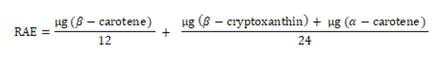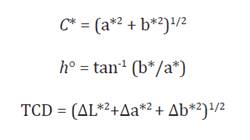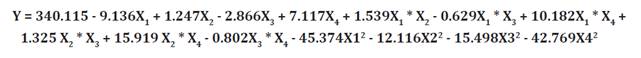Revista de la Facultad de Ciencias
Agrarias. Universidad Nacional de Cuyo. Tomo 57(1). ISSN (en línea) 1853-8665.
Año 2025.
Original article
Obtaining
a lipid extract from peach palm (Bactris gasipaes Kunth) epicarp. Quantification of
carotenoid content and application as a food additive
Obtención
de un extracto lipídico a partir del epicarpio de chontaduro (Bactris
gasipaes Kunth):
Cuantificación del contenido de carotenoides y aplicación como aditivo
alimentario
Coralia Osorio2,
Luis Eduardo
Ordoñez-Santos3
1Universidad del Valle Seccional Palmira. Tecnología de
Procesamiento de Alimentos. Carrera 31A N° 60 - 135. Palmira. Valle del Cauca.
Colombia.
2Universidad Nacional de Colombia. Departamento de Química. AA
14490. Bogotá. Colombia.
3Universidad Nacional de Colombia-Sede Palmira. Facultad de
Ingeniería y Administración. Departamento de Ingeniería. Carrera 32 N° 12 - 00.
Palmira. Valle del Cauca. Colombia.
* jader.martinez@correounivalle.edu.co
Abstract
The agro-industrial
assessment of fruit by-products as food additives would allow compliance with
Sustainable Development Goals. This research aimed at the homogenizer-assisted
extraction of total carotenoids from peach palm (Bactris gasipaes) peel
(epicarp) with sunflower oil. We also studied its application as a natural
additive in white corn flour food. The response surface methodology and the
rotational composite central design quantified the extraction process. The
studied factors were extraction speed, temperature, time, and liquid-solid
ratio. Total carotenoid content in the extract (336.06 μg/g dried epicarp) was
optimized at 50°C, with 76 seconds, extraction speed of 19200 rpm, and
liquid-solid ratio of 48.75 mL/g. The green extract obtained from
homogenizer-assisted extraction constitutes a natural additive with
agro-industrial potential for use in roasted corn cake, increasing carotenoid
(30.60 μg/g of β-carotene), provitamin A (4.14 μg/g) and antioxidant
activity (11.57 % DPPH).
Keywords: Bactris gasipaes, β-carotene,
corn agroindustry, natural dye, homogenizer-assisted extraction
Resumen
El uso
agroindustrial de los subproductos de las frutas, como aditivos alimentarios,
podría ser una alternativa para el cumplimiento de los Objetivos de Desarrollo
Sostenible. Por lo tanto, el objetivo de esta investigación fue la extracción
asistida por homogeneización de carotenoides totales de la cáscara (epicarpio)
de chontaduro (Bactris gasipaes) con aceite de girasol y su aplicación
como aditivo natural en un producto alimenticio elaborado con harina de maíz
blanco. Se aplicó la metodología de superficie de respuesta junto con el diseño
central compuesto rotacional para cuantificar el proceso de extracción. Los
factores de estudio fueron la velocidad de extracción, temperatura, tiempo y
relación líquido-sólido. Los carotenoides totales en el extracto obtenido
(336,06 μg/g de epicarpio seco) se optimizaron a una temperatura de 50°C, con
un tiempo de 76 s, velocidad de extracción de 19200 rpm y relación
líquido-sólido de 48.75 mL/g. El extracto verde obtenido de la extracción
asistida por homogeneización es un aditivo natural con potencial agroindustrial
para uso en alimentos como la arepa de maíz, debido al incremento en los
valores de carotenoides (30,60 μg/g de β-caroteno), provitamina A (4,14
μg/g) y actividad antioxidante (11,57 % DPPH).
