring and winter cultivars. This study
characterized changes in rapeseed phenology with varying sowing dates to
determine critical periods in both winter and spring cultivars. The trial took
place in Balcarce, Argentina, where a winter-type variety and a spring-type
were sown on eight different dates in a randomized complete block design with
three replicates. Phenology was monitored weekly, and yield was evaluated at
the end of the season. Changes in sowing dates and cultivars led to variations
in the timing of critical periods. Considering the experimental conditions, the
optimal sowing window was between April and July for sowing either rapeseed
cultivar. However, the winter variety did not bloom for sowing dates after
July, while the spring variety showed yield reductions due to frosts for sowing
dates before the end of April. Changes in sowing date resulted in differences
in timing and duration of vegetative and reproductive stages, generally leading
to shorter crop cycles. However, in late sowing, winter cultivars lengthened
their life cycle to the point of not reaching flowering during the growing
season.
Keywords: autumn sowing date,
winter sowing date, vernalization, frost
Resumen
La
fecha óptima de siembra debe hacer coincidir el período crítico del cultivo con
condiciones favorables. Existen diferencias en la ubicación de las etapas de
desarrollo de colza entre las variedades primaverales e invernales. El presente
estudio se realizó para caracterizar los cambios en la fenología de la colza
al variar la fecha de siembra para determinar el momento del período crítico en
ambas variedades invernales y primaverales. El ensayo se llevó a cabo en
Balcarce, Argentina, donde se sembraron una variedad tipo invernal y otra de
tipo primaveral en ocho fechas diferentes. Se utilizó un diseño de bloques
completos al azar con tres repeticiones y se monitoreó la fenología semanalmente,
además de evaluar el rendimiento de cada tratamiento al final del ciclo. El
período crítico se desplazó a diferentes períodos dependiendo de la fecha de
siembra y la variedad. Bajo las condiciones experimentales, hubo una ventana
ambiental óptima entre abril y julio para sembrar, tanto la variedad primaveral
como la invernal. Sin embargo, la variedad invernal no floreció en siembras
posteriores a julio, y la variedad primaveral experimentó reducciones en el
rendimiento debido a las heladas en siembras antes del fin de abril. Las
variedades ante las diferentes fechas de siembra presentaron diferencias tanto
en la ubicación como en la duración de las etapas vegetativas y reproductivas,
tendiendo a acortar su ciclo. Sin embargo, en las siembras más tardías, la
variedad invernal alargó su ciclo hasta el punto de no alcanzar la floración
durante la temporada de crecimiento.
Palabras
clave: fecha de siembra otoñal, fecha de siembra invernal,
vernalización, heladas
Originales: Recepción: 04/06/2024- Aceptación: 18/10/2024
Introduction
Brassica
napus L., commonly known as rapeseed or canola (particularly the
“double-zero” variety with low erucic acid and glucosinolates), holds
substantial economic value, primarily attributed to its edible oil and significance
in bioenergy (18).
Over the last decade, rapeseed has been cultivated on approximately 35 million
hectares globally (38),
with key production regions in the European Union, Asia, and North America (35).
Effective optimization of rapeseed crop productivity depends on selecting the
sowing date and cultivar type. Rapeseed cultivars are categorized into winter
and spring varieties (37).
Winter cultivars require vernalization and are sown in the autumn, with their
development rate being temperature-dependent within specific ranges (5,
10, 21, 32). Winter cultivars are mainly cultivated in areas that meet the
required temperature thresholds, particularly in Northern Europe, the United
States, and China. In contrast to winter cultivars, spring cultivars do not
require vernalization and are sown at later dates (16).
Primary production areas for spring cultivars include Canada, with minor
cultivation areas in India and South America at earlier sowing dates (17).
Nevertheless, no significant differences in productivity have been reported
between winter and spring cultivars in environments that fulfill their specific
requirements (31).
However, a wider period of sowing dates could modify cultivar relative performance.
Rapeseed
development has various growth stages, from leaf rosette formation to seed
maturation. Commonly used phenological scales for the study of rapeseed growth
and development are Silvester-Bradley and Makepeace (1984) and Arnoud
(1989).
