Revista de la Facultad de Ciencias
Agrarias. Universidad Nacional de Cuyo. En prensa. ISSN (en línea) 1853-8665.
Original article
Enterococcus
gallinarum CRL 1826 as a probiotic for ranaculture: in vitro safety,
technological, and physiological properties
Enterococcus
gallinarum CRL 1826 como probiótico para ranicultura: seguridad in vitro,
propiedades tecnológicas y fisiológicas
María Andreína Acevedo1,
María Claudia Otero1,
María Constanza Lizárraga2,
Cesar Emmnuel Ale1,
Ricardo Javier Llanos2,
María Elena Nader-Macías3,
1 Instituto Superior de Investigaciones Biológicas
(INSIBIO-CONICET). Chacabuco 461. T4000ILI. San Miguel de Tucumán. Argentina.
2 Universidad Nacional de Tucumán. Facultad de Bioquímica,
Química y Farmacia. Instituto de Biología “Dr. Francisco D. Barbieri”.
Chacabuco 461. T4000ILI. San Miguel de Tucumán. Argentina.
3 Centro de Referencia para Lactobacilos (CERELA-CONICET).
Chacabuco 145. T4000ILC. San Miguel de Tucumán. Argentina.
* sergio.pasteris@fbqf.unt.edu.ar
Abstract
This study aimed to
progress in designing a probiotic containing autochthonous Enterococcus
gallinarum CRL 1826 for application during the life cycle of Lithobates
catesbeianus in hatchery conditions. We assessed bacterial resistance to
chemotherapeutics used in ranaculture, the presence of genes encoding virulence
factors (VF) and vancomycin resistance, and bacterial survival and maintenance
of beneficial properties after freeze-drying and storage. The strain exhibited
resistance to antiseptics, sensitivity to most chemotherapeutics, presence of vanC,
and absence of VF genes. It demonstrated resistance to freeze-drying and the
highest survival when using skim milk+sucrose and storage at 4°C for 24 months.
It also displayed bacteriocin activity against Listeria monocytogenes.
Pre-lyophilized and lyophilized cultures grew/resisted individual
gastrointestinal conditions and simulated gastrointestinal digestion, keeping
bacteriocin activity and surface properties. For the first time, we
demonstrated that E. gallinarum CRL 1826 is a safe bacterium with
technological and physiological properties that would allow bullfrog gut
colonization. These studies are essential for progressing towards selecting E.
gallinarum CRL 1826 as a probiotic to prevent epizootics during bullfrog
breeding and control foodborne bacteria, potentially improving growth
performance of L. catesbeianus.
Keywords: Enterococcus
gallinarum, safety characteristics, lyophilization, probiotics for
aquaculture, bullfrog
Resumen
En este estudio se
avanzó en el diseño de un probiótico con Enterococcus gallinarum CRL
1826, autóctono de Lithobates catesbeianus, para su aplicación durante
el ciclo de vida de este anfibio en criaderos. Evaluamos: resistencia
bacteriana a quimioterapéuticos utilizados en ranicultura, presencia de genes
que codifican para factores de virulencia (FV) y resistencia a vancomicina, y
sobrevida y mantenimiento de propiedades benéficas luego de la liofilización y
almacenamiento. La cepa fue resistente a antisépticos, sensible a la mayoría de
los quimioterapéuticos, posee el gen vanC y ausencia de genes de FV.
Resistió la liofilización, con la mayor sobrevida con leche descremada+sacarosa
durante su almacenamiento a 4°C, 24 meses. También presentó actividad
bacteriocina frente Listeria monocytogenes. Los cultivos
pre-liofilizados y liofilizados crecieron/resistieron en las condiciones
individuales y luego de la digestión gastrointestinal simulada, manteniendo la
actividad bactericiona y propiedades de superficie. Por primera vez, demostramos
que E. gallinarum CRL 1826 es una bacteria segura con propiedades
tecnológicas y fisiológicas que le permitirían colonizar el intestino de rana
toro. Estos estudios permiten avanzar en la selección de E. gallinarum CRL
1826 como probiótico para prevenir epizootias durante la cría de rana toro,
controlar bacterias transmitidas por alimentos y, potencialmente, mejorar el
rendimiento de crecimiento de L. catesbeianus.