Palabras clave: Bactris gasipaes, β-caroteno,
agroindustria de maíz, colorante natural, extracción asistida por
homogeneización
Originales: Recepción: 13/06/2023 - Aceptación: 03/12/2024
Introduction
The peach palm (Bactris
gasipaes) is cultivated in Nicaragua, Honduras, Costa Rica, Panama,
Colombia, Venezuela, French Guiana, Brazil, Bolivia, Hawaii, Indonesia,
Malaysia and Reunion Island (8). The common name
of this fruit changes among countries. It is called pupunha (Brazil), pejibaye
(Costa Rica and Nicaragua), pijuayo (Peru), person (Guyana), chontaduro
(Colombia and Ecuador), and peach palm (English-speaking countries) (8). The fruit’s pulp
is consumed mainly cooked with salt, or processed into flour for bakery or
animal feed (8, 13). Additionally,
indigenous communities in Peru, Bolivia and Brazil obtain fermented beverages (8).
Even though peels constitute an important source of carotenoids (330 μg/g) (12), and represent
10-12 % of total fruit weight, they are eliminated during consumption and
processing (11, 15).
Carotenoids like β-carotene,
α-carotene, β-cryptoxanthin, zeaxanthin, and lycopene, provide
fruits and vegetables with characteristic yellow, red, and orange colors (9). At a functional
level, they have antioxidant activity, and some are a source of provitamin A,
with potential applications for health and nutrition (14). Furthermore,
carotenoids in processed foods highlight color and encourage consumption (5,
22). The agro-industrial use of peels as by-product would
significantly contribute to the food, pharmaceutical and cosmetic industries (17) while reducing
waste generation, avoiding economic losses (25,
27) and supporting the reduction of greenhouse gases (27).
The green
extraction methodology can obtain molecules of interest in different plant
matrices (1, 10, 16, 17, 18, 24). The interaction
of emerging technologies with biodegradable solvents makes these extraction
processes environmentally friendly while reducing health risks. Additionally,
these processes become more efficient when fewer solvents, less extraction
time, and less energy are used (16, 18). Despite
extraction efficiency, green carotenoid extraction studies using
homogenizer-assisted extraction (HAE) are still scarce (1,
3). HAE, also known as high-shear homogenization, is a mechanical
method based on high-speed homogenization, generating a shear effect between
analyte and solvent, causing cell wall rupture and releasing the active
compound of interest (24).
Therefore, new research is needed on food enriched with
bioactive compounds, such as carotenoid pigments (7). This research
aimed to obtain a carotenoid-rich extract using the homogenizer-assisted
extraction from peach palm epicarp with sunflower oil and study its application
as a natural additive.
Materials
and methods
Sample
collection and preparation
Red peach palm
fruits with commercial maturity were acquired in the local market of Palmira,
Department of Valle del Cauca, Colombia. Whole, healthy fruits were washed with
water and disinfected with sodium hypochlorite at 150 ppm. The fruits were
conventionally cooked in water for 60 min at boiling temperature (kg fruit/2 L
water). Then, peels (epicarp) were removed using a disinfected, manual,
stainless steel fruit peeler. Epicarp flour was produced according to previous
studies (11). The epicarp was dehydrated in a
convection oven (Binder ED 53 UL, Germany) at 60 ± 2°C until 10-11 % moisture.
Dehydrated samples were crushed in an electric mill to particle size ≤ 0.25 mm.
This flour was refrigerated in a sterile amber glass bottle at 4°C for later
use.
Homogenizer-assisted
extraction of epicarp carotenoids
The homogenizer-assisted extraction (HAE) was carried out in an
ultra-turrax (T 18 digital, IKA, Janke & Kunkel, Germany) using sunflower
oil as extraction solvent. Treatments were processed according to the
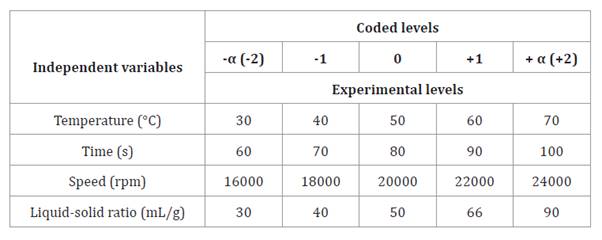
established extraction parameters shown in table 1.
Table 1. Central
composite rotatable design with independent variables and coded levels.
Tabla
1. Diseño central compuesto rotacional
con variables independientes y niveles codificados.