Temperature plays a primary role in determining the duration of growing stages (12,
34), although rapeseed exhibits a long-day photoperiodic response (26). The number of
leaves is contingent on the length of the vegetative phase, although immature
pods can also perform photosynthetic functions (28). Plants can
develop multiple floral branches, which form pods containing oil-rich seeds (19). The optimal
sowing date is one where the crop critical period matches with the most
favorable environmental conditions. This critical period starts with the onset
of flowering, lasting approximately four weeks, and is a crucial phase during
which pod numbers per plant and flower abortion levels are determined (22). Additionally,
crop physiological status during the first half of seed filling is a key factor
(27). Regardless of the
cycle duration and available water supply, which can affect the productivity of
any crop, rapeseed, in particular, exhibits a high sensitivity to elevated
temperatures during the flowering period (24,
29). Furthermore, the crop remains vulnerable to frost events even
during the seed-filling stage (31). Late sowings are
not recommended because as temperature increases during seed filling, seed oil
content decreases (13, 36).
In temperate environments, grain yield can reach 4000 kg ha-1
under optimal conditions (10, 15). Where winters are
mild, it is feasible to use either early-sown spring genotypes or winter
varieties (11, 16). Nevertheless,
inappropriate sowing dates could potentially shorten the crop cycle for both
types of cultivars (7). The success of
the rapeseed crop depends on choosing a suitable sowing date to avoid delays in
flowering and suboptimal environmental conditions during seed filling. In
temperate regions, winter varieties should have autumn sowing dates, and spring
varieties should have winter sowing dates. However, meteorological and
operational factors could delay the sowing date and compromise the optimal
conditions for crop growth. Therefore, there is a need to evaluate how
different sowing dates could modify the phenology and stages of the rapeseed
crop using both spring and winter cultivars across a wide range of dates.
Moreover, it is crucial to analyze how the management of the sowing date
optimizes crop yield depending on the type of cultivar. This study aimed to
characterize changes in rapeseed phenology by varying sowing dates to determine
the timing of critical periods and yield in both winter and spring cultivars.
Materials and methods
The
experiment was conducted during the 2015/2016 growing season at the
Experimental Research Station in Balcarce, Province of Buenos Aires, Argentina
(37°45’ S- 58°18’ W) 130 meters above sea level. Two rapeseed cultivars were
sown: a winter type (Vectra, from QualityCrops) and a spring type (Bioaureo
2386, from Nuseeds), using an experimental seeder under conventional tillage.
Sowing was conducted at a high density to establish the initial plant stand,
and thinning was performed after crop emergence to achieve the target density
(70 pl/m2). Experimental design consisted of randomized complete
blocks with three replicates, conducted in parallel strips across eight
different sowing dates, corresponding to different days of year (DOY): April
23, 2015 (F1: DOY 113), May 13, 2015 (F2: DOY 141), June 1, 2015 (F3: DOY 152),
June 24, 2015 (F4: DOY 175), July 10, 2015 (F5: DOY 191), August 5, 2015 (F6:
DOY 217), August 27, 2015 (F7: DOY 239), and September 14, 2015 (F8: DOY 257).
Each experimental unit had seven furrows 5 m long with 0.21 m distance between
furrows, with the total surface area of each experimental unit being 7.35 m2.
Throughout
the crop development, phenology was closely monitored twice a week, with a
specific focus on the rosette stages with 4 (B4) to 6 (B6) developed leaves,
the onset of stem elongation (D1), flowering (F1), and physiological maturity
(MF), following the European phenological classification INRA-CETIOM (4).
Each growth stage was considered to start when 50% of the plants showed their
specific characteristics. Thermal sum calculations were calculated using a base
temperature of 0°C (21).
Meteorological data, including air temperature, incident radiation,
precipitation, and potential evapotranspiration, were obtained from a
meteorological station located approximately 500 meters from the experimental
site. To estimate the water deficit throughout the crop growing season, a water
balance was conducted using potential evapotranspiration (ETP) calculated
through the Penman-Monteith method (2).
