Revista de la Facultad de Ciencias
Agrarias. Universidad Nacional de Cuyo. Tomo 57(2). ISSN (en línea) 1853-8665.
Año 2025.
Original article
Comparison
of Fatty Acid Profiles of Sacha Inchi Oil (Plukenetia huayllabambana), Sesame
Oil (Sesamum indicum), and Peanut Oil (Arachis hypogaea) Using
Two Extraction Methods for Food Purposes
Comparación
de los perfiles de ácidos grasos del aceite de sacha inchi (Plukenetia
huayllabambana) aceite de sésamo (Sesamum indicum) y aceite de
cacahuete (Arachis hypogaea) utilizando dos métodos de extracción con
fines alimentarios
Jhoan Plua Montiel1,
Juan Alejandro
Neira Mosquera1, 2,
3,
Sungey Naynee
Sanchez Llaguno1,
Jhonnatan Placido
Aldas Morejon4,
Karol Yannela
Revilla Escobar4, 5*,
Edgar
Caicedo-Álvarez6
1 Universidad de las Fuerzas Armadas-ESPE, Sede Santo Domingo de
los Tsáchilas. Departamento de Ciencias de la Vida y la Agricultura, Luz de
América Vía Quevedo km 24. Ecuador.
2 Universidad Pública de Sango Domingo de los Tsachilas. UPSDT.
km 28. vía Quevedo. Santo Domingo. Ecuador.
3 Universidad Técnica Estatal de Quevedo. Facultad de Ciencias de
la Industria y Producción. Quevedo. Ecuador.
4 Universidad Nacional de Cuyo. Facultad de Ciencias Aplicadas a
la Industria. San Rafael. M5600APG. Argentina.
5 Pontificia Universidad Católica del Ecuador. Carrera de
Agroindustrias. SEDE Esmeraldas. Esmeraldas. Ecuador.
6 Universidad Estatal del Sur de Manabí. Facultad de Ciencias
Naturales y de la Agricultura. Jipijapa. Ecuador.
* kyrevilla@pucese.edu.ec
Abstract
Vegetable oil
consumption has increased in recent decades due to the high content of
monounsaturated (Omega 9) and polyunsaturated (Omega 3 and 6) fatty acids. For
this reason, this research compared the fatty acid profile of sacha inchi,
sesame and peanut oils under two extraction methods for food purposes. A
completely randomized experimental design considered an A*B factorial
arrangement with 3 repetitions. Factor A corresponds to oilseed type and Factor
B is extraction method. The results showed that both factors significantly
influenced (p<0.05) bromatological characteristics (pH, acidity, peroxide
value, relative density and ash). The lowest concentration of saturated fatty
acids was obtained in sacha inchi oil + cold pressing (6.80 g/100 g), while
monounsaturated fatty acids increased in peanut oil + hot pressing (51.51 g/100
g). Sacha inchi oil + cold pressing had the highest content of polyunsaturated
fatty acids (84.36 g/100 g).
Keywords: fatty acids,
agri-food, monounsaturated, polyunsaturated, saturated, oilseeds
Resumen
El consumo de
aceites vegetales ha aumentado en las últimas décadas debido a su alta
composición de ácidos grasos monoinsaturados (Omega 9) y poliinsaturados (Omega
3 y 6). Por esta razón, la presente investigación comparó el perfil de ácidos
grasos del aceite de sacha inchi, ajonjolí y maní a partir de dos
métodos de extracción con fines alimentarios. Se utilizó un diseño experimental
completamente aleatorizado, con arreglo factorial A*B con 3 repeticiones, donde
el Factor A correspondió al tipo de oleaginosa y el Factor B es igual a los
métodos de extracción. Los resultados mostraron que los factores de estudio
influyeron significativamente (p<0,05) en los valores de las características
bromatológicas (pH, acidez, índice de peróxidos, densidad relativa y cenizas).
Por otro lado, la menor presencia de ácidos grasos saturados se obtuvo en el
aceite de sacha inchi + prensado en frío (6,80 g/100g), mientras que, los
ácidos grasos monoinsaturados incrementaron en el aceite de de maní + prensado
en caliente (51,51 g/100g) y el aceite de sacha inchi + prensado en frío,
presentó el mayor contenido de ácidos grasos poliinsaturados (84,36 g/100g).
Palabras clave: ácidos grasos,
agroalimentario, monoinsaturados, poliinsaturados, saturados, oleaginosas
Originales: Recepción: 03/09/2024 - Aceptación: 06/02/2024
Introduction
In the constant
search for food sources promoting health and well-being, vegetable oils provide
unique fatty acid compositions and potential health benefits (36). Additionally, the
Amazon region is home to various plant species with crucial roles in global
agriculture (36). However, among
lesser-known oilseed species with potential economic value due to their
chemical properties, sacha inchi oil (P. huayllabambana), sesame oil (S.
indicum), and peanut oil (A. hypogaea) provide diverse nutritional
profiles and versatile culinary applications (3).
