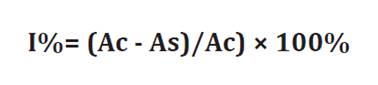Revista de la Facultad de Ciencias
Agrarias. Universidad Nacional de Cuyo. Tomo 57(1). ISSN (en línea) 1853-8665.
Año 2025.
Original article
Antimicrobial
and antioxidant properties of the woody endocarp of native and commercial
walnuts from Argentina
Propiedades
antimicrobianas y antioxidantes del endocarpio leñoso de nogales nativos y
comerciales de Argentina
Ingrid Georgina
Orce1,
Gabriela Inés
Furque2,
Emilia Lorenzo3,
Gretel Rodriguez
Garay4,
Fiamma Pereyra3,
María Rosa Alberto5,
Patricia Elizabeth
Gomez Kamenopolsky1, 3,
1 Centro Regional de Energía y Ambiente para el Desarrollo
Sostenible. CREAS (UNCA-CONICET) Prado 366. San Fernando del Valle de
Catamarca. CP K4700AAP. Catamarca. Argentina.
2 Universidad Nacional de Catamarca. Facultad de Ciencias Exactas
y Naturales. Departamento de Química. Av. Belgrano al 300. San Fernando del
Valle de Catamarca. CP K4700AAP. Catamarca. Argentina.
3 Universidad Nacional de Catamarca. Facultad de Ciencias
Agrarias. Departamento de Química. Av. Maestro Quiroga 50. San Fernando del
Valle de Catamarca. CP K4700AAP. Catamarca. Argentina.
4 Instituto Nacional de Tecnología Agropecuaria (INTA). Estación
Experimental Agropecuaria Catamarca. Ruta 33 km 4.5 (4705). Valle Viejo.
Catamarca. Argentina.
5 Instituto de Biotecnología Farmaceútica y alimentaria
(INBIOFAL) Predio Universitario Ingeniero Herrera. Av. Kirchner 1900. CP 4000.
San Miguel de Tucumán. Tucumán. Argentina.
*
mario.arena@fbqf.unt.edu.ar
Abstract
Juglans australis is a tree from the Juglandaceae
family found in the southernmost region of America. Its small edible nuts
are not commercialized, and their bioactive characteristics are unknown. This
study first reports the antioxidant, antiradical, and antibacterial activity of
extracts from this native walnut against phytopathogenic bacteria and compared
with its commercial counterpart, J. regia L. Different extracts from the
woody endocarp (shells) were obtained using methanol and ethyl acetate.
Methanolic extracts significantly inhibited phytopathogenic growth at all
concentrations tested (0.1, 1, and 10 mg/mL). The best activity was reported
against Xanthomonas. Highest total phenolics and the most significant
antioxidant activity were determined in methanolic extracts (TPC: 121 mg gallic
acid equivalent (GAE)/g of dried peel, FRAP: 58.6 mmol Trolox/100 g of peel
dried and 9.7 mM Trolox/100 g of dried peel). Extracts from both species
demonstrated congruent patterns. Gallic acid was the most abundant compound in
the methanolic extract. However, extracts demonstrated superior efficiency,
suggesting a potential synergistic effect among their components. Antioxidant
and antimicrobial activity of methanolic extracts against Xanthomonas make
them potential control agents.
Keywords: Juglans australis, phytopathogens,
polyphenols, bioactive compounds, sustainable agriculture, Xanthomonas sp.
Resumen
Juglans australis es un árbol
perteneciente a la familia Juglandaceae que se encuentra en la región
más austral del continente americano. Aunque las nueces también son
comestibles, son pequeñas y no se comercializan, sus características bioactivas
son desconocidas. Este estudio constituye el primer informe sobre la actividad
antioxidante, antirradical y antibacteriana de extractos de la nuez nativa
frente a bacterias fitopatógenas, y su comparación con la especie comercial, J.
regia L. Se obtuvieron diferentes extractos a partir del endocarpio leñoso
(cáscaras) utilizando metanol y acetato de etilo. El extracto metanólico
resultó ser la fracción más activa e inhibió significativamente el crecimiento
de los fitopatógenos en todas las concentraciones analizadas (0,1, 1 y 10
mg/mL). La mejor actividad se registró para el género Xanthomonas. El
mayor contenido de fenoles totales y la actividad antioxidante más significativa
se determinó en el extracto metanólico (TPC: 121 mg de ácido gálico equivalente
(GAE)/g de cáscara seca, FRAP: 58,6 mmol de Trolox/100 g de cáscara seca y 9,7
mM de Trolox/100 g de cáscara seca). Los extractos de ambas especies se
comportaron de manera similar. Al analizar la composición química, el ácido
gálico fue el compuesto más abundante en el extracto metanólico. Sin embargo,
los extractos mostraron una eficiencia superior, lo que sugiere un posible
efecto sinérgico entre sus componentes. La actividad antimicrobiana de los
extractos metanólicos contra Xanthomonas, junto con su capacidad
antioxidante, resalta su potencial aplicación como agentes de control de
fitopatógenos.