The
trial was monitored weekly to ensure disease and weed control. In addition to
phenology tracking, the yield of each treatment was determined. At the end of
the growing season, harvesting was conducted by collecting 1-meter from each of
the three central rows within the plots. Harvested seeds were then processed
using a stationary thresher, and their weight was recorded to calculate the
hectare yield based on the harvested area.
All the variables were analyzed using the statistical software
INFOSTAT (8). An analysis of variance was
performed, and the Fisher Least Significant Difference (LSD) test for mean
comparison was used, with a significance level of P < 0.05.
Results and discussion
Environmental conditions
Rainfall was comparatively low during June, September, and
December. However, there was a soil profile recharge in August. The water
balance analyzed the relationship between rainfall and potential
evapotranspiration (figure
1).

Las
precipitaciones acumuladas al mes en columnas azules, la demanda atmosférica en
forma de evapotranspiración potencial (ETP) en columnas naranjas y el saldo resultante
de su diferencia en columnas celestes.
Figure 1.
Water balance during the 2015-16 season for Balcarce, in the Buenos Aires
province, Argentina.
Figura
1. Balance hídrico durante la temporada 2015-16 para Balcarce,
provincia de Buenos Aires, Argentina.
Mild water deficits were consequently recorded in May, with
moderate deficits in September and November, and severe deficits persisting in
December. Water deficits may affect flowering and later grain filling under
some sowing dates.
Crop development
As
the sowing date was delayed, the average length of the crop cycles became
shorter (figure
2A).

2B Number
of days per decade with minimum temperatures below 3 (blue), 7 (light blue),
and 9°C (red) and photothermal quotient (dashed green) for the year 2015.
2B Número
de días por década con temperaturas mínimas inferiores a 3 (azul), 7 (azul
claro) y 9°C (rojo) y cociente fototérmico (verde discontinuo) para el año
2015.
Figure
2A. Duration of the stages Sowing-6 leaves (S-B6 in
red), 6 leaves-to inflorescence period (B6-D1 in green), appearance of
inflorescence-beginning of flowering (D1-F1 in purple) and flowering -
physiological maturity (F1-MF in light blue) expressed in days of the year for
two cultivars analyzed and eight sowing dates evaluated.
Figura 2A. Duración
de las etapas Siembra - 6 hojas (S-B6 en rojo), 6 hojas - aparición de la
inflorescencia (B6-D1 en verde), aparición de la inflorescencia - inicio de
floración (D1-F1 en morado) y floración - madurez fisiológica ( F1-MF en celeste) expresado en días del año para los dos
cultivares analizados y las ocho fechas de siembra evaluadas.
The
winter cultivar generally exhibited a longer cycle than the spring cultivar,
but after the sixth sowing date, the winter cultivar did not complete its
cycle. Critical growth periods occurred at different times when changing the
sowing date and between the cultivars. These results align with previous
studies from the 2008/2009 season in Paraná, where the cultivars exhibited different
responses due to the delayed sowing dates (7).
From the fifth sowing date onward, the growth cycle of the
winter cultivar changed from shortening to extending because the vernalization
requirements were not satisfied. Vernalization-requiring cultivars increase
their development rate as temperature accumulates from 4°C to 9°C. From the
250th day of the year (coinciding with the emergence of the winter cultivar
sown on the sixth sowing date), a noticeable decrease in the number of days
with minimum temperatures below 7 and 9°C was observed (figure 2B). Consequently,
the winter variety sown after July did not flower by the time of harvest of the
other treatments.
The duration from emergence to flowering period expressed in
days presented a significant interaction between the sowing date and cultivar
type (p ≤ 0.0001). For the spring cultivar, the vegetative period was reduced
for seven of the eight sowing dates because of delayed sowing (figure 3A).