P. huayllabambana belongs to the Euphorbiaceae
family, native to the Amazon, known as “wild peanut”, “Inca peanut”, “Inca
inchi” or “mountain peanut” (24). It is widely
distributed in South America, particularly in the Amazon River basin.
Currently, Peru leads the production and industrialization of this plant
material, with annual seed production of approximately 1200 tons (14). However,
countries such as Colombia, Ecuador and Bolivia have also begun to venture into
agriculture and economy (10).
On the other hand, S.
indicum is an oilseed plant cultivated in China, India, Sudan, Japan,
Mexico, countries in West and Central Africa, and Central America (30). The growing
interest in the nutritional value of sesame has led to a significant increase
in its consumption and use in baking (9). This shift in
consumption habits is reflected in the increasing use of seeds in food products
at both domestic and industrial levels (9). Furthermore, S.
indicum is the sixth most economically important oilseed crop globally,
with nutritional value (fats, proteins, minerals, and vitamins) in food
security (24).
Recent research
stresses the importance of differentiating the fatty acid profiles of these
oils to optimize their use in nutrition. Notably, sacha inchi oil is
characterized by high alpha-linolenic acid (ALA), an essential omega-3 fatty
acid with cardiovascular protective effects and contribution to cognitive
development (37). Sesame oil is
rich in polyunsaturated fatty acids, particularly linoleic and oleic acids with
antioxidant and anti-inflammatory properties, positively influencing
cardiovascular and metabolic health (25). In comparison,
peanut oil has oleic and linoleic acids associated with reduced cardiovascular
risk and improved lipid profiles (2). However, the
instability of polyunsaturated fatty acids, especially in oils such as sacha
inchi, can lead to oxidation and harmful compounds when exposed to high
temperatures or improper storage. This instability can negatively affect
nutritional quality and safety (37).
Oil extraction
methods, such as cold and hot extraction, are crucial in determining oil
nutritional quality and sensory properties while influencing the stability of
fatty acids, antioxidants, and other bioactive compounds (31). Cold extraction
is a mechanical process that better preserves heat-sensitive compounds and
maintains oil nutritional and sensory quality (22). In contrast, hot
extraction uses high temperatures, accelerating extraction rates and increasing
oil yield but degrading heat-sensitive compounds and affecting quality (32).
This study aimed to
compare fatty acid profiles of sacha inchi oil (P. huayllabambana), sesame
oil (S. indicum), and peanut oil (A. hypogaea) using two
extraction methods for food purposes.
Materials
and methods
Plant
Material
For this study,
sacha inchi was obtained from the Lago Agrio canton, Sucumbíos province,
Ecuador, located 600 m a. s. l. with coordinates 0°05’05” N 76°52’58” W. Annual
temperature ranges between 20 and 35°C, ideal for its cultivation. Sesame was
acquired from the Quevedo canton, Los Ríos province, at 150 m a. s. l. with
coordinates 1°02’00” S 79°27’00” W, featuring a monsoonal tropical climate and
temperatures between 23°C and 32°C, enhancing its quality. Peanut seeds
were obtained from the Pichincha canton, Manabí province, with an average
altitude of 350 m a. s. l. and coordinates 1°02’50” S 79°49’07” W, dry tropical
climate and temperatures between 24°C and 30°C, suitable for peanut
cultivation.
Oil
Extraction Methods
Cold Press
Extraction
The seeds were
dried at room temperature until 7% humidity. Once dried, 20 kg of each plant
material were equally distributed for the different extraction methodologies.
The oils were obtained by subjecting the nuts to a hydraulic pressing process
between 246 and 250 Bar, with a piston-cylinder mechanism controlled by an
electric panel. The nuts were introduced into a perforated basket and pressed.
The expelled oil falls onto a stainless steel tray, where it is collected and
filtered through a cloth before storage.
Hot Press
Extraction
Similarly to cold
pressing, seeds were subjected to indirect heating at 90°C for 20 minutes
before pressing.
Bromatological
Analysis
Oil physicochemical
analysis included emulsifying the oils with water to determine pH, and acidity
according to NTE INEN 0038:1973 standard (16). Oleic acid was
considered the predominant acid. Peroxide evaluation followed the NTE INEN
277:1978 standard (17), and relative
density followed the NTE INEN 0035:2012 standard (19). Humidity was
analyzed by the Colombian Technical Standard NTC 287:2018 (15). Animal and
vegetable fats and oils along with moisture and volatile matter content.
Finally, ashes were quantified by the AOAC standard method (920,153).