Palabras claves: Juglans australis, fitopatógenos,
polifenoles, compuestos bioactivos, agricultura sustentable, Xanthomonas sp.
Originales: Recepción: 23/06/2023 - Aceptación: 05/12/2024
Introduction
Phytopathogens
cause significant economic loss in agriculture (35). Even considering
synthetic pesticides imply detrimental environmental and human health
consequences, several products are still being used. In the last decade,
eco-friendly compounds have emerged as alternative pesticides (8,
19). These natural antimicrobial agents are safer and cheaper than
chemical agents (48) contributing to
the economy and environment (9, 19, 44).
Several plant
bioactive compounds have shown antimicrobial activity (14). Phenolic-rich
plant extracts have shown significant activity against phytopathogens (22,
42), including Xanthomonas spp., a major threat to crops
like rice and citrus, which have developed resistance to chemicals and
antibiotics (25, 29). Some studies
report the effects of extracts as natural antimicrobials against Xanthomonas
sp. (3, 23, 24, 30).
Other pathogens,
like Clavibacter michiganensis, are responsible for significant losses
in tomato (32), and the bacterium
Erwinia amylovora mainly affects pome fruit trees like pear, apple,
quince, and loquat (5, 28). Carnaval
et al. (2022) observed the inhibition effect of seriguela (Spondias
purpurea L.) extract on Clavibacter michiganensis pv michiganensis
and Xanthomonas phaseoli.
The genus Juglans
includes over 20 species, being J. regia L. majorly significant due
to its extensively studied nutritional and functional properties (7,
15). Walnut by-products, including shells, are rich in bioactive
phytochemicals with antimicrobial potential for medicine, food preservation,
and agroindustry (1, 4, 26, 27). Although their
potential as biopesticides is recognized, the antimicrobial activity of these
compounds is still unexplored. Meanwhile, shell biomass is often undervalued
despite being a cost-effective, renewable resource.
On the other hand, Juglans australis is a native walnut
tree from the Juglandacea family inhabiting the most austral region of South
America, and the Northwestern subtropical rainforest in Argentina, locally
known as “Yungas” (11),
in Jujuy, Salta, Tucumán, La Rioja, and Catamarca (figure 1).
Its fruit is an indehiscent, subglobose drupe with a thick, adherent mesocarp
and a rigid shell (endocarp) containing the embryo (39) (figure
1).
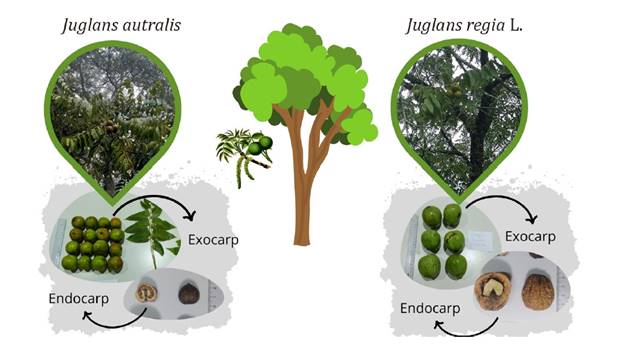
Figure 1. Juglans
australis tree in Ancasti (Catamarca) city,
Argentina (left) and J. regia (right). Morphological comparison of
native J. australis and commercial J. regia L. fruits.
Figura 1 Árbol
y frutos de la especie y Juglans australis (izquierda) y Juglans
regia (derecha), correspondientes a la localidad de Ancasti, provincia de
Catamarca, Argentina.
Contrasting with the extensive knowledge about commercial
walnuts, information on this native species remains scarce. To date, no
research reports antimicrobial or antioxidant activity of Juglans australis;
except for its activity against herpes virus (37).
We
aimed to analyze the antimicrobial and antioxidant properties of walnut shell
extracts from the commercial J. regia and the native J. australis for
future uses in agricultural management. These extracts would contribute to
waste valorization while generating added value to the autochthonous species.
Furthermore, extract chemical compositions allowed deeper comprehension of
their bioactive activities.
Materials and methods
Sample collection
In
2021, samples of J. regia and J. australis walnut shells were
collected in Ancasti, Catamarca, Argentina. Walnut shells were cleaned and
dried under shaded conditions for a week. Selected samples were ground into
small particles using a grinder.
Solvent extraction
Solvent
extraction involved 50 g of the powdered walnut shells extracted with 250 mL of
absolute methanol (MeOH) and ethyl acetate (AcOEt) for 45 min at room
temperature and filtered through Whatman n° 4 (48).
The solvents were evaporated under a vacuum in a Büchi R-210 rotavapor. The
extracts obtained were redissolved in dimethyl sulfoxide (DMSO) to a final
concentration of 0.1, 1, and 10 mg/mL and stored in the dark at 4°C for further
use. All extractions were done in duplicate.
Determination of total phenolic content and antioxidant
activities
Total
polyphenol content (TPC) was determined colorimetrically using
Folin-Ciocalteu’s reagent at 765 nm. A standard curve was performed with gallic
acid. The results were expressed as μg gallic acid/g dry weight (DW) (41). In
vitro antioxidant activity was measured using the free radical elimination
activity assay on 1,1, -diphenyl-2-picrylhydrazylradical (DPPH) (10),
and ferric reduction capacity of plasma assay (FRAP) (6).