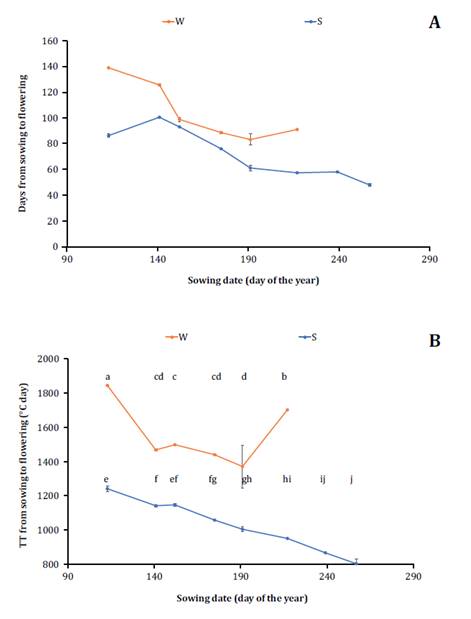
Cada
punto representa la fecha promedio de las tres repeticiones. Letras diferentes
indican diferencias significativas entre las medias de los tratamientos.
Figure
3.
Duration of the Sowing-Flowering period expressed in days 3A and in
thermal time 3B for two cultivars analyzed (winter in red and spring in
blue) and eight sowing dates evaluated expressed according to the day of the
year in which sowing took place.
Figura
3. Duración del período siembra - floración expresado en días 3A
y expresado en unidades de tiempo térmico 3B para los dos cultivares
analizados (invernal en rojo y primaveral en azul) y las ocho fechas de siembra
evaluadas expresadas según el día del año en que se realizaron.
The winter cultivar also tended to shorten its vegetative period
when the sowing date was delayed, up to its fifth sowing date (corresponding to
July). From the seventh sowing date onward, the winter cultivar did not flower
before the harvest date of the other treatments. These results partially align
with results obtained by Takashima et al. (2013), who assessed a
narrower range of sowing dates. Furthermore, the winter cultivar accumulated a
higher thermal sum for the emergence-flowering period (p ≤ 0.0001) than the
spring cultivar on all sowing dates. In the spring cultivar, the vegetative
period expressed in thermal units shortened as the sowing date was delayed (figure 3B), whereas the
winter cultivar displayed a similar trend up to the fifth sowing date
(corresponding to July), after which the thermal sum for phenological change
significantly increased.
Shortening the
vegetative period in the spring cultivar would be compatible with a
quantitative photoperiodic effect. In crops with a quantitative response to
long-day photoperiod, increases in day length from June 21st to December 21st
imply reductions in thermal sums required for growth stage change
up to a threshold where the thermal sum is fixed. Since the threshold of
approximately 14 hours proposed by Nanda et al. (1996) is not reached in
the studied sowing dates, inductive photoperiod is not achieved. Hence, the
thermal time to flowering was shortened by gradually delaying the sowing date
due to the increased day length. At sowing dates where the day length does not
exceed 14 hours, changes in day length caused by different sowing dates will be
proportional to changes in thermal sum until flowering. In the winter cultivar,
starting from the fifth sowing date, thermal sums shift from decreasing with
delayed sowing to extending due to their vernalization requirements.
Sensitivity to this factor also varies among cultivars (25), and it is unknown
what the threshold temperature for vernalization of the analyzed cultivar is or
within which period the stimulus is captured. Although cultivars requiring
vernalization have accelerated development rates due to temperature accumulation
between the range of 4°C to 9°C, the upper defined limit is at 13°C (11). Among plants in
which flowering is promoted by the accumulation of cold hours, the effective
temperature range is 1-7°C (21). In previous
studies on rapeseed varieties, the optimal vernalization temperature was
reported within the range of 6-9°C (33). Therefore, sowing
winter cultivars after August 5th in the southeastern
Buenos Aires region will not allow the accumulation of sufficient cold hours to
meet the vernalization requirement for flowering during the growing season.
On the same sowing date, the spring cultivar showed a longer
seed-filling duration than the winter cultivar. The duration from flowering to
physiological maturity, expressed in days, exhibited an interaction between the
sowing date and cultivar type (p ≤ 0.0060). Both cultivars gradually reduced
their filling period duration (figure 4A).