Fatty Acid
Analysis
Before HPLC according to Oubannin et al. (2024), all samples were
esterified with 2 mL methanol and 0.5% KOH at 60 °C for 10 minutes. Then, fatty
acid methyl esters were extracted with 2 mL hexane. This mixture was
centrifuged at 3000 rpm for 5 minutes. The upper phase obtained after
centrifugation was filtered with a 0.45 μm filter for later analysis. A C18
column (250 mm × 4.6 mm, 5 μm) was mounted in the HPLC system isocratically at
35°C column temperature and operating pressure of 2000 to 2500 psi.
Acetonitrile and methanol (70:30 v/v) were passed through the mobile phase at a
flow rate of 1 mL/min and detection was performed with a UV-Vis detector at 220
nm. Volumes of 10-20 μL were injected automatically with 30 minutes of analysis
time.
Statistical
Analysis
An ANOVA was
conducted using a completely randomized block design with an A*B factorial
arrangement in triplicate. Factors were oilseed (a0: sacha inchi, a1: sesame,
and a2: peanut), and extraction method (b0: cold pressing and b1: hot pressing,
table
1).
The data obtained were analyzed with Statistica (39) including Tukey
test at p<0.05 and Statgraphics (40).
Table 1. Factors
involved in vegetable oil extraction.
Tabla
1. Factores que intervienen en la
extracción de aceite vegetal.
Results
and discussion
Bromatological Analysis
of Oils From Three Oilseeds (sacha inchi, sesame, and peanut) Extracted by Cold
and Hot Pressing
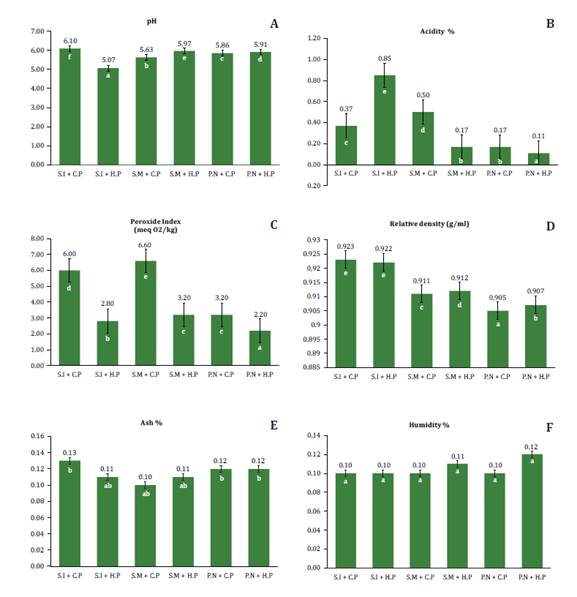
Figure 1A shows pH
variability of oils obtained by cold and hot extraction methods. We
demonstrated that both extraction methods significantly (p<0.05) affect pH
values. The highest and lowest pH values were 6.10 and 5.07, observed in sacha
inchi oil extracted by cold pressing (S.I + C.P) and hot pressing (S.I + H.P),
respectively. These results are consistent with previous studies reporting 6.11
for sacha inchi oil, 5.86 for peanut oil and 5.64 for sesame oil (29).
Sacha
inchi + prensado en frío (S.I + CP), Sacha inchi + prensado en caliente (S.I +
HP), Sésamo + prensado en frío (S.M + CP), Sésamo + prensado en caliente (S.M +
H.P), Maní + prensado en frío (P.N + C.P), Maní + prensado en caliente (P.N +
H.P).
Figure
1. Oil bromatological analyses obtained by two extraction
methods.
Figura
1. Análisis bromatológicos de aceites
obtenidos por dos métodos de extracción.
Figure 1B shows that
oilseed type significantly (p<0.05) affects acidity. Sacha inchi oil
extracted by hot-pressing showed higher acidity (0.85%). In contrast, peanut
oil showed lower acidity, with values of 0.11% and 0.17% for both extraction
methods. These results indicate that oilseed type and extraction process
determine free fatty acid content in vegetable oils. A higher free fatty acid
content, indicated by higher acidity, can affect oil stability, shelf life and
nutritional and sensory quality (12). Additionally Peroné et
al. (1999) mention that cold extraction methods generally produce oils
with lower acidity than methods involving high temperatures and solvents (28).
Figure 1C shows how cold
extraction significantly increased (p < 0.05) the peroxide content. Sesame
oil extracted by cold pressing presented the highest value, with 6.60, while
peanut oil extracted by hot pressing (P.N + H.P) showed the lowest value, 2.20.