Results are expressed in mmol Trolox equivalents/100 g dry weight (DW). All
samples were analyzed in triplicate.
Identification of extract phenolic compounds by UHPLC-MS/MS
Phenolic compounds
of extracts from walnut shells were identified by a UHPLC (Ultimate 3000 RSLC,
Dionex - Thermo Scientific) equipped with a diode array detector and coupled to
a TSQ Quantum ultra-triple-quadrupole mass spectrometer (TSQ Quantum Access
Max, Thermo Scientific) and column Hypersil GOLD aQ (150 x 2.1 mm, 5 um)
(Thermo Scientific). The mobile phase was a binary mixture of solvents: mobile
phase A corresponded to ultrapure water/formic acid solution (Merck, Darmstadt,
Germany) (0.1% v/v), and mobile phase B corresponded to an acetonitrile /
formic acid solution (Merck, Darmstadt, Germany) (0.1% v/v). Gradient
conditions were as follows: 0-18 min, 97% A; 18-21 min, 90% B; 21-26 min, 97%
A. Electrospray source of the MS was performed in negative mode. Eluate was
monitored at 250 nm, flow rate was 0.3 mL min-1, injection volume was 15 μL,
and the column was maintained at 35°C. Polyphenols were tentatively identified
according to their retention times, UV/Vis spectra, high-resolution MS, and
MS/MS spectra by comparison with pure compounds. We searched for gallic acid,
cumaric acid, caffeic acid, ferulic acid, rutin, and eriocitrin (Sigma-Aldrich,
St. Louis, MO, USA). The linearity of each calibration curve was confirmed by
plotting the ratio of peak areas of phenolic compounds to the internal standard
against compound concentration. Data were analyzed via LC-MS Xcalibur
workstation software (Version 2.6, Thermo Fisher Scientific).
Antibacterial activity
Antibacterial activity of the different walnut shell extracts
was evaluated against Gram-negative bacteria Erwinia amilovora, Xanthomonas
axonopodis pv. phaseolus, and X.
campestris pv. campestris 8004 and a Gram positive
bacteria, Clavibacter michiganensis. Bacterial suspensions were prepared
in Tryptic Soy Broth (TSB). By microdilution, microtiter plate wells were
filled with bacterial suspension (107 CFU/mL) and the extract solution (final
concentrations 0.1; 1 and 10 mg/ mL). Pure gallic acid, the main extract
component, was incorporated in the antimicrobial assays at three different
concentrations: 34 ppm (higher concentration detected in the extracts), 100
ppm, and 500 ppm. The extracts and gallic acid suspensions were prepared by
diluting stock solutions in DMSO. Vehicle controls were prepared with DMSO and
each bacterial culture. The 1% vehicle did not affect bacterial growth and was
used as negative control. Streptomycin sulfate was also used as positive
control. The microplates were incubated at 28°C for 24 h, and growth was
detected using a microplate reader (Multiskan Go, Thermo) at 560 nm (45).
Inhibition percentages were also calculated according to the Equation:
where
Ac
= absorbance of the control sample (phytopathogens without extract and
antibiotic)
As
= absorbance of phytopathogens + extract samples.
All
assays were conducted three times and analyzed in triplicate.
Statistical analysis
ANOVA
and Tukey test evaluated differences between treatments using INFOSTAT
(Student version, 2020e).
Results and discussion
Total Phenolic compounds and Antioxidants activities
Table 1,
shows total phenolic content and antioxidant activity of organic extracts (ME:
methanolic extract, EAE: ethyl-acetate extract) of J. australis and J.
regia.
Tabla 1. Contenido
fenólico total (TPC), actividades antirradicalias (DPPH) y reductoras (FRAP) de
extractos metanólicos y de acetato de etilo obtenidos a partir del endocarpio
de J. australis y J. regia.
Table 1. Total
phenolic content (TPC), antiradical (DPPH) and reducing (FRAP) activities of
methanolic and ethyl acetate of endocarp extracts from J. australis and J.
regia.
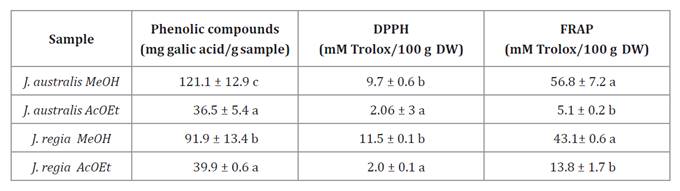
Value is expressed as mean ±
standard error. Different letters indicate statistically significant
differences (p<0.05).
Los valores corresponden a la
media ± error estándar. Letras diferentes indican diferencias estadísticas
(p<0,05).
Solvent extraction capacities varied significantly (p <
0.05). The highest phenolic content and reducing activity were observed in the
methanolic extract corresponding to J. australis (121 mg GAE/g d.w. and
56.8 mmol Trolox/100 g d.w., respectively). However, the strongest antiradical
activity was measured in the methanolic extract of J. regia (p <
0.01).