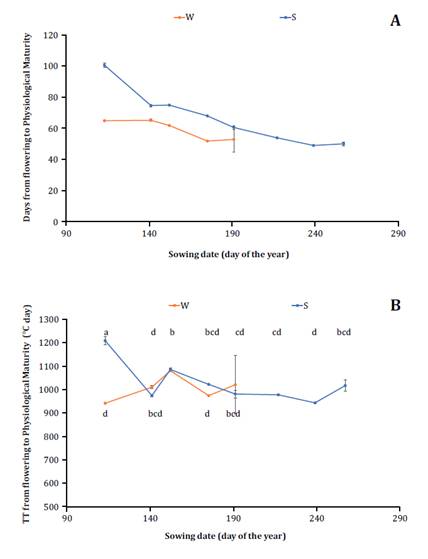
Cada
punto representa la fecha promedio de las tres repeticiones. Letras diferentes
indican diferencias significativas entre las medias de los tratamientos.
Figure
4. Duration of the period from Flowering to
Physiological Maturity expressed in days 4A and in thermal time 4B for
two cultivars analyzed (winter in red lines and spring in blue lines) and eight
sowing dates evaluated expressed according to the day of the year in which were
carried out.
Figura
4. Duración del período de Floración a
Madurez Fisiológica expresado en días 4A y expresado en tiempo térmico 4B
para los dos cultivares analizados (invernal en rojo y primaveral en azul)
y las ocho fechas de siembra evaluadas expresadas según el día del año en que
se realizaron.
Significant differences were determined among the combinations
of sowing date and cultivar for the duration from the flowering to
physiological maturity period expressed in thermal time (p ≤ 0.0013). The
spring cultivar sown in April had the longest seed-filling period, while the
remaining combinations of sowing dates and cultivars did not show significant
differences in the duration of the period expressed as thermal time, with an
approximate value of 1000°C day (figure 4B). The findings suggest that the spring cultivar, when sown on
the earliest date, may prolong its grain-filling period in thermal time because
of occasional frosts during the reproductive phase. This consideration is
important because the spring cultivar flowered 50 days earlier than the winter
cultivar on the same sowing date (figure 2A). The hypothesis regarding the frost effect suggests that the
occurrence of frosts may extend grain filling by inducing senescence in main
branches, which results in greater development of lateral pods and a delay in
crop maturity. It was hypothesized that postponing the sowing date by 20 days
after March 1st would decrease the number
of frost days by one during the critical period (31).
Crop
yield
Sowing dates that
resulted in the highest yields were May (date 2) for winter varieties and the
end of June (date 4) for spring varieties (p ≤ 0.0001). For the experimental
conditions, an optimal sowing timeframe for both types of rapeseed cultivars
was determined to be between late April and July (figure 5).
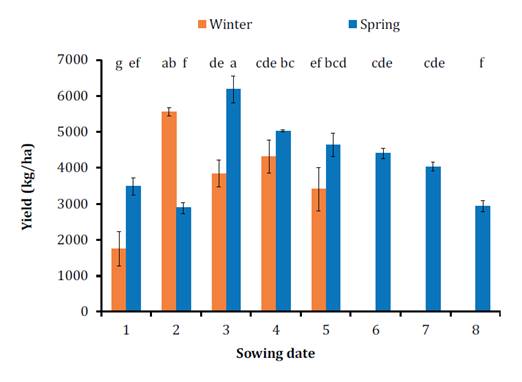
Cada
barra representa el rendimiento promedio de las tres repeticiones y su error
estándar. Letras diferentes indican diferencias significativas entre las medias
de los tratamientos (P<0,05).
Figure
5. Grain yield expressed in kilograms per hectare on a
dry basis for the two cultivars analyzed (winter in orange and spring in blue)
and the eight sowing dates evaluated.
Figura
5. Rendimiento expresado en kilogramos
por hectárea en base seca para los dos cultivares analizados (invernal en
naranja y primaveral en azul) y las ocho fechas de siembra evaluadas.
Notably, the winter
variety failed to flower when sown late, while the spring variety experienced reduced
yields due to early frosts when sown too early. Consequently, the highest
yields were achieved during a specific flowering window between day 270 and 300
of the year, roughly from mid-September to October, with yields exceeding 5000
kg ha-1. These findings
are consistent with prior studies showing increases in grain yields for spring
varieties when sowing dates were delayed (15). However, in later
sowing dates, the highest yield was associated with the shorter-cycle variety,
as observed in a previous study 7. Our findings contrast those previously
reported by Agostini
(2011),
who suggested that environments with longer grain-filling periods would lead to
higher returns. Therefore, studying how different cultivar types respond to
various environmental variables when sowing dates are adjusted becomes crucial
for optimizing critical periods and increasing crop yield (23).