This agrees with previous studies suggesting that increasing temperature and
heating time favors hydroperoxide formation. Varying conditions from 80°C for
10 minutes to 200°C for 20 minutes, peroxide content increased from 1.91 to
3.25 mEqO2/kg (6). This increase
reflects a greater production of primary oxidation products, attributed to the
action of free radicals on unsaturated fatty acids, such as linolenic acid,
predominant in sacha inchi oils (37, 38). Our results are
within the limit established by the Ecuadorian Technical Standard NTE INEN
34:2012 (20), which stipulates that peroxide index
of oils for human consumption must not exceed 10 mEqO/kg.
In the relative density analysis (figure 1D), a significant
influence of the type of oilseed on the variability of this property was
observed (p<0.05), highlighting the sacha inchi oil obtained by the cold and
hot extraction methods (S.I + C.P and S.I + H.P), with the highest densities of
0.923 g/ml and 0.922 g/ml, respectively. On the other hand, peanut oil
presented lower densities, with values of 0.905 g/ml and 0.907 g/ml for the
mentioned methods. These results are consistent with previous research
indicating that the density of Moringa stenopetala seed oil is 0.9 g/ml and
values ranging from 0.99 to 0.97 g/ml for sacha inchi oil when different
temperatures (90 to 110°C) are applied (18, 34). In addition, the
oil extracted from pumpkin seeds (Cucurbita pepo) presented a density of
0.09 g/ml (1).
Figure 1E details
ash contents ranging between 0.11% and 0.13%. No variability was found among
oilseeds and extraction methods (p>0.05). These values are lower than the
reported by Bonku
et al. (2020), who determined ranges from 1.2% and 2.3% in peanut oil (A.
hypogaea). Similarly, a study on sesame oil reported ash values from 1.44%
to 5.93%, considering Mida and Woremog, two different study regions (5). Discrepancies in
our crude ash content and literature values could be attributed to topographic
and climatic differences, and variations in extraction methods.
No significant
differences were found for moisture content between groups (p > 0.05), with
average values ranging from 0.10% to 0.12% (figure 1E). These findings
are consistent with previous studies reporting similar levels for oils from the
same species (13). Other studies
showed non-significant differences across production areas of sesame oil,
(5.43% - 5.81%) (38), and peanut (4.2 ±
0.5% and 3.8 ± 0.37%) for Huaquechula and Tlapanalá varieties (8). However, other
studies reported moisture variability in microencapsulated sacha inchi oil (P.
huayllabambana and P. volubilis), ranging from 3.20% to 5.87% (3).
Profile
of Fatty Acids
Saturated Fatty
Acids
Most important saturated fatty acids in vegetable oils include
C11:0 (undecanoic acid), C16:0 (palmitic
acid), C17:0 (margaric acid), C18:0 (stearic acid), C20:0 (arachidic
acid), C22:0 (behenic acid) and C24:0 (lignoceric acid). We showed that cold-pressed and
hot-pressed peanut oils had the highest values of these acids, with 17.54 g/100
g and 18.05 g/100 g, respectively. In contrast, cold-pressed sacha inchi oil
showed a lower value of 6.79 g/100 g, while hot-pressed sacha inchi oil had a
similar value, of 7.57 g/100 g (table 2). These values for sacha inchi oils, obtained by both methods,
are relatively low compared to those reported by Seid and Mehari (2022), who found a
saturated fatty acid composition of 9.38 g/100 g (38), warned that
excessive consumption of saturated fatty acids can increase cardiovascular
risk. Therefore, the aforementioned oils are interesting alternatives for human
diet, keeping cholesterol levels under control (35).
Table 2. Saturated
fatty acids in oils (sacha inchi, sesame, and peanut) obtained by cold and hot
extraction.
Tabla
2. Ácidos grasos saturados presentes
en aceites (sacha inchi, ajonjolí y maní) obtenidos por extracción en frío y
caliente.
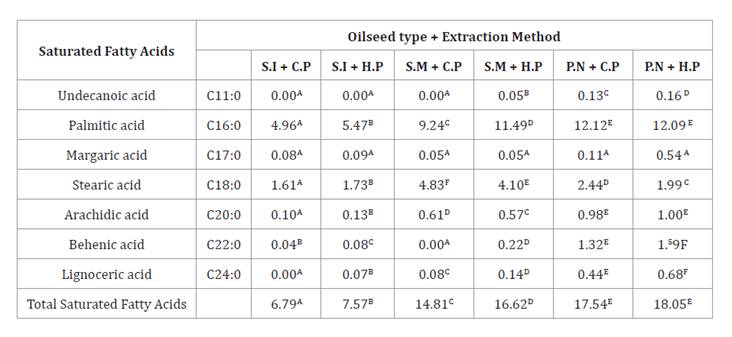
Different letters represent
statistically significant differences (Tukey p < 0.05).
Diferentes letras representan
diferencias estadísticamente significativas (Tukey p < 0,05).