Total carotenoids
(μg/g dried epicarp) were determined according to the spectrophotometric method
(15), using a molar
extinction coefficient of 7.10 × 104 M-1cm-1
and sunflower oil as blank (15). The HAE was
optimized via the response surface methodology combined with the rotational
composite central design (RCCD). Table 1 shows coded factors, central points, and extreme values.
Preliminary experiments identified central points, confirming that the
liquid-solid ratio, temperature, time, and extraction speed significantly
affected extraction.
Extract
application in corn griddle cake
The optimized
extract was used as a natural additive in corn griddle cake. Two treatments
were elaborated: a control with precooked white corn flour (WCF), and another
with white corn to which 50 mL of the lipid extract was added as a natural
additive. In all cases, 100 g of flour were mixed with 2 g of salt and 145 g of
water. Kneading time was 5 minutes, and standing time was 3 minutes. All
samples were 4 cm diameter and 1 cm thick. They were cooked on a preheated plate
at 180 °C for 10 minutes (5 minutes on each side) obtaining the brownish and
crunchy texture of traditional corn griddle cake.
Concentration
of carotenoid and provitamin A in corn griddle cake
Carotenoids (μg /g of corn grilled cake) were determined by spectrophotometry
(17). Absorbance of the organic phase was measured at 444, 450, and 451 nm and
compared to hexane with a spectrophotometer (Genesys 20 UV-Vis, Thermo Electron
Scientific Instruments LLC, Madison, WI, USA). Carotenoid concentration (μg/g
of sample) was calculated using extinction coefficients (E% 1 cm) in hexane:
2460, 2480, 2560, and 2800 for β-cryptoxanthin, zeaxanthin, β-carotene
and α -carotene, respectively. The provitamin A, expressed as retinol
activity equivalents (RAE, μg/g of corn grilled cake), was calculated using a
conversion factor of 12 for β-carotene and 24 for the other provitamins
according to equation
1,
as reported by the standard method (20).
Determination
of antioxidant activity in corn griddle cake
Antioxidant
activity AA (%) was determined as inhibition percentage of the radical DPPH
(2,2-diphenyl-1-picrylhydrazyl) according to the colorimetric method (26).
Color
parameters in corn griddle cake
Sample surface color was evaluated using the CIEL*a* b*
coordinates, measured with a CR-400 Colorimeter, Konica Minolta Tokyo, Japan,
with 2° observer settings and D65 deuterium lamp. The equipment
was calibrated using a standard measurement plate: Y = 89.50, x = 0.3176, y =
0.3347. In addition, the Chroma (C *), hue angle (h °), and total
color difference, TCD, were calculated with equations 2-4:
Experimental
design and statistical analysis
The HAE was
optimized with the response surface methodology combined with rotational
central design of 29 experiments, where 16 were factorial points, 8 were axial
points, and 5 were central points. The study factors were the liquid-solid
ratio, temperature, time, and extraction speed. Factor effects and their
interaction were evaluated with a second-order polynomial model to estimate the
variables. Factor effects were identified with ANOVA (p < 0.05), and
model reliability was evaluated with the coefficient of determination, R2, lack of Fit, and
coefficient of variation. The statistical software Design Expert (Version 11,
Stat-Easy, Godward, MN, USA) was used in optimization design. The t-Student
test validated the optimization model, and evaluated the two grilled corn cake
formulations. The statistical analysis was run in the Minitab version 18
statistical package for Windows.
Results
and discussion
Response
surface optimization and contour plots
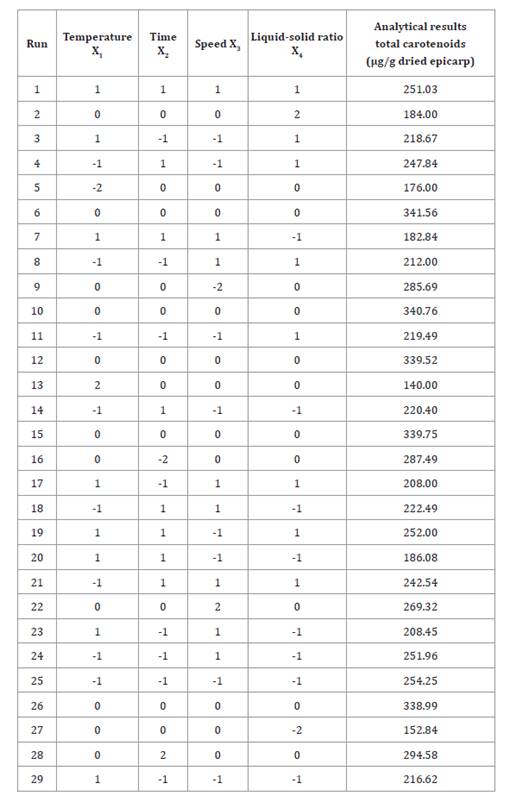
Table 2, shows total
carotenoids in each treatment evaluated during the HAE. Results ranged from
140.00 to 341.56 μg/g dried epicarp. These values are lower than the 440-670
μg/g obtained in peach palm epicarp (13).