Methanolic
extracts had the highest total phenols content and the strongest antiradical
and antioxidant activities. Extracts using polar solvents usually exhibit
higher phenolic content and positively correlate with antioxidant potential (48).
Among different factors in the extraction process, total phenolic compounds in
walnut shell varies from 1 mg/g shell to 32.76 mg GAE mg/g shell (2,
26, 48). Here, we obtained 121 mg GAE mg/g shell in methanolic extracts
of J. australis, significantly higher-more than two to ten times-than
the reported (19).
When
DPPH and FRAP activities were analyzed, methanolic extract of J. australis showed
similar activity to J. regia and cited in the literature (42). Yang
et al. (2014) analyzed the antioxidant and antiradical properties of
walnut-shell extracts with different polarity solvents, demonstrating that
methanol also shows the strongest antioxidant activity and reducing power.
These established methods are reliable indicators of antioxidant potential in
the native walnut, comparable with commercial J. regia activities,
making it a potential source of bioactive compounds.
Solvent
selection is crucial for antioxidant isolation by extraction methods. The
chosen solvent significantly affects extract yield and its antioxidant activity
due to the varying polarities of the extracted compounds (31).
Methanolic and ethyl acetate extracts are commonly used in phytochemical
studies. Methanol is a polar solvent that effectively extracts water-soluble
compounds, including phenolics, flavonoids, and alkaloids. Ethyl acetate, on
the other hand, is a less polar solvent that targets more lipophilic compounds,
such as terpenes, steroids, and some fatty acids.
This
difference in composition might depend on the genotype and environmental
conditions during development, and maturity at harvest (2).
Identification and quantification of phenolic compounds
Phenolic
compounds of walnut shell extract in J. australis and J. regia were
determined using the UHPLC-MS/MS method. Results observed for J. regia extracts
coincide with previous reports (2, 16, 18).
The methanolic extract had the highest concentration of GLC (26.8 mg/L),
followed by CMR (3.1 mg/L) and CFC (762 μg/L). Notably, rutin (RTN) was only
detected in J. regia methanolic extract, albeit at a lower concentration
(103 μg/L). In the ethyl acetate extract of J. regia, the most abundant
were GLC (0.1 mg/L), followed by CMR (607 μg/L) and CFC (μg/L).
RTN in J. regia extracts suggests the potential unique
properties of this species. Studies conducted on several parts of fruit and
leaves consistently reveal that gallic acid is among the most abundant
components in these extracts (2, 16, 18). Fernandez
Argulló et al. (2021) recently reported that
gallic, ellagic, and ferulic acids were the major phenolic compounds in walnut
wood waste extracts. However, our study did not detect ferulic acid.
On the other hand, Gallic acid (GLC), caffeic acid (CFC), and
cumaric acid (CMR) were identified in both J. australis extracts
(methanolic and ethyl acetate) (table 2).
The methanolic extract presents the highest GLC content (34000 μg/L), followed
by CMR (672 μg/L) and CFC (351 μg/L). In ethyl acetate extracts, GLC was most
abundant (9000 μg/L), followed by CMR (269 μg/L) and CFC (103 μg/L). Table 2
shows GLC content was significantly higher than CFC and CMR compounds for both
extracts. Ours is the first characterization and quantification of phenolic
compounds in this native walnut.
Table 2. Phenolic
compounds in walnut shell extracts, and quantitative analysis of phenolic
components in methanolic and ethyl acetate extracts of J. regia and J.
australis.
Tabla 2. Compuestos
fenólicos en extractos de cáscaras de nuez, y análisis cuantitativo del
contenido de componentes fenólicos en extractos de acetato de etilo y
metanólico presentes en J. regia y J. australis.
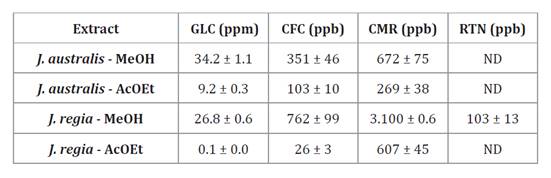
ppb = parts per billion = μg/L; ppm
= parts per million = mg/L.
Note: AcOEt: ethyl-acetate;
MetOH: methanolic; ppb = parts per billion = μg/L; ppm = parts per million =
mg/L, GLC: gallic acid; CFC: caffeic acid; CMR: cumaric acid; RTN: rutin. ND:
not detectable.
ppb = partes por billón = μg/L; ppm
= partes por millón = mg/L.
Nota: AcOEt: acetato de etilo; MetOH: metanol; LOD:
Límite de detección; ppb = partes por mil millones = μg/L; ppm = partes por
millón = mg/L; GLC: ácido gálico; CFC: ácido cafeico; CMR: ácido cumárico; RTN:
rutin. ND: no detectable.
Since
studies on phenolic compounds are mainly conducted in J. regia, more
information on J. australis extracts is needed. Considering differences
between species, essential in-depth studies would allow understanding
phytochemical profiles while identifying lost or gained compounds during crop
domestication.