Monounsaturated
Fatty Acids
Table 3 shows
monounsaturated fatty acids in the analysed oils, highlighting the main ones:
C16:1 (palmitoleic acid), C18:1 (oleic acid Omega 9), C20:1 (eicosenoic acid)
and C24:1 (nervonic acid).
Table 3. Monounsaturated
fatty acids in oils (sacha inchi, sesame, and peanut) obtained by cold and hot
pressing.
Tabla
3. Ácidos grasos monoinsaturados
presentes en aceites (sacha inchi, ajonjolí y maní) obtenidos por diferentes
métodos de extracción (prensado en frío y en caliente).
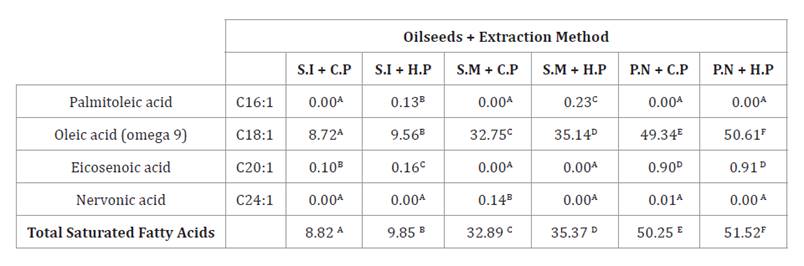
Different letters represent
statistically significant differences (Tukey p < 0.05).
Diferentes letras representan
diferencias estadísticamente significativas (Tukey p < 0,05).
The highest values of these fatty acids were observed in
hot-pressed peanut oil (51.52 g/100 g) and cold-pressed peanut oil (50.25 g/100
g), close to the monounsaturated fatty acid content of virgin olive oil (73.90
g/100 g), as reported in Spanish diets (37). Hot-pressed
sesame oil (35.37 g/100 g) and cold-pressed sesame oil (32.89 g/100 g) present
intermediate values, comparable to the 39 g/100 obtained after roasting
temperature (4). In this study,
the predominant monounsaturated fatty acid is omega-9, known for its ability to
improve resistance to LDL oxidation (a crucial factor in atherosclerosis),
given its phenolic compounds (23). According to the
White Paper on Nutrition in Spain, consuming more than 51 g/100 g of
monounsaturated fatty acids per day is inadvisable. In this context, oils
obtained from different extraction methods comply with said report (11,
21).
Polyunsaturated
Fatty Acids
Oils derived from various oilseeds constitute a significant
source of polyunsaturated fatty acids, particularly linoleic acid (C18:2,
omega-6) and alpha-linolenic acid (C18:3, omega-3). Our results showed that
sacha inchi oil, cold-pressed or hot-pressed, presented the highest
concentrations of polyunsaturated fatty acids, with 84.36 g/100 g and 82.55
g/100 g, respectively. In contrast, hot-pressed peanut oil showed a
significantly lower concentration, reaching 30.83 g/100 g (table 4).
Table 4. Polyunsaturated
fatty acids in oils (sacha inchi, sesame, and peanut) obtained by cold and hot
pressing.
Tabla
4. Ácidos grasos poliinsaturados
presentes en aceites (sacha inchi, sésamo y maní) obtenidos por diferentes
métodos de extracción (prensado en frío y en caliente).
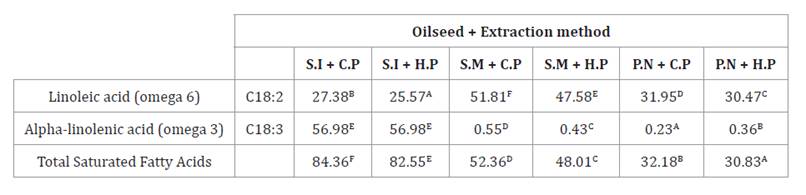
Different letters represent statistically
significant differences (Tukey p < 0.05).
Diferentes letras representan
diferencias estadísticamente significativas (Tukey p < 0,05).
These findings underline the critical influence of extraction
methods on preserving polyunsaturated fatty acids in oilseeds. Comparatively,
these results exceeded values reported for avocado oil (Persea americana) in
Ecuador (27), with total polyunsaturated fatty
acid content of 62.33 g/100 g, even considering genotype and extraction
conditions. The consumption of polyunsaturated fatty acids prevents various
chronic diseases, like diabetes mellitus, obesity and cardiovascular diseases.
These fatty acids activate the PPARα receptor (peroxisome
proliferator-activated receptor alpha), which stimulates lipid oxidation,
reduces insulin resistance and prevents hepatic steatosis (33).