Table 2. Central
composite rotatable design with experimental total carotenoids.
Tabla
2. Diseño central compuesto rotacional
con resultados experimentales de carotenoides totales.
The ANOVAmodelpresenteda p < 0.0001,
monitoringHAEoptimizationoftheresponse variable of interest (total
carotenoids). Factors, interactions (temperature*time, temperature*ratio,
time*speed, and time*ratio) and quadratic effect on the independent variables
significantly affected carotenoid extraction (table 3).
Table 3. ANOVA
for the fitted quadratic polynomial model estimated for total carotenoid
content of peach palm epicarp.
Tabla
3. Análisis de varianza del modelo polinomial cuadrático
estimado para el contenido total de carotenoides a partir del epicarpio de
chontaduro.
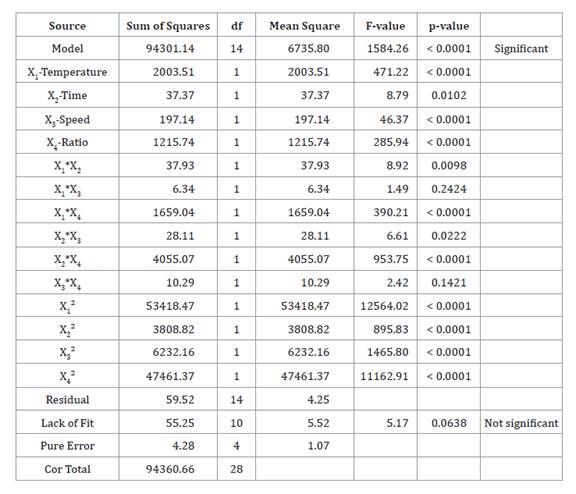
R2 = 0.9994, R2 adj = 0.9987, R2 pred = 0.9966, and CV% = 0.844
Lack of fit was not significant (p > 0.05). The R2
= 0.9994, R2 adj = 0.9987, R2
pred = 0.9966 and CV% = 0.844 (table 3) indicated good
regression fit according to the following equation:
Figure 1 A-F, shows
response surfaces of interaction and quadratic effects in carotenoid
extraction.
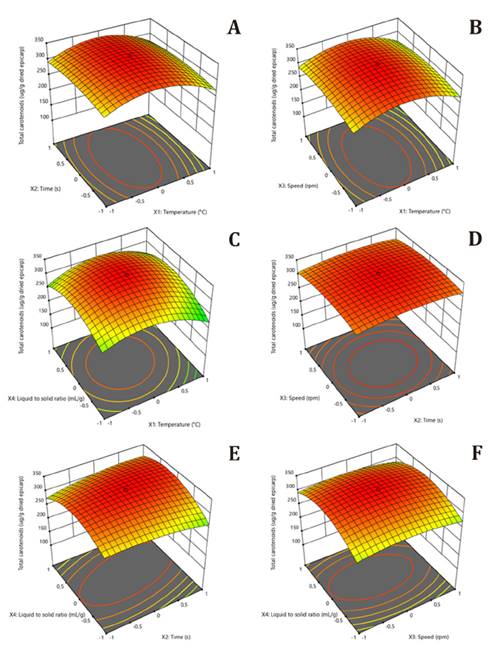
Time vs temperature
(A), speed vs. temperature (B),
liquid-solid ratio vs. temperature (C),
speed vs. time (D), liquid-solid ratio vs.
time (E) and liquid-solid ratio vs. speed (F).
Tiempo
vs temperatura (A), velocidad vs temperatura (B), relación
líquido-sólido vs. temperatura (C),
velocidad vs tiempo (D), relación líquido-sólido vs tiempo (E) y
relación líquido-sólido vs. velocidad (F).