Palabras clave: Enterococcus
gallinarum, características de seguridad, liofilización, probióticos para
acuicultura, rana toro
Originales: Recepción: 17/09/2024
- Aceptación: 12/12/2024
Introduction
In aquaculture,
ranaculture is committed to amphibian breeding. Over recent decades,
ranaculture has experienced significant growth, driven by conservation efforts (46) and commercial
interests.
The American
bullfrog (Lithobates catesbeianus) provides meat for human consumption
and various by-products such as gut, liver, adipose tissue oil, and carcasses (50). Additionally, L.
catesbeianus is reared in captivity to obtain skin for the pharmaceutical
industry as a source of biologically active molecules including biogenic
amines, hormones (54), anti-tumor
agents, antimicrobials, and antioxidants (22,
51).
Profitability of
amphibian hatcheries is linked to animal health status, as demonstrated in
other aquaculture activities (17). The microbiome
includes the microbiota, microbial metabolites, and genetic elements (3), exerting
physiological functions for the host, including maintenance of ecological
equilibrium, immunological modulation, and infectious disease prevention (18). However, in
intensive breeding systems like bullfrog production, with high animal densities
and consequent crowded conditions, the microbiome may undergo modifications
favoring epizootics caused by pathogens or potential pathogens that usually
belong to native microbiota (31, 35, 36).
The Red-Leg
Syndrome (RLS) represents a major infectious disease affecting bullfrog
hatcheries, resulting in high mortality and significant economic losses (11). The RLS-related
pathogens include Aeromonas hydrophila, Citrobacter freundii, Pseudomonas
aeruginosa, and Proteus vulgaris, entering the host via the
gastrointestinal tract or skin, and affecting the animals at different ages (55). Therapeutic and
preventive measures often involve using antiseptics and antibiotics (19,
52). However, these procedures can affect native microbiota and
contribute to antibiotic resistance (52). Consequently, a
novel alternative introduces the application of probiotics to restore the
microbiome (18).
Our research group
has investigated the microbial population of bullfrog hatcheries, revealing
that lactic acid bacteria (LAB) and RLS-related pathogens are part of the
native microbiota of animals and hatchery environments (31,
35, 36). Among LAB, Lactococcus lactis CRL 1584, CRL 1827, Lactiplantibacillus
plantarum CRL 1606, and Enterococcus gallinarum CRL 1826 were
preselected as potential probiotics for ranaculture (31,
33, 36, 37, 38). However, E. gallinarum CRL 1826 emerged as a promising
strain with antimicrobial activity against native RLS-related pathogens and Listeria
monocytogenes (responsible for bullfrog meat spoilage), adhesion and
colonization properties (31, 34), and in vivo safety
when administered to bullfrog embryos (38). Nevertheless,
additional studies are needed to fully validate the hypothesis that E.
gallinarum CRL 1826 exhibits suitable properties as a probiotic for
bullfrog breeding.
This study aimed to
assess the in vitro safety profile to chemotherapeutics used in bullfrog
farms, the presence of virulence factors and antibiotic-encoding genes, and the
bacterial resistance and maintenance of beneficial properties after
freeze-drying, storage, and exposure to gastrointestinal conditions.
Materials
and methods
Bacterial
strains and culture conditions
Enterococcus
gallinarum CRL 1826 was isolated from healthy L. catesbeianus and
identified by phenotypic and genotypic approaches (31). The LAB
strain was grown on de Man, Rogosa & Sharpe (MRS) broth pH 6.8 (10 h at
37°C) in microaerophilia. Pseudomonas aeruginosa 1007 and 1047, and C.
freundii CFb (RLS-related pathogens from ranaculture) were grown in
nutritive broth pH 6.9 for 7 h, while L. monocytogenes Scott A was grown
in BHI broth, pH 7.4 for 6 h (29, 34). All cultures were incubated
at 37°C in microaerophilia. Bacterial strains were stored at -20°C in their
specific growth media supplemented with 20% (v/v) glycerol.
Chemotherapeutics
resistance of native microorganisms from bullfrog hatcheries
Vancomycin
resistance of E. gallinarum CRL 1826 was determined via the methodology
proposed by “Clinical
and Laboratory Standards Institute” (CLSI) (2015). The strain was
grown in MRS broth (10 h at 37°C) and adjusted to an OD625nm of 0.08-0.10, corresponding
with 0.5 of McFarland scale (0.5% BaSO4).