Conclusions
This study demonstrated that oilseed type and extraction method
significantly influenced bromatological characteristics (pH, acidity, peroxide
index, relative density, and ash), except moisture content. Regarding fatty
acid profile, sesame and peanut oils (both cold-pressed and hot-pressed) are
excellent sources of monounsaturated fatty acids, with higher concentrations of
omega-9 than sacha inchi oil. On the other hand, sacha inchi oil constitutes a
source of polyunsaturated fatty acids, particularly omega-3 and omega-6.
Consequently, these oilseeds may enrich the human diet, while offering
industrial and food applications.
1. Abubakar, M.;
Mohammed-Adewumi, A.; Amina-Ladidi, M.; Jibril -Hassan, L.; Agatha, N. 2024.
Physicochemical properties of oil extracted from pumpkin (Cucurbita pepo)
Seeds. Lafia Journal of Scientific & Industrial Research. 5-9.
https://doi.org/10.62050/ljsir2024. v2n1.276
2. Akhtar, S.;
Khalid, N.; Ahmed, I.; Shahzad, A.; Ansar, H.; Suleria. 2014. Physicochemical
characteristics, functional properties, and nutritional benefits of peanut oil:
A review. Critical Reviews in Food Science and Nutrition. 4.
https://doi.org/10.1080/10408398.2011.644353
3. Alarcón-Rivera,
R.; Pérez-Camino, M. C.; Chasquibol-Silva, N. 2019. Evaluation of the shelf
life of microencapsulated sacha inchi oils (Plukenetia huayllabambana and
Plukenetia volubilis). Journal of the Peruvian Chemical Society. 85(3).
http://www.scielo.org.pe/scielo. php?script=sci_arttext&pid=S1810-634X2019000300005
4. Arab, R.; Casal,
S.; Pinho, T.; Cruz, R.; Lamine Freidja, M.; Lorenzo, J.; Hano, C.; Mafani, K.;
Makhlouf, L. 2022. Effects of seed roasting temperature on sesame oil fatty
acid composition, lignan, sterol and tocopherol contents, oxidative stability
and antioxidant potential for food applications. Moleculas. 27(4).
https://doi.org/https://doi.org/10.3390/molecules27144508
5. Beshaw, T.;
Demssie, K.; Tefera, M.; Guadie, A. 2022. Determination of proximate
composition, selected essential and heavy metals in sesame seeds (Sesamum
indicum L.) from Ethiopian markets and assessment of the associated health
risks. Toxicology Reports, 1806-1812. https://doi.org/10.1016/j.
toxrep.2022.09.009
6.
Bocanegra-Morales, N.; Galeano-Garcia, P. 2023. Chemical composition, fatty
acid profile, and optimization of the sacha inchi (Plukenetia volubilis L.)
seed-roasting process using response surface methodology: Assessment of
oxidative stability and antioxidant activity. Foods. 12(18).
https://doi.org/https://doi.org/10.3390%2Ffoods12183405
7. Bonku, R.; Yu,
J. 2020. Health aspects of peanuts as an outcome of its chemical composition.
Food Science and Human Wellness. 9(1): 21-30.
https://doi.org/10.1016/j.fshw.2019.12.005
8. Bravo, A.;
Navarro, E.; Rincón, C.; Soriano, M. 2018. Physicochemical characteristics and
fatty acid profile of two cultivars. Revista de Ciencias Naturales y Agropecuarias.
5(15): 9-18. https://
www.ecorfan.org/bolivia/researchjournals/Ciencias_Naturales_y_Agropecuarias/
vol5num15/Revista_de_Ciencias_Naturales_y_Agropecuarias_V5_N15_3.pdf
9. Edmund, H.; Sam,
P. 2017. Anti-inflammatory and antioxidant effects of sesame oil on
atherosclerosis: A descriptive literature review. Cureus. 9(7).
https://doi.org/10.7759%2Fcureus.1438
10. FEN (Spanish
Nutrition Foundation). 2013. White paper on nutrition in spain madrid: Spanish
Foundation. Spanish Food Safety and Nutrition Agency.
https://www.sennutricion.org/
media/Docs_Consenso/Libro_Blanco_Nutricion_Esp-2013.pdf
11. Haile, M.;
Duguma, H. T.; Chameno, G.; Kuyu, C. G. 2019. Effects of location and
extraction solvent on physico chemical properties of Moringa stenopetala seed
oil. Heliyon. 5(11). https://doi. org/10.1016/j.heliyon.2019.e02781
12. Hu, T.; Zhou,
L.; Kong, F.; Wang, S.; Hong, K.; Lei, F.; He, D. 2023. Influence of oilseed
type and extraction method on free fatty acid content in vegetable oils. Foods.
12(18): 3351. https://doi.org/https://doi.org/10.3390/foods12183351
13. ICONTEC
(Instituto Colombiano de Normas Técnicas). 2018. NTC 287:2018 - Animal and
vegetable fats and oils. Determination of moisture and volatile matter content.