Figure
1. 3D surface plots of the effect of temperature, time,
speed and liquid-solid ratio in the homogenizer-assisted extraction (HAE) of total
carotenoids from Bactris gasipaes epicarp.
Figura
1. Gráficos de superficie 3D del efecto de la temperatura, el
tiempo, la velocidad y la relación líquido-sólido en la extracción asistida por
homogeneizador (HAE) de carotenoides totales del epicarpio de Bactris
gasipaes.
Figures 1A and 1B, show response
surfaces generated by significant effects of time and temperature, and speed
and temperature. Augmented extractions were observed with increasing time,
speed, and temperature, while at over 50°C, extraction was reduced. This was
previously observed on HAE in oligosaacharides from banana pulp (2,
18). However, these authors did not observe a significant impact on
extraction speed of phenolic compounds and chlorophylls during this process (2,
16).
Carotenoids increase during HAE due to a higher mass transfer
coefficient between carotenoid pigments and sunflower oil. This generates a
mechanical breakdown of the biological matrix through shearing during HAE,
reducing viscosity and accelerating diffusion and breakdown of
protein-carotenoid bonds in the plant matrix (16,
21). Carotenoid reduction with increasing temperature during HAE
may be associated with isomerization and oxidative degradation (21).
Figure 1C, shows the
response surface generated by temperature and liquid-solid ratio effects on the
total carotenoid extraction. A quadratic effect was evidenced, at the beginning
by a significant increase in extraction after an increase in liquid-solid ratio
and temperature. Later, extraction levels were reduced when the liquid-solid
ratio surpassed 50 mL/g, and temperature exceeded 50°C. Liquid-solid ratio
effects in HAE of bioactive compounds in plant by-products were reported by Eyiz et
al. (2020) in red grape pomace. The results presented here are consistent
with the principles of mass transfer exposed by Wong et al. (2015), who stated that
the concentration gradient between liquid and solid constitutes the driving
force, which is greater for a higher liquid-solid ratio. On the other hand,
extraction reduction of total carotenoids for ratios above 50 mL/g could
prolong solvent diffusion distance into the matrix (23,
29).
Figure 1D, shows the
response surface generated by speed and time effects on total extraction.
Factors interaction positively affected extraction. Increased concentrations
may have resulted from shearing and mechanical damage, transferring pigments to
the solvent (16). Figures 1E and
1F,
validate liquid-solid ratios, time, and speed effects in extracting total
carotenoids from peach palm epicarp. A quadratic and interaction effect was
observed on the response variable. The optimal HAE point of total carotenoids
was 336.06 μg/g dried epicarp, with 50°C, 76 s time, 19200 rpm and a
liquid-solid ratio of 48.75 mL/g. When experimentally validating the process
factors established in HAE optimization, a carotenoid content of 334.97 ± 1.06
μg/g dried epicarp was obtained, not significantly different (p >
0.05, n = 4) from the theoretical one. Therefore, experimental values
were adjusted to the quadratic model. When comparing optimized values with the
maceration method (sunflower oil for 24 h), HAE exceeds the concentration of
the conventional method (113. 94 μg/g dried epicarp) by 2.95 times. Extraction
efficiency of bioactive compounds with HAE in plant matrices was previously
described (16). These authors
achieved high extraction rates with shearing, rupturing the plant matrix in a
few seconds, increasing mass transfer coefficient (16). In addition, this
method uses agitation, accelerating extraction and increasing mass transfer
from the plant matrix to the solvent with diffusion and osmotic processes.
Application
of the optimized extract in corn griddle cake
All response
variables were significantly affected (p < 0.05, table 4).
Table 4. Carotenoids,
provitamin A, antioxidant activity and color attributes in two corn griddle
cake formulations.
Tabla
4. Carotenoides, provitamina A, actividad antioxidante
y atributos de color en dos formulaciones de arepas de maíz.