Antibacterial activity and gallic acid effects on bacterial
growth
The
effects of J. regia and J. australis extract and gallic acid
(main compound in all extracts) were evaluated against phytopathogen growth (figure
2). All extracts tested showed the highest inhibition against Xanthomonas
(figure
2 A and B).
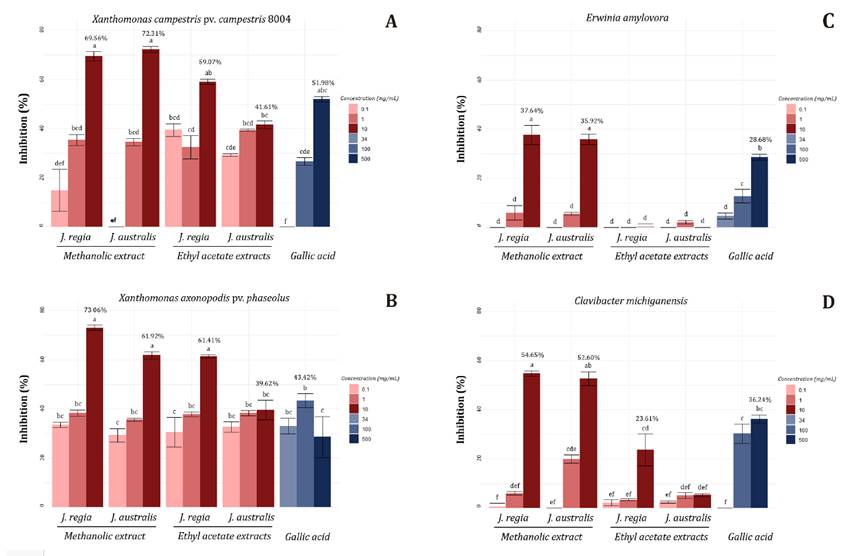
Cada
valor se expresa como media ± error estándar. Las diferencias estadísticamente
significativas se indican con letras diferentes (p < 0,01).
Figure
2. Antibacterial activity of shell extracts from
walnuts.
Figura
2 Actividades antibacterianas contra
bacterias fitopatógenos de los diferentes extractos de cáscara de nuez.
For
Xcc 8004, methanolic extracts of J. regia (69.56%) and J.
australis (72.31%) exhibited maximum inhibition at 10 mg/mL. Ethyl acetate
extracts exhibited inhibitory activity against Xcc 8004, with J.
regia demonstrating higher inhibition percentage (59.07%) than J.
australis (41.61%).
All
extracts from both Juglans sp. inhibited Xanthomonas
axonopodis pv. phaseoli, exceeding 40% (figure
2B).
Considering
Erwinia amylovora, methanolic extracts from both species diminished
bacterial development in 37.64% (J. regia) and 35.92% (J. australis)
at the highest tested concentration (figure 2C).
Once
more, methanolic extracts at 10 mg/mL reached 54.65% and 52.60% inhibition
against Clavibacter michiganensis, for J. regia and J.
australis, respectively (figure 2D).
Gallic
acid did not show substantial inhibition on the phytopathogens evaluated. At
the highest concentration, it inhibited X. axonopodis (51.95%) (figure 2).
Other studies mention antimicrobial activity of different parts of J. regia principally
against pathogens of importance in human health (38),
but none on antimicrobial effect against phytopathogens. Here, we observe that
the methanolic extract from walnut shells had the best antimicrobial activity
against the phytopathogens assayed even at the minimum concentration (0.1
mg/mL).
Shell
extracts have inhibitory effects against Xanthomonas sp. and the highest amount of gallic acid (table 2),
probably involved in antibacterial activities. Gallic acid is extensively
studied, and its mechanism of action as an effective antimicrobial is well
known (13, 21, 40).
Vu et al. (2017)
report that gallic acid in walnuts can be found either in its free form or as
part of hydrolyzable tannins. Nevertheless, gallic acid was less effective at
inhibiting the phytopathogens assayed, suggesting a synergistic effect of the
minor components.
Few studies have
examined walnut shell extracts’ antimicrobial effects, typically requiring
higher concentrations (1-100 mg/mL) (31, 32, 44). Several reports
used minimum bactericidal concentrations above 20 mg/mL for J. regia extracts
(43) with notable
activity against gram-negative bacteria like E. coli and P.
aeruginosa (36). In our study,
methanolic extracts effectively inhibited Gram-positive and Gram-negative
phytopathogens at 10 mg/mL, particularly Xanthomonas spp, as previously
reported with extracts from six walnut cultivars against Gram-positive (Bacillus
cereus, Bacillus subtilis, Staphylococcus aureus) and Gram-negative
bacteria (P. aeruginosa, E. coli, Klebsiella pneumoniae) (34).
Recently, the
search for new natural compounds has gained interest given antioxidant and
antimicrobial properties. Native plants are valued for economic and ecological
benefits, with preservation playing a vital role (22).
The scarce
information about using J. australis phytochemicals evaluates the effect
of leaves and stem extracts on Herpes simplex virus (37). Here, we could
demonstrate the efficacy of the native extracts over various phytopathogens.