Towards the end of September, the water scenario (figure 1), and photothermal
conditions improve, and frost incidence decreases, making it the optimal time
for the critical period (figure
2B).
During this period, the photothermal quotient (Q) increases, enhancing
radiation capture and allowing for a high accumulation of carbohydrates. The
potential photothermal effect suggests that Q could also be used to explain
changes in yield in rapeseed, as is the case in various species (3,
6, 9). In addition to the photothermal component, post-flowering
rainfall is crucial in rainfed crops and was positively associated with yield
and grain oil content (29, 33). Under our
experimental conditions, water availability was favorable during October,
making it the ideal time for the critical period to take place.
In summary,
variations in sowing dates and cultivar selection influenced the timing of the
critical period. Alterations in sowing dates resulted in shifts in the
flowering date, ranging from day 200 to day 300, thus delaying the flowering
period from June to approximately October. The optimal sowing dates identified
in this research differ slightly from those recommended for no-tillage systems
in the region, which typically occur in March and April. The study was
conducted under conventional tillage in only one season, potentially mitigating
frost damage during crop emergence with sowing dates after May. Winter
cultivars show less flexibility in sowing dates than spring varieties, as they
may not flower in late sowings due to the absence of vernalization conditions.
For the evaluated experimental conditions, the only observed limitation in the
sowing date of spring cultivars is to avoid planting before April due to the
high risk of frequent frost during the flowering period.
Conclusion
Changes in sowing date presented differences in both location
and duration of vegetative and reproductive stages, tending to shorten their
cycle. However, in late sowing, winter cultivars lengthened their cycle to the
point of not reaching flowering during the growing season. For the tested
environmental experiment conditions, optimal sowing dates were between the end
of April and July for both spring and winter cultivars.
1. Agosti, M. B.
2011. Fertilización nitrógeno-azufrada y variabilidad genotípica en el
rendimiento y la calidad del grano en colza-canola (Brassica napus L.).
Tesis Magister. Universidad de Buenos Aires. 130 p.
2. Allen, R. G.; Pereira, L. S.; Raes, D.; Smith, M. 1998. Crop
evapotranspiration: guidelines for computing crop water requirements. In: FAO
Irrigation and Drainage Paper N° 56. FAO. Rome. Italy. 300 p.
3. Arisnabarreta,
S.; Miralles, D. J. 2008. Critical period for grain number establishment of
near isogenic lines of two- and six-rowed barley. Field Crops Research. 107:
196-202. https:// doi.org/10.1016/j.fcr.2008.02.009
4. Arnoud, F. 1989.
Colza: selection, varietés. Cahier Technique. CETIOM. París, Francia. 28 p.
5. Bouché, F.;
Woods, D. P.; Amasino, R. M. 2017. Winter memory throughout the plant kingdom:
Different paths to flowering. Plant Physiology. 173(1): 27-35.
https://doi.org/10.1104/ pp.16.01322
6. Cantagallo, J.
E.; Chimenti, C. A.; Hall, A. J. 1997. Number of seed per unit area in
sunflower correlates well with a phototermal quotient. Crop Science. 37: 1780-
1786. https://doi.org/10.2135/ cropsci1997.0011183X003700060020x
7. Coll, L.;
Larrosa, L. M. 2010. Efecto de la fecha de siembra y el ciclo sobre el rendimiento
de colza. Actualización Técnica N° 1. EEA INTA Paraná. 36 p.
8. Di Rienzo, J.
A.; Casanoves, F.; Balzarini, M. G.; Gonzalez, L.; Tablada, M.; Robledo, C. W.
2008. InfoStat, Grupo InfoStat. FCA. Universidad Nacional de Córdoba.
Argentina.