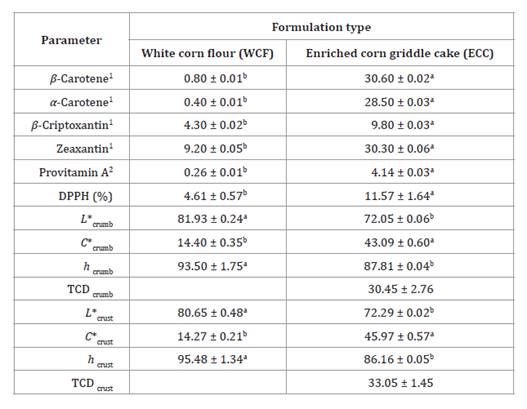
1 μg of compound/g of corn grilled
cake, 2 RAE μg/g
of corn grilled cake, averages on the same column followed by different letters
vary significantly from each other (p < 0.01) according to t-Student
test.
1 μg de compuesto/g de arepa de maíz, 2 RAE μg/g de arepa de maíz, los valores
promedios en la misma columna seguidos de letras diferentes varían
significativamente entre sí (p < 0,01) según la prueba t-Student.
The enriched corn
griddle cake (ECC) presented statistically higher carotenoids, provitamin A,
and antioxidant activity than white corn flour (WCF). These differences are
mainly due to the incorporation of the lipid extract in the ECC formulation.
Other studies have used peach palm lipid extract in food matrices as bakery
products, emulsions, and Frankfurt sausages (5,
17, 19). For example, de Souza Mesquita et al. (2020) reported
increasing carotenoid pigments and provitamin A in mayonnaise made with this
lipid extract. Bioactive compounds can influence antioxidant capacity in food
matrices, and the addition of carotenoid pigments in the samples may increase
antioxidant capacity, as stated in guava pulp after homogenization treatment (4).
Color attributes
evaluated in crumb and crust showed L* and h° were statistically
reduced in ECC, and C* significantly increased compared to WCF. The TCD
had a greater difference between ECC and WCF (table 4). These results
are explained by the higher concentration of carotenoid pigments in ECC.
These pigments absorb part of the visible spectrum, favoring the
yellow color in ECC. Meanwhile, low carotenoid concentration in WCF resulted in
greater reflection of the visible spectrum, generating a white color in the
samples. Suo
et al. (2023) confirm changes in the white color of French fries to reddish
tones when fried in corn oil enriched with carotenoid.
Conclusion
HAE was adequate for carotenoid extraction in peach palm
epicarp. Maximum extraction of total carotenoids was reached when processing
the samples at 50°C, 76 s, 19200 rpm, and liquid-solid ratio of 48.75 mL/g. In
addition, the HAE method presented the best extraction performance for total
carotenoids compared to extraction with maceration. The green extract obtained
from homogenizer-assisted extraction is a natural additive with agro-industrial
potential for use in roasted corn cake, increasing carotenoids (30.60 μg/g of β-carotene),
provitamin A (4.14 μg/g) and antioxidant activity (11.57 % DPPH).
Acknowledgment
Authors gratefully acknowledge the financial support provided by
the Universidad Nacional de Colombia-sede Palmira (Hermes project: 57499),
Universidad del Valle seccional Palmira and C # 909 Minciencias-Colombia. The
ANLA and Ministry of Environment and Sustainable Development granted permission
to collect samples (Framework Agreement for Access to Genetic Resources and
their Derivative Products No. 357 of November 17, 2022 signed between the
Ministry of Environment and Sustainable Development and the National University
of Colombia).
1.
Baria, B.; Upadhyay, N.; Singh, A. K.; Malhotra, R. K. 2019. Optimization of
green extraction of carotenoids from mango pulp using split plot design and its
characterization. LWT. 104: 186-194. doi.org/10.1016/j.lwt.2019.01.044
2.
Bilgin, M.; Elhussein, E. A. A.; Özyürek, M.; Güçlü, K.; Şahin, S. 2018.
Optimizing the extraction of polyphenols from Sideritis montana L. using
response surface methodology. Journal of Pharmaceutical and Biomedical
Analysis. 158: 137-143. doi.org/10.1016/j.
jpba.2018.05.039
3.
Dall’Acqua, S.; Kumar, G.; Sinan, K. I.; Sut, S.; Ferrarese, I.; Mahomoodally,
M. F.; Zengin, G. 2020. An insight into Cochlospermum planchonii extracts
obtained by traditional and green extraction methods: Relation between chemical
compositions and biological properties by multivariate analysis. Industrial
Crops and Products. 147: 112226. doi.org/10.1016/j.
indcrop.2020.112226
4.
da Silva Lima, R.; Nunes, I. L.; Block, J. M. 2020. Ultrasound-assisted
extraction for the recovery of carotenoids from Guava’s pulp and waste powders.