Conclusion
In this study, we first report antibacterial activity of
extracts from J. australis against phytopathogenic bacteria, and first
findings on their particular antioxidant and antiradical activities. This
contributions could enhance regional value. This research first characterizes
walnut shell extracts from J. australis. Our findings demonstrate that
methanolic extracts exhibit significant antimicrobial activity against Xanthomonas
sp., suggesting natural biocontrol alternatives to copper-based
formulations.
Acknowledgments
This research
received financial support from the Agencia Nacional de Promoción Científica y
Técnica ANPCyT (PICT 2020-03408) and the Consejo Nacional de Investigaciones
Científicas y Técnicas, CONICET (PIBAA 0208CO).
The authors are grateful to the researcher, Dr. Osvaldo Delgado (PROIMI),
for providing the bacterial strains used in the antimicrobial assays.
1. Acquaviva, R.;
D’Angeli, F.; Malfa, G. A.; Ronsisvalle, S.; Garozzo, A.; Stivala, A.; Ragusa,
S.; Nicolosi, D.; Salmeri, M. and Genoveseet, C. 2021. Antibacterial and
anti-biofilm activities of walnut pellicle extract (Juglans regia L.)
against coagulase-negative staphylococci. Nat Prod Res. 35(12): 2076-81.
2. Akbari, V.;
Jamei, R.; Heidari, R.; Esfahlan, A. J. 2012. Antiradical activity of different
parts of Walnut (Juglans regia L.) fruit as a function of genotype. Food
Chemistry. 135: 2404-2410.
3. Bajpai, V. K.;
Dung, N. T.; Suh, H. J.; Kang, S. C. 2010. Antibacterial activity of essential
oil and extracts of Cleistocalyx operculatus Bbds against the bacteria
of Xanthomonas spp. JAOCS, Journal of the American Oil Chemists Society.
87: 1341-1349.
4. Barekat, S.;
Nasirpour, A.; Keramat, J.; Dinari, M.; Meziane-Kaci, M.; Paris, C.; Desobry,
S. 2022. Phytochemical composition, antimicrobial, anticancer properties, and
antioxidant potential of green husk from several walnut varieties (Juglans
regia L.). Antioxidants. 12(1): 52.
5. Bennet, R. A.;
Billing, E. 1978. Capsulation and virulence in Erwinia amylovora. Annals
of Applied Biology. 89: 41-45.
6. Benzie, I. F.
F.; Strain, J. J. 1996. The ferric reducing ability of plasma (FRAP) as a
measure of antioxidant power: The FRAP assay. Analytical Biochemistry. 239:
70-76.
7. Bernard, A.;
Lheureux, F.; Dirlewanger, E. 2018. Walnut: past and future of genetic
improvement. Vol. 14. Tree Genetics and Genomes. Springer Verlag.
8. Boiteux, J.;
Espino, M.; Fernández, M. de los Á.; Pizzuolo, P.; Silva, M. F. 2019.
Eco-friendly postharvest protection: Larrea cuneifolia-nades extract
against botrytis cinerea. Revista de la Facultad de Ciencias Agrarias.
Universidad Nacional de Cuyo. Mendoza. Argentina. 51(2): 427-437.
9. Boiteux, J.;
Fernández, M. de los Á.; Espino, M.; Fernanda Silva, M. F.; Pizzuolo, P. H.;
Lucero, G. S. 2023. In vitro and in vivo efficacy of Larrea
divaricata extract for the management of Phytophthora palmivora in
olive trees. Revista de la Facultad de Ciencias Agrarias. Universidad Nacional
de Cuyo. Mendoza. Argentina. 55(2): 97-107. DOI: https://doi. org/10.48162/rev.39.112
10. Brand-Williams,
W.; Cuvelier, M. E.; Berset, C. 1995. Use of a free radical method to evaluate
antioxidant activity. LWT-Food Science and Technology. 28: 25-30.
11. Brown, A. D.;
Grau, H. R.; Malizia, L. R.; Grau, A. 2001. In: Kappelle M, Brown AD (eds)
Bosques nublados del neotrópico. Instituto Nacional de Biodiversidad. Costa
Rica. p 623-659.
12. Carnaval, L. S. C.; Cerboneschi, M.; Tegli, S.; Yoshida, C.
M. P.; Melo, E.; Santos, A. M. P. 2022. Potential agrifood application of seriguela
(Spondias purpurea L.) residues extract and nanoZnO as antimicrobial,
antipathogenic and antivirulence agents. Research, Society and Development 11:
e37211125033. https://doi.org/10.33448/rsd-v11i1.25033
13. Cavalca, L. B.;
Zamuner, C. F. C.; Saldanha, L. L.; Polaquini, C. R.; Regasini, L. O.; Behlau,
F.; Ferreira, H. 2020. Hexyl gallate for the control of citrus canker caused by
Xanthomonas citri subsp citri. Microbiology open. 9: 1-8.
14. Daglia, M. 2012.
Polyphenols as antimicrobial agents. Current Opinion in Biotechnology. 23:
174-181.