9. Fischer, R. A.
1985. Number of kernels in wheat crops and the influence of solar radiation and
temperature. Journal of Agriculture Science. Cambridge. 105: 447-461.
https://doi. org/10.1017/S0021859600056495
10. Gómez, N. V.;
Miralles, D. J. 2011. Factors that modify early and late reproductive phases in
oilseed rape (Brassica napus L.): Its impact on seed yield and oil
content. Industrial Crops and Products. 34: 1277-1285.
https://doi.org/10.1016/j.indcrop.2010.07.013
11. Gómez, N. V.;
Miralles, D. J.; Mantese, A. I.; Menéndez, Y. C.; Rondanini, D. P. 2018. Colza:
un cultivo con historia en la FAUBA. Universidad de Buenos Aires. Facultad de
Agronomía; Agronomía & Ambiente. 38(1): 23-36.
12. Habekotté, B.
1997. Evaluation of seed yield determining factors of winter oilseed rape (Brassica
napus L.) by means of crop growth modelling. Field Crops Research. 54:
137-151. https://doi.org/10.1016/S0378-4290(97)00044-0
13. Hocking, P. J.;
Kirkegaard, J. A.; Angus, J. F.; Gibson, A. H.; Koetz, E. A. 1997. Comparison of
canola, Indian mustard and Linola in two contrasting environments. I. Effects
of nitrogen fertilizer on dry-matter production, seed yield and seed quality.
Field Crops Research. 49: 107-125.
https://doi.org/10.1016/S0378-4290(96)01063-5
14. Iriarte, L. B.
2014. Cultivo de colza: fecha de siembra, densidad y distancia entre surcos.
INTA Barrow. 11 p.
15. Iriarte, L. B.;
Valetti, O. 2008. Cultivo de Colza. INTA. Buenos Aires. 152 p.
16. Iriarte, L. B.;
López, Z. B. 2014. El cultivo de colza en Argentina. Situación actual y
perspectivas. 1° Simposio Latinoamericano de Canola. Passo Fundo. Brasil. 1-7.
17. Kirkegaard, J.
A.; Lilley, J. M.; Berry, P. M.; Rondanini, D. P. 2021. Canola. In: Sadras VO
and Calderini DF. Crop Physiology Case Histories for Major Crops, eds and
(Academic Press). 518-549.
18. Lin, L.;
Allemekinders, H.; Dansby, A.; Campbell, L.; Durance-Tod, S.; Berger, A.;
Jones, P. J. 2013. Evidence of health benefits of canola oil. Nutr. Rev. 71:
370-385. https://doi.org/10.1111/ nure.12033
19. McWilliam, S.
C.; Stafford, J. A.; Scott, R. K.; Norton, G.; Stokes, D. T. 1995. The
relationship between canopy structure and yield in oilseed rape. In:
Proceedings of the 9th International
Rapeseed Congress. Cambridge. UK. 491-493.
20. Mendham, N. J.;
Salisbury, P. A. 1995. Physiology: crop development, growth and yield. In:
Kimber D.; McGregor, D. I. (Eds.), Brassica Oilseed, Production, Utilization,
Camb. 11-64.
21. Michaels, S.;
Amasino, R. 2000. Memories of winter: vernalization and the competence to
flower. Plant Cell Environ. 23: 1145–1154.
https://doi.org/10.1046/j.1365-3040.2000.00643.x
22. Mingeau, M.
1974. Comportement du colza e printemps a la sécheresse. Informations
Techniques (Paris, France). 36: 1-11.
23. Miralles, D.
J.; Ferro, B.; Slafer, G. 2001. Developmental responses to sowing date in
wheat, barley and rapeseed. Field Crops Research. 71: 211-223.
https://doi.org/10.1016/S0378- 4290(01)00161-7
24. Morrison, M.
J.; Stewart, D. W. 2002. Heat stress during flowering in summer brassica. Crop
Science. 42: 797-803. https://doi.org/10.2135/cropsci2002.7970
25. Murphy, L.;
Scarth, R. 1991. Vernalization response of spring canola (Brassica napus L.)
In: Mc Gregor DI (eds.). Proceedings of the Eight International Rapeseed
Congress. Saskatoon, Saskatchewan. Canadá. 1764-1768.