Plant Foods for Human Nutrition. 75(1): 63-69.
doi.org/10.1007/s11130-019-00784-0
5.
de Souza Mesquita, L. M.; Neves, B. V.; Pisani, L. P.; de Rosso, V. V. 2020.
Mayonnaise as a model food for improving the bioaccessibility of carotenoids
from Bactris gasipaes fruits. LWT. 122: 109022.
doi.org/10.1016/j.lwt.2020.109022
6.
Eyiz, V.; Tontul, I.; Turker, S. 2020. Optimization of green extraction of
phytochemicals from red grape pomace by homogenizer assisted extraction.
Journal of Food Measurement and Characterization. 14(1):39-47.
doi.org/10.1007/s11694-019-00265-7
7.
Khalid, M.; Rahman, S. U.; Bilal, M.; Iqbal, H. M. N.; Huang, D. 2019.
Biosynthesis and biomedical perspectives of carotenoids with special reference
to human health related applications. Biocatalysis and Agricultural
Biotechnology. 17: 399-407. doi.org/10.1016/j.
bcab.2018.11.027
8.
Kramer, Y. V.; Clement, C. R.; de Carvalho, J. C.; Fernandes, A. V.; da Silva,
C. V. A.; Koolen, H. H. F.; Aguiar, J. P. L.; Nunes-Nesi, A.; Ramos, M. V.;
Araújo, W. L.; de Carvalho Gonçalves, J. F. 2023. Understanding the
technical-scientific gaps of underutilized tropical species: The case of Bactris
gasipaes Kunth. Plants. 12(2): 337. doi.org/10.3390/plants12020337
9.
López, A.; Montaño, A.; Garrido, A. 2005. Provitamin A carotenoids in table
olives according to processing styles, cultivars, and commercial presentations.
European Food Research and Technology. 221(3): 406-411.
doi.org/10.1007/s00217-005-1190-8
10.
Martínez-Girón, J.; Ordoñez-Santos, L. E.; Rodríguez-Rodríguez, D. X. 2019.
Extraction of total carotenoids from peach palm fruit (Bactris gasipaes)
peel by means of ultrasound application and vegetable oil. DYNA. 86(209):
91-96. doi.org/10.15446/dyna.v85n207.74840
11.
Martínez-Girón, J.; Osorio, C.; Ordoñez-Santos, L. E. 2022. Effect of
temperature and particle size on physicochemical and techno-functional
properties of peach palm peel flour (Bactris gasipaes, red and yellow
ecotypes). Food Science and Technology International. 28(6): 535-544.
doi.org/10.1177/10820132211025133
12.
Matos, K. A. N.; Lima, D. P.; Barbosa, A. P. P.; Mercadante, A. Z.; Chisté, R.
C. 2019. Peels of tucumã (Astrocaryum vulgare) and peach palm (Bactris
gasipaes) are by-products classified as very high carotenoid sources. Food
Chemistry. 272: 216-221. doi.org/10.1016/j.
foodchem.2018.08.053
13.
Menezes Silva, J. V.; Silva Santos, A.; Araujo Pereira, G.; Campos Chisté, R.
2023. Ultrasound-assisted extraction using ethanol efficiently extracted
carotenoids from peels of peach palm fruits (Bactris gasipaes Kunth)
without altering qualitative carotenoid profile. Heliyon. 9: 14933.
doi.org/10.1016/j.heliyon.2023.e14933
14.
Namitha, K. K.; Negi, P. S. 2010. Chemistry and biotechnology of carotenoids.
Critical Reviews in Food Science and Nutrition. 50(8): 728-760. doi: 10.1080/10408398.2010.499811
15.
Ordoñez-Santos, L. E.; Pinzón-Zarate, L. X.; González-Salcedo, L. O. 2015.
Optimization of ultrasonic-assisted extraction of total carotenoids from peach
palm fruit (Bactris gasipaes) by-products with sunflower oil using
response surface methodology. Ultrasonics Sonochemistry. 27: 560-566.
doi.org/10.1016/j.ultsonch.2015.04.010
16.
Ordoñez-Santos, L. E.; Garzón-García, A. M. 2021a. Optimizing homogenizer-assisted
extraction of chlorophylls from plantain epicarp (Musa paradisiaca L).