Animal and vegetable fats and oils. Determination of moisture and volatile
matter content. https://tienda. icontec.org/gp-grasas-y-aceites-animales-y-vegetales-determinacion-del-contenido-dehumedad-y-materia-volatil-ntc287-2018.html
14. INEN
(Ecuadorian Institute for Standardization). 1973. Ecuadorian Technical Standard
0038. Edible fats and oils. Determination of acidity. Ecuadorian
standardization service.
https://www.academia.edu/8969698/NTE_INEN_0038_Grasas_y_aceites_comestibles_
Determinaci%C3%B3n_de_la_acidez
15. INEN (Ecuadorian Institute for Standardization). 1978.
Ecuadorian Technical Standard 277. Fats and oils. Determination of the Peroxide
Index. Ecuadorian standardization service.
https://es.scribd.com/document/405847035/Inen-277-Indice-de-Peroxido
16. INEN
(Ecuadorian Institute for Standardization). 2012. Ecuadorian Technical Standard
0035. Animal and vegetable oils and fats determination of relative density.
Ecuadorian standardization service.
https://es.scribd.com/document/339261140/NTE-INEN- 35-1#:~:text=informaci%C3%B3n%20del%20documento-,Esta%20norma%20
describe%20el%20m%C3%A9todo%20del%20picn%C3%B3metro%20para%20
determinar%20la,relativa%20utilizando%20una%20f%C3%B3rmula%20dada.
17. INEN
(Ecuadorian Institute for Standardization). 2012. Ecuadorian Technical Standard
34. Blend of edible vegetable oils. Requirements. Ecuadorian standardization
service. https://es.scribd.com/document/339261140/NTE-INEN-35-https://es.scribd.com/
document/534182662/nte-inen-34-2-Mezcla-de-aceites
18. Kittibunchakul,
S.; Hudthagosol, C.; Sanporkha, P.; Sapwarobol, S.; Temviriyanukul, P.;
Suttisansanee, U. 2022. Evaluation of sacha inchi (Plukenetia volubilis L.)
by-products as valuable and sustainable sources of health benefits.
Horticulturae. 344: 8. https://doi.
org/https://doi.org/10.3390/horticulturae8040344
19. León-Sánchez,
G.; Monteagudo-Borges, R.; Rodríguez-Jiménez, E. 2022. Characterization of the
oil extraction process of Moringa oleifera in relation to seed type.
Tecnología Química. 42(1).
http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S2224-61852022000100024
20. Misganaw-
Gedlu, A.; Amare -Aregahegn, D.; Atlabachew, M.; Abebe, W. 2021. Fatty acid
composition, total phenolic contents and antioxidant activity of white and
black sesame seed varieties from different localities of Ethiopia. Chemical and
Biological Technologies in Agriculture. 8(1). https://doi.org/https://doi.org/10.1186/s40538-021-00215-w
21. Mohamed, A.;
Ferhat, B.; Meklati, F. C. 2007. Comparison of different extraction methods:
cold pressing, hydrodistillation, and solvent free microwave extraction, used
for the isolation of essential oil from Citrus fruits. Journal of
Chromatography A. 1210(2): 139-147. https://
doi.org/10.1016/j.chroma.2008.09.085.
22. Montero-Torres,
J. 2020. Nutritional and economic importance of peanut (Arachis hypogaea L.).
Journal of Agricultural Research and Innovation. 7(2).
http://www.scielo.org.bo/scielo. php?pid=S240916182020000200014&script=sci_abstract
23.
Nagendra-Prasad, M.; Sanjay, K.; Deepika, S.; Vijay, N.; Kothari, R.;
Nanjunda-Swamy, S. 2012. A review on nutritional and nutraceutical properties
of sesame. Nutrition & Foods Sciences. 2(127).
https://doi.org/http://dx.doi.org/10.4172/2155-9600.1000127
24. Neira Mosquera,
J. A.; Coello Culluzpuma, A.; Sanche LLaguno, S. N.; Plua Montiel, J.; Viteri
García, I. P. 2021. Study on the effect of variety and extraction conditions of
avocado (Persea americana) oil for food purposes in Ecuador. Clinical
Nutrition and Hospital Dietetics. 94-98.
https://www.revistanutricion.org/articles/study-of-the-effects-ofvariety-and-conditions-of-the-avocado-oil-persea-americana-extraction-process-forfood-purposes-.pdf
25. Neira-Mosquera,
J. A.; Menéndez-Viteri, O. F.; Ullón-Arcia, J. A.; Sánchez-Llaguno, S. N. 2022.
Study of the vegetable oils of sacha inchi (Plukenetia huayllabambana), sesamum
indicum and peanuts (Arachis hypogaea) and their influence on the
preparation of “Frankfurt” type vegetable sausages, considering bromatological
and organoleptic characteristic. Journal of Pharmaceutical Negative Results.