Bacterial suspensions were seeded by spread on Müeller Hinton (MH) agar discs
containing 30 μg vancomycin (Britania Laboratories, Argentina). The plates were
incubated for 24 h at 37°C. Vancomycin susceptibility was determined by
considering breakpoint values proposed by CLSI (2022). The MIC for
vancomycin and other antibiotics (Amikacin, Ceftazidime, Ciprofloxacin,
Chloramphenicol, Oxytetracycline, Penicillin), and Metronidazole (Britania
Laboratories, Argentina) frequently applied in bullfrog hatcheries, was also
determined via agar dilution method. Thus, antibiotic solutions were prepared
to final concentrations in MH plates from 0.25 to 512 μg/mL. In each plate, 104 CFU/mL of the strain
were inoculated and incubated at 37°C for 24 h. Susceptibility determination
considered breakpoint values (8, 20, 48). Finally, we
evaluated the MIC of P. aeruginosa strains, C. freundii, and L.
monocytogenes to antiseptics (methylene blue, malachite green, benzalkonium
chloride, CuSO4,
KMnO4) commonly
used in ranaculture (4). Final antiseptic
concentrations in MH plates ranged from 0.25 to 16,384 μg/mL. Later, indicator
strains were inoculated and incubated as previously indicated. The MIC was
defined as the lowest antiseptic concentration completely inhibiting growth of
the tested microorganisms.
Vancomycin
resistance and virulence factors genes in E. gallinarum CRL 1826
For DNA isolation,
cells from a 10 h culture of E. gallinarum on MRS broth were harvested
(3,000 x g, 10 min, at 4°C), washed with TE buffer (10 mM Tris-HCl pH 8,
10 mM EDTA pH 8), and processed according to Pospiech and Neumann (1995). The polymerase
chain reaction (PCR) technique amplified genes encoding vancomycin resistance (vanA,
vanB, and vanC) and those for virulence factors (agg, gelE,
esp, efa, and cylA) in E. gallinarum CRL 1826.
Primers and PCR conditions are described in Supplementary Table S.1. The reaction
mixture was prepared with DNA sample, 20 ng; PCR buffer 10X, 2.5 μL; MgCl2
(1.5 mM), 1.25 μL; dNTPs (200 μM), 1 μL each; Taq DNA
polymerase (0.1 U/mL), 0.25 μL; primer forward/f/ and primer reverse/r/ (0.5
μM), 2.5 μL each; MilliQ water to achieve 25 μL.
10X PCR buffer: 200
mM Tris-HCl pH 8.4; 500 mM KCl.
A MyCycler thermal cycler from BioRad (BioRad, Richmond,
California, USA) was used for the PCR reaction.
Separation of the
amplification products was performed according to Sambrook and Gething (1989). The run was
carried out with 1X TAE buffer (0.04 M Tris-Acetate; 1 mM EDTA, pH 8) by
applying 85 V. A 1 Kb DNA Ladder (Invitrogen, Argentina) was used as a
molecular weight marker. Gels were then stained with ethidium bromide (1 μg/mL)
and DNA visualized with a U.V. light transilluminator (λ=320 nm).
Viability
and bacteriocin activity of E. gallinarum CRL 1826 after freeze-drying
and storage
The LAB strain was
grown in MRS (10 h at 37°C). Cells were harvested by centrifugation (3,000 x g,
10 min at 4°C), washed twice with sterile distilled water, and centrifuged. The
pellets were re-suspended in lyoprotectants (w/v): 10% lactose (L), 10% sucrose
(S), 10% skim milk (SM), 10% whey protein concentrate (WPC; Lacprodan 35,
Arla-Foods Ingredients, Argentina), 5% L + 5% S (L-S), 5% SM + 5% L (SM-L), 5%
SM + 5% S (SM-S), 5% WPC + 5% L (W-L), and 5% WPC + 5% S (W-S) to obtain ~1011
CFU/mL. Cells were also re-suspended in neutral sterile distilled
water (control). Finally, samples were frozen at -20°C for 12 h, and
lyophilized according to Montel Mendoza et al. (2014). Dried cells were
distributed in glycogelatin capsules, packed in plastic bottles with silica
gel, and stored at 4 and 25°C for 24 months. The number of viable cells (CFU)
before and after freeze-drying was determined by the serial dilution method and
plated on MRS agar (1.5% w/v). Cell viability for each lyoprotectant was
expressed as Survival Factor (SF), and calculated as follows:
SF=1 - (Log CFUbefore - Log CFUafter)
/ Log CFUbefore
CFUbefore=CFU/mL
x total culture volume (mL)
CFUafter=CFU/mL
x [total weight pre-lyophilized sample (g) / weight lyophilized sample (g)]
Cell viability
during storage was expressed as Survival Factor during t month of
Storage (SFSt), and calculated
as follows:
SFSt=1
- (Log CFU0 -Log CFUt)
/ Log CFU0
CFU0=initial
CFU/g x total weight of dried sample (g); CFUt=t
time CFU/g x total weight of dried sample (g).