15. Delaviz, H.;
Mohammadi, J.; Ghalamfarsa, G.; Mohammadi, B.; Farhadi, N. 2017. A review study
on phytochemistry and pharmacology applications of Juglans regia plant. Phcog Rev. 11: 145-52.
16.
Fernández-Agulló, A.; Castro-Iglesias, A.; Freire, M. S.; González-Álvarez, J.
2020. Optimization of the extraction of bioactive compounds from walnut (Juglans
major 209 x Juglans regia) leaves: Antioxidant capacity and phenolic
profile. Antioxidants. 9: 4-6.
17.
Fernández-Agulló, A.; Freire, M. S.; Ramírez-López, C.; Fernández-Moya, J.;
González-Álvarez, J. 2021. Valorization of residual walnut biomass from forest
management and wood processing for the production of bioactive compounds.
Biomass Convers Biorefin. 11(2): 609-18.
18. Hu, Q; Liu, J.;
Li, J.; Liu, H.; Dong, N.; Geng, Y.; Yang, L.; Wang, Y. 2020. Phenolic
composition and nutritional attributes of diaphragma juglandis fructus and
shell of walnut (Juglans regia L.). Food Sci Biotechnol. 29(2): 187-96.
19. Hussain, T.;
Singh, S.; Danish, M.; Pervez, R.; Hussain, K.; Husain, R. 2020. Natural
Metabolites: An eco-friendly approach to manage plant diseases and for better
agriculture farming. In natural bioactive products in sustainable agriculture.
https://doi.org/10.1007/978-981- 15-3024-1_3
20. INFOSTAT
Analytical Software version 2020e. Universidad Nacional de Córdoba. Córdoba.
Argentina.
21. Krol, E.; De
Sousa Borges, A.; Da Silva, I.; Polaquini, C. R.; Regasini, L. O.; Ferreira,
H.; Scheffers, D. J. 2015. Antibacterial activity of alkyl gallates is a
combination of direct targeting of FtsZ and permeabilization of bacterial
membranes. Frontiers in Microbiology. 6: 390.
22. Lorenzo, M. E.;
Casero, C. N.; Gómez, P. E.; Segovia, A. F.; Figueroa, L. C.; Quiroga, A.;
Werning, M. L.; Wunderlin, D. A.; Baroni, M. V. 2020. Antioxidant
characteristics and antibacterial activity of native woody species from
Catamarca, Argentina. Nat Prod Res. DOI: 10.1080/14786419.2020.1839461.
23. Luján, E. E.;
Torres-Carro, R.; Fogliata, G.; Alberto, M. R.; Arena M. E. 2019. Fungal
Extracts as Biocontrol of Growth, Biofilm Formation, and Motility of Xanthomonas
citri subsp. citri. Global Journal
of Agricultural Innovation, Research & Development. 6: 25-37.
24. Macioniene, I.;
Cepukoit, D.; Salomskiene, J.; Cernauskas, D.; Burokiene, D.; Salaseviciene, A.
2022. Effects of Natural Antimicrobials on Xanthomonas Strains Growth.
Horticulturae. 8: 7.
25. Mansfield, J.;
Genin, S.; Magori, S.; Citovsky, V.; Sriariyanum, M.; Ronald, P.; Dow, M.;
Verdier, V.; Beer S. V.; Machado, M. A. 2012. Top 10 plant pathogenic bacteria
in molecular plant pathology. Mol. Plant Pathol. 13: 614-629.
26. Masek, A.;
Latos-Brozio, M.; Chrzescijanska, E.; Podsedek, A. 2019. Polyphenolic profile
and antioxidant activity of Juglans regia L. leaves and husk extracts.
Forests. 10: 988. doi:10.3390/f10110988
27. Mateș, L.;
Rusu, M. E.; Popa, D. S. 2023. Phytochemicals and Biological Activities of
Walnut Septum: A Systematic Review. J Antioxidants. 12(3): 604.
28. Merlin, E.;
Lopez, J.; Sarmiento, H. 2014. Control del tizón del fuego en manzano. Folleto
Técnico Núm. 73. INIFAP.
29. Miller, S. A.;
Ferreira, J. P.; Lejeune, J. T. 2022. Antimicrobial use and resistance in plant
agriculture: A one health perspective. Agriculture (Switzerland). 12(2): 1-27.
30. Mohana, D. C.;
Raveesha, K. A. 2006. Antibacterial activity of Caesalpinia coriaria (Jacq.)
Willd. against plant pathogenic Xanthomonas
pathovars: an ecofriendly approach. Journal of Agricultural Technology. 2:
31.
31. Moure, A.;
Cruz, J. M.; Franco, D.; Domínguez, J. M.; Sineiro, J.; Domínguez, H. 2001.
Natural antioxidants from residual sources. Food Chem. 72: 145-171.
32. OEPP/EPPO.
2005. Clavibacter michiganensis subsp. michiganensis.
Bull. OEPP-EPPO Bull. 35: 275-283.
33. Oliveira, I.;
Sousa, A.; Ferreira, I. C. F. R.; Bento, A.; Estevinho, L.; Pereira, J. A.
2008. Total phenols, antioxidant potential and antimicrobial activity of walnut
(Juglans regia L.) green husks. Food and Chemical Toxicology. 46:
2326-2331.