13(3): 623-627.
26. Oubannin, S.;
Bijla, L.; Ahmed, M.; Ibourki, M.; El Kharrassi, Y.; Devkota, K.; Bouyahya, A.;
Maggi, F.; Caprioli, G.; Sakar, E.; Gharby, S. 2024. Recent advances in the
extraction of bioactive compounds from plant matrices and their use as
potential antioxidants for vegetable oils enrichment. Journal of Food
Composition and Analysis. 128. https://doi.
org/https://doi.org/10.1016/j.jfca.2024.105995
27. Panpan Wei, F.
W.; Xiaoyun, C.; Guige, H.; Qingguo, M. 2022. Sesame (Sesamum indicum L.):
A comprehensive review of nutritional value, phytochemical composition, health
benefits, development of food, and industrial applications. Nutrients. 14(19):
4079. https://doi. org/10.3390/nu14194079
28. Peroné, J.;
Ruiz-Gutiérrez, V.; Barrón, L. 1999. High performance liquid chromatography for
the separation of triglycerides from complex animal fats. Fats and Oils. 50(4):
298-311.
29. Rivera, M.;
Ramos, M.; Silva, M.; Briceño, J.; Álvarez, M. 2022. Effect of pre-extraction
temperature on the yield and fatty acid profile of morete (Mauritia
flexuosa L. F.) oil. La Granja. 35(1): 98-111.
https://doi.org/10.17163/lgr.n35.2022.08
30. Rodríguez, G.;
Villanueva, E.; Glorio, P.; Baquerizo, M. 2015. Oxidative stability and shelf
life estimation of sacha inchi (Plukenetia volubilis L.) oil. Scientia
Agropecuaria. 6(3): 155-163. http://dx.doi.org/10.17268/sci.agropecu.2015.03.02
31. Rodriguez Cruz,
M.; Tovar Armando, R.; Del Prado, M.; Torres, N. 2005. Molecular mechanisms of
action of polyunsaturated fatty acids and their health benefits. Journal of
Clinical Research. 57(3): 457-472.
https://www.scielo.org.mx/pdf/ric/v57n3/v57n3a10.pdf
32. Romero- Hidalgo, L. E.; Valdiviezo- Rogel, C. J.; Bonilla
-Bermeo, S. M. 2019. Characterization of sacha inchi (Plukenetia
volubilis) seed oil from San Vicente, Manabí, Ecuador, obtained using non-thermal
extrusion processes. La Granja. 30(2): 77-78. https://doi.org/https://
doi.org/10.17163/lgr.n30.2019.07
33. Ruiz, S.;
Sánchez, E.; Tabares Villareal, E.; Prieto, A.; Arias, J.; Gómez, R.;
Castellanos, D.; García, P.; Chaparro, S. 2007. Biological and cultural
diversity of the southern colombian amazon-diagnosis. Bogotá, Colombia:
Coporamazonia, Humboldt Institute, Sinchi Institute.
https://doi.org/https://repository.humboldt.org.co/entities/publication/a8f09059-
7552-4fa8-968f-cd7a553af361
34. Schwingshackl,
L.; Bogensberger, B.; Benčič, A.; Knüppel, S.; Boeing, H.; Hoffmann, G. 2018 . Effects of oils and solid fats on blood lipids: a
systematic review and network meta-analysis. J Lipid Res. 59(9): 1771-1782.
https://doi.org/10.1194/jlr.p085522
35. Seid, F.;
Mehari, B. 2022. Elemental and proximate compositions of sesame seeds and the
underlying soil from Tsegede, Ethiopia. Int J Anal Chem.
https://doi.org/10.1155%2F2022%2F1083196
36. Suri, K.;
Singh, B.; Kaur, A.; Singh, N. 2019. Impact of roasting and extraction methods
on chemical properties, oxidative stability and maillard reaction products of
peanut oils. J. Food Sci. Technol. 56: 2436-2445.
37. Torrejón, C.;
Uauy, R. 2011. Fat quality, atherosclerosis and coronary heart disease: effects
of saturated fatty acids and trans fatty acids. Journal of Medicine of Chile.
139(7): 924-931.
38. Xu, B.; Chang,
K. 2008. Total phenolics, phenolic acids, isoflavones, and anthocyanins and
antioxidant properties of yellow and black soybeans as affected by thermal
processing. J. Agric. Food Chem. 56: 7165-7175.
https://doi.org/10.1021/jf8012234
39. TIBCO Software.
2023. Software version 15.0 [Software]. TIBCO Software Inc.
40. StatPoint Technologies. 2024. STATGRAPHICS Centurion XVII
[Software]. StatPoint Technologies Inc.