26. Nanda, R.;
Bhargava, S.; Tomar, D.; Rawson, H. M. 1996. Phenological development of Brassica
campestris, B. juncea, B. napus and B. carinata grown
in controlled environments and from 14 sowing dates in the field. Field Crops
Res. 46: 93-103. https://doi. org/10.1016/0378-4290(95)00090-9
27. Permingeat, M.
P. 2013. Rendimiento de colza 00: determinación del período crítico. Tesis de
grado. Facultad de Ciencias Agrarias. Universidad de Mar del Plata. Argentina.
20 p.
28. Rondanini, D.
P.; Menendez, Y. C.; Gomez, N. V.; Miralles, D. J.; Botto, J. F. 2017.
Vegetative plasticity and floral branching compensate low plant density in
modern spring rapeseed. Field Crops Research. 210: 104-113.
https://doi.org/10.1016/j.fcr.2017.05.021
29. Secchi, M. A.; Fernandez, J. A.; Stamm, M. J.; Durrett, T.;
Prasad, P. V. V.; Messina, C. D.; Ciampitti, I. A. 2023. Effects of heat and
drought on canola (Brassica napus L.) yield, oil, and protein: a
meta-analysis. Field Crops Res. 293. Article 108848. 10.1016/j. fcr.2023.108848
30.
Silvester-Bradley, R.; Makepeace, R. J. 1984. A code for stages of development
in oilseed rape (Brassica napus L.). Aspects of Applied Biology. 6:
399-419.
31. Takashima, N.
E.; Rondanini, D. P.; Puhl, L. E.; Miralles, D. J. 2013. Environmental factors
affecting yield variability in spring and winter rapeseed genotypes cultivated
in the southeastern Argentine Pampas. European Journal of Agronomy. 48: 88-100.
https://doi.org/10.1016/j. eja.2013.01.008
32. Tommey, A. M.;
Evans, E. J. 1991. Temperature and daylength control of flower initiation in
winter oilseed rape (Brassica napus L.). Annals of Applied Biology. 118:
201-208. https://doi. org/10.1111/j.1744-7348.1991.tb06098.x
33. Walton, G.; Si,
P.; Bowden, B. 1999. Environmental impact on canola yield and oil. In:
Proceedings of the 10th International
Rapeseed Congress. Camberra, Australia. http://www. regional.org.
au/au/gcirc/2/136.htm (Accessed: Sept 2023).
34. Weymann, W.;
Bottcher, U.; Sieling, K.; Kage, H. 2015. Effects of weather conditions during
different growth phases on yield formation of winter oilseed rape. Field Crops
Res. 173: 41-48. https://doi.org/10.1016/j.fcr.2015.01.002
35. Woźniak, E.;
Waszkowska, E.; Zimny, T.; Sowa, S.; Twardowski, T. 2019. The rapeseed
potential in Poland and Germany in the context of production, legislation, and
intellectual property rights. Front Plant Sci. 2019 Nov 5;10:1423.
doi: 10.3389/fpls.2019.01423. PMID: 31749825; PMCID:
PMC6848278.
36. Yaniv, Z.;
Schafferman, D.; Zur, M. 1995. The effect of temperature on oil quality and
yield parameters of high- and low-erucic acid Cruciferae seeds (rape and
mustard). Industrial Crops and Products. 3: 247-251.
https://doi.org/10.1016/0926-6690(94)00041-V
37. Zhang, Z.;
Cong, R. H.; Ren, T.; Li, H.; Zhu, Y.; Lu, J. W. 2020. Optimizing agronomic
practices for closing rapeseed yield gaps under intensive cropping systems in
China. Journal of Integrative Agriculture. 19(2020): 1241-1249.
https://doi.org/10.1016/S2095-3119(19)62748-6
38. Zheng, Q.; Liu, K. 2022. Worldwide rapeseed (Brassica
napus L.) research: A bibliometric analysis during 2011-2021. Oil Crop
Science. 7(4): 157-165. https://doi.org/10.1016/j.
ocsci.2022.11.004.
Conflict of interest
The authors declare the absence of any commercial or financial
relationships that could be construed as a potential conflict of interest.