The maintenance of bacteriocin activity of E. gallinarum CRL
1826 was determined after lyophilization and during storage by the plate
dilution method (34). Thus, the LAB
strain was inoculated in MRS broth, and incubated for 72 h at 37°C with
subsequent cultures every 24 h. When cultures reached an OD⁓1.0 (540 nm),
cell-free supernatants were obtained and stored (-20°C) until bacteriocin
activity determination (33). The antimicrobial
titer was defined as the reciprocal of the greatest two-fold dilution producing
a clear inhibition zone of ~1 mm, expressed as arbitrary units per milliliter
of culture supernatant (AU/mL). Besides, bacteriocin activity was expressed as
relative activity to freeze-dryng (RA) and storage (RAS), and calculated by equations:
Growth
and viability of E. gallinarum CRL 1826 at different pH values, bile
salts, and digestive enzymes
Assays were
conducted with pre-lyophilized (PL) and lyophilized (L) (5% SM + 5% S) E.
gallinarum CRL 1826 cells. Thus, 106 CFU/mL
were inoculated in the following media: LAPTg broth at pH 2, 3, 4, 5, 6, 6.8
(optimal bacterial growth), 7, and 8; LAPTg broth + pepsin 0.05; 0.15; 0.3, and
0.6% at pH 2; LAPTg broth + bile 0.1; 0.3; 0.5; 1; 1.5; 3; 6, and 10% at pH 8,
and LAPTg broth + pancreatin: 0.01; 0.05; 0.1; 0.15, and 0.2% at pH 8. Cultures
were incubated at 37°C and bacterial growth was determined by optical density
(OD540 nm) for
24 h. In media without OD changes, 109 CFU/mL
were inoculated and incubated for 18 min at different pH and pepsin
concentrations, 10 min with 1 to 10% bile, and 90 min with 0.3 to 0.5% bile.
Subsequently, CFU/mL were determined on LAPTg agar (incubation at 37°C, 24 h,
in microaerophilia) and a Survival Factor (SF) was calculated. Likewise, cells
from each medium were grown as described, determining AU/mL.
Simulated
gastrointestinal digestion model
For these
experiments, we considered the highest individual concentrations of each factor
allowing high E. gallinarum CRL 1826 viability and bacteriocin activity,
as well as gastrointestinal conditions reported for adult amphibians (47). Therefore, 109
CFU/mL of PL and L cultures were resuspended in PBS solution (pH
7.4) + pepsin 0.6%, and incubated for 90 min at 37°C. During this time, a
gradual pH decrease (7.4 to 2) was induced using 1N HCl and samples were taken
at 0, 30, 60, and 90 min (Phase 1: stages a, b, c, and d, respectively). Then,
the suspensions were centrifuged and cells were resuspended in PBS solution pH
8 + 1% bile (OX-bile, FLUKA) for 10 min (Phase 2: stage e). Subsequently, cells
were collected and inoculated in PBS solution pH 8 containing 0.3% bile + 0.1%
pancreatin (SIGMA-ALDRICH), and samples were taken at 30, 60, and 90 min (Phase
3: f, g, and h, respectively). At each stage, SF and AU/mL were evaluated.
Finally, surface properties (hydrophobicity and autoaggregation) were determined
according to Niederle
et al. (2019).
Statistical
analysis
Data processing was
done using Minitab (30) and Infostat (13) softwares. Results
are the average of three independent assays, evaluated by ANOVA or General
Linear Models. When residuals showed a normal distribution, a post-test (at
0.05 significant levels) for multiple comparisons was performed. When
assumptions were not met, nonparametric variance analysis was applied (Mood’s
median test).