34. Pereira, A. P.;
Ferreira, C. F. R.; Marcelino, F, Valentao, P.; Andrade, P.; Seabra, R.;
Estevinho, L.; Bento A.; Pereira, J. A. 2007. Phenolic compounds and
antimicrobial activity of olive (Olea europaea L. Cv. Cobrançosa)
leaves. Molecules. 12: 1153-1162.
35. Pontes, J. G.
D. M.; Fernandes, L. S.; dos Santos, R.; Tasic, L.; Fill, T. P. 2020. Virulence
factors in the Phytopathogen-Host Interactions: An overview. Journal of
Agricultural and Food Chemistry. 68: 7555-7570.
36. Raaman, N.; Mathiyazhagan,
K.; Jegadeesh, R.; Divakar, S.; Vennila, S.; Balasubramanian, K. 2011.
Antimicrobial activities of different organic extracts of nut shells of Juglans
regia (walnut). Herbal Tech Industry. 20: 22.
37. Ruffa, M. J.; Wagner, M. L.; Suriano, M.; Vicente, C.;
Nadinic, J.; Pampuro, S.; Salomón, H.; Campos, R. H.; Cavallaro, L. 2004.
Inhibitory effect of medicinal herbs against RNA and DNA viruses. Antiviral
Chemistry and Chemotherapy. 15(3): 153-159. https://doi.
org/10.1177/095632020401500305
38. Sandu-Bălan,
A.; Ifrim, I. L.; Patriciu, O. I.; Ștefănescu, I. A.; Fînaru, A. L. 2024.
Walnut by-products and elderberry extracts-sustainable alternatives for human
and plant health. Molecules. 29(2). https://doi.org/10.3390/molecules29020498
39. Sharma, M.;
Sharma, M.; Sharma. M. 2022. A comprehensive review on ethnobotanical,
medicinal and nutritional potential of walnut (Juglans regia L.).
Proceedings of the Indian National Science Academy.
https://doi.org/10.1007/s43538-022-00119-9
40. Silva, I. C.;
Polaquini, C. R.; Regasini, L. O.; Ferreira, H.; Pavan, F. R. 2017. Evaluation
of cytotoxic, apoptotic, mutagenic, and chemo- preventive activities of
semi-synthetic esters of gallic acid. Food and Chemical Toxicology. 105:
300-307.
41. Singleton, V.
L.; Rossi, J. A. 1965. Colorimetry of total phenolics with
phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 16: 144-158.
42. Soto-Maldonado,
C.; Caballero-Valdés, E.; Santis-Bernal, J.; Jara-Quezada, J.; Fuentes-Viveros,
L.; Zúñiga-Hansen ME. 2022. Potential of solid wastes from the walnut industry:
Extraction conditions to evaluate the antioxidant and bioherbicidal activities.
Electronic Journal of Biotechnology 58: 25-36.
43. Vieira, V.;
Pereira, C.; Abreu, R. M.; Calhelha, R. C.; Alves, M. J.; Coutinho, J. A. P.;
Ferreira, O.; Barros L.; Ferreira ICFR. 2020. Hydroethanolic extract of Juglans
regia L. green husks: A source of bioactive phytochemicals. Food and
Chemical Toxicology. 137: 111189. https:// doi.org/10.1016/j.fct.2020.111189
44.
Villamil-Galindo, E.; Piagentini, A. 2024. Green solvents for the recovery of
phenolic compounds from strawberry (Fragaria x ananassa Duch) and apple
(Malus domestica) agro-industrial bio-wastes. Revista de la Facultad de
Ciencias Agrarias. Universidad Nacional de Cuyo. Mendoza. Argentina. 56(1):
149-160. DOI: https://doi.org/10.48162/rev.39.131
45. Viola, C. M.;
Torres-Carro, R.; Cartagena, E.; Isla, M. I.; Alberto, M. R.; Arena, M. E. 2018.
Effect of wine wastes extracts on the viability and biofilm formation of Pseudomonas
aeruginosa and Staphylococcus aureus Strains. Evidence-Based
Complementary and Alternative Medicine. https://doi.org/10.1155/2018/9526878
46. Vu, T. T.; Kim,
H.; Tran, V. K.; Vu, H. D.; Hoang, T. X.; Han, J. W.; Choi, Y. H.; Jang, K. S.;
Choi, G. J.; Kim J. C. 2017. Antibacterial activity of tannins isolated from Sapium
baccatum extract and use for control of tomato bacterial wilt. PLoS ONE.
12: 1-12.
47. Yabalak, E.;
Erdogan Eliuz, E. A. 2021. Green synthesis of walnut shell hydrochar, its
antimicrobial activity and mechanism on some pathogens as a natural sanitizer.
Food Chemistry. 366. https://doi.org/10.1016/j.foodchem.2021.130608
48. Yang, J.; Chen, C.; Zhao, S.; Ge, F.; Liu, D. 2014. Effect
of solvents on the antioxidant activity of walnut (Juglans regia L.)
s.hell extracts. Journal of Food and Nutrition Research. 2: 621-626.