Journal of Food Measurement and Characterization. 5: 1108-1115.
doi.org/10.1007/s11694-020-00703-x
17.
Ordóñez-Santos, L. E.; Esparza-Estrada, J.; Vanegas-Mahecha, P. 2021b. Ultrasound-assisted
extraction of total carotenoids from mandarin epicarp and application as
natural colorant in bakery products. LWT. 139: 110598.
doi.org/10.1016/j.lwt.2020.110598
18.
Pereira, G. A.; Arruda, H. S.; Molina, G.; Pastore, G. M. 2018. Extraction optimization
and profile analysis of oligosaccharides in banana pulp and peel. Journal of
Food Processing and Preservation. 42(1): 13408. doi.org/10.1111/jfpp.13408
19.
Pinzón-Zárate, L. X.; Hleap-Zapata, J. I.; Ordóñez-Santos, L. E. 2015. Análisis
de los parámetros de color en salchichas Frankfurt adicionadas con extracto
oleoso de residuos de chontaduro (Bactris gasipaes). Información
Tecnológica. 26(5): 45-54. doi.org/10.4067/S0718- 07642015000500007
20.
Stinco, C. M.; Fernández-Vázquez, R.; Escudero-Gilete, M. A.; Heredia, F. J.;
Meléndez-Martínez, A. J.; Vicario, I. M. 2012. Effect of orange juice’s
processing on the color, particle size, and bioaccessibility of carotenoids.
Journal of Agricultural and Food Chemistry. 60(6): 1447-1455. doi: 10.1021/jf2043949
21.
Strati, I. F.; Oreopoulou, V. 2014. Recovery of carotenoids from tomato
processing by-products-a review. Food Research International. 65: 311-321. doi.org/10.1016/J. FOODRES.2014.09.032
22.
Suo, A.; Fan, G.; Wu, C., Li, T.; Cong, K. 2023. Green extraction of
carotenoids from apricot flesh by ultrasound assisted corn oil extraction:
Optimization, identification, and application. Food Chemistry. 420: 136096.
doi.org/10.1016/j.foodchem.2023.136096
23.
Tao, Y.; Sun, D. W. 2015. Enhancement of food processes by ultrasound: a
review. Critical Reviews in Food Science and Nutrition. 55(4): 570-594.
doi.org/10.1080/10408398.2012.667849
24.
Tiwari, S.; Upadhyay, N.; Singh, A. K.; Meena, G. S.; Arora, S. 2019. Organic
solvent-free extraction of carotenoids from carrot bio-waste and its
physico-chemical properties. Journal of Food Science and Technology. 56(10):
4678-4687. doi.org/10.1007/ s13197-019-03920-5
25.
Trigo, J. P.; Alexandre, E. M.; Saraiva, J. A.; Pintado, M. E. 2020. High
value-added compounds from fruit and vegetable by-products - Characterization,
bioactivities, and application in the development of novel food products.
Critical Reviews in Food Science and Nutrition. 60(8): 1388-1416.
doi.org/10.1080/10408398.2019.1572588
26.
Turkmen, N.; Sari, F.; Velioglu, Y. 2005. The effect of cooking methods on
total phenolics and antioxidant activity of selected green vegetables. Food
Chemistry. 93(4): 713-718. doi.
org/10.1016/j.foodchem.2004.12.038
27.
Uekert, T.; Dorchies, F.; Pichler, C. M.; Reisner, E. 2020. Photoreforming of
food waste into value-added products over visible-light-absorbing catalysts.
Green Chemistry. 22(10): 3262-3271. doi.
org/10.1039/D0GC01240H
28.
Wong, W. H.; Lee, W. X.; Ramanan, R. N.; Tee, L. H.; Kong, K. W.; Galanakis, C.
M.; Prasad, K. N. 2015. Two level half factorial design for the extraction of
phenolics, flavonoids and antioxidants recovery from palm kernel by-product.
Industrial Crops and Products. 63: 238-248. doi.
org/10.1016/J.INDCROP.2014.09.049
29.
Xu, Y.; Pan, S. 2013. Effects of various factors of ultrasonic treatment on the
extraction yield of all-trans-lycopene from red grapefruit (Citrus paradise
Macf.). Ultrasonics Sonochemistry. 20(4): 1026-1032.
doi.org/10.1016/j.ultsonch.2013.01.006