Results
Susceptibility
to antimicrobials of E. gallinarum CRL 1826, RLS-related pathogens, and L.
monocytogenes
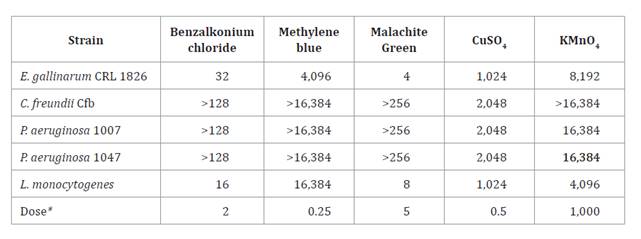
Table 1 shows MIC values
for chemotherapeutics frequently used in bullfrog hatcheries.
Table 1. Minimum
inhibitory concentration (μg/mL) of antimicrobials frequently used in
ranaculture.
Tabla
1. Concentración inhibitoria mínima
(μg/mL) de antimicrobianos frecuentemente utilizados en ranicultura.

a (7); b (20); c (48).
Based on breakpoint values, E. gallinarum CRL 1826 was
sensitive to most antimicrobials except ceftazidime and metronidazole, whose
MIC values were 512 and >512, respectively. Concerning vancomycin, the disc
diffusion assay revealed that the LAB strain was sensitive (data not shown),
and the MIC value below the breakpoint. Moreover, MIC of antiseptics for E.
gallinarum CRL 1826, C. freundii, P. aeruginosa, and Listeria
monocytogenes was also determined (table 2).
Table 2. MIC
(μg/mL) of antiseptics frequently used in ranaculture.
Tabla
2. CIM (μg/mL) de antisépticos
frecuentemente utilizados en ranicultura.
* Antiseptic concentrations used
in bullfrog hatcheries (4).
* Concentraciones de antisépticos
utilizados en la cría de rana toro (4).
Therefore, all
strains showed MIC values greater than the dose usually used in ranaculture,
excepting E. gallinarum CRL 1826 when using malachite green. Therefore, E.
gallinarum CRL 1826 is sensitive to antibiotics and resistant to
antiseptics used in bullfrog farms.
Vancomycin
resistance and virulence factors genes in E. gallinarum CRL 1826
The presence of vanA, vanB, and vanC genes
was determined by PCR amplification (figure 1A). In lane 3, an
approximately 800 bp band was detected and matched with the amplification
product of vanC gene (822 bp). Concerning virulence factor genes
described for Enterococcus, the LAB strain did not display any tested
factor (figure
1B)
when compared to the standard band size (Supplementary, Table S.1). Thus, our
results demonstrate that E. gallinarum CRL 1826 is a safe LAB strain.
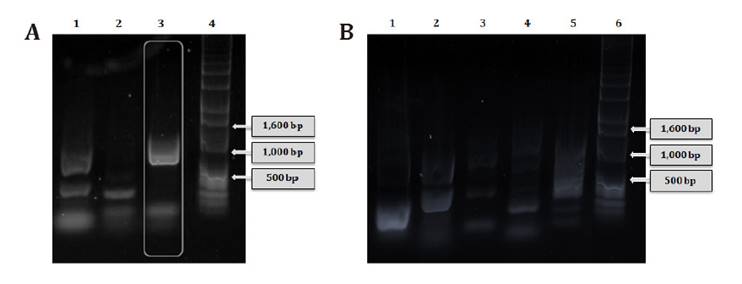
(A)
Genes de resistencia a vancomicina 1: producto de amplificación del gen vanA;
2: vanB; 3: vanC; 4: marcador de peso molecular de ADN de 1 Kb.
(B) Genes de factores de virulencia. 1: agg; 2: cylA; 3: gelE;
4: esp; 5: efa; 6: marcador de peso molecular de ADN de 1 Kb.
Figure
1. Vancomycin resistance and virulence factor genes in E.
gallinarum CRL 1826.
Figura
1. Genes de resistencia a vancomicina
y de factores de virulencia en E. gallinarum CRL 1826.
Effect
of the drying medium on survival and bacteriocin activity to lyophilization of E.
gallinarum CRL 1826
We determined the freeze-drying resistance of the LAB strain
using nine lyoprotectants. The three average SF values ranged between 0.81 to
0.97 (figure
2).
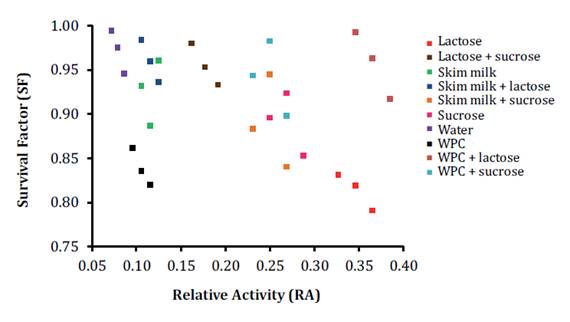
Figure 2. Viability
(SF) and bacteriocin activity (RA) scatter plot of lyophilized E. gallinarum
CRL 1826. WPC: whey protein concentrate.
Figura
2. Diagrama de dispersión de
viabilidad (SF) y actividad bacteriocina (RA) de E. gallinarum CRL 1826
liofilizado. WPC: concentrado proteico de suero.
Optimal cell viability recuperation was detected in water, SM-L,
WPC-L, L-S, WPC-S, and SM, with SF values from 0.93 to 0.97, without
significant differences (p≤0.05, Fisher test). Bacteriocin production in
lyophilized cultures was calculated as relative activity (RA) (table 3).
Table 3. Bacteriocin
activity of lyophilized E. gallinarum CRL 1826.
Table
3. Actividad bacteriocina de E.
gallinarum CRL 1826 liofilizado.
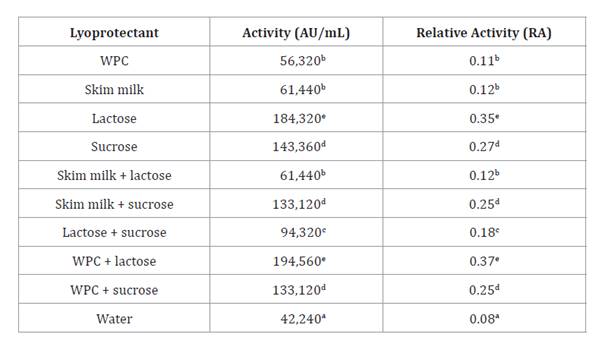
a-b: indicates significant
differences (p>0.05); WPC: whey protein concentrate.
a-b: indica
diferencias significativas (p>0,05). WPC: concentrado proteico de
suero.
Thus, the nine lyoprotectant solutions increased RA values
(0.11-0.37) when compared with those obtained in water (0.08) after
freeze-drying (figure
2).
However, the highest RA values were detected when the LAB strain was
lyophilized in L (RA=0.35) and WPC-L (RA=0.37), without significant differences
(p≤0.05, Fisher test). Considering RA and SF values, the optimal
freeze-drying condition was WPC-L (figure 2). However, no correlation was found between both factors
(Pearson correlation coefficient: 0.08).
Viability
and bacteriocin activity of lyophilized E. gallinarum CRL 1826 during
storage
Survival of freeze-dried cultures in different lyoprotectants
during storage at 4°C was analyzed by general linear models (figure 3).
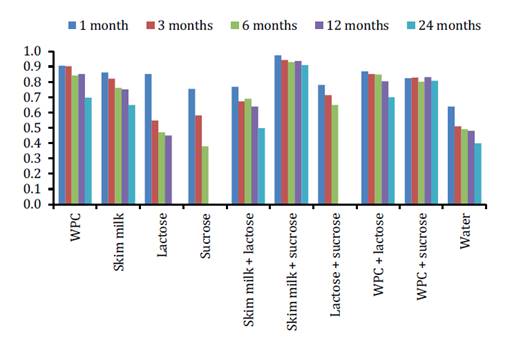
* Indicates
significant differences compared to 1-month storage for each lyoprotectant (p>0.05).
WPC:
whey protein concentrate.
*
Indica diferencias significativas respecto del almacenamiento durante 1 mes
para cada lioprotector (p>0,05).
WPC:
concentrado proteico de suero.
Figure
3. Viability (SF) of lyophilized E. gallinarum CRL
1826 during 24 months of storage at 4°C.
Figura
3. Viabilidad (SF) de E. gallinarum
CRL 1826 liofilizado y almacenado durante 24 meses a 4°C.
Therefore, cell viability values over 0.90 during 24 months were
obtained in SM-S, without significant differences up to 12 months (p≤0.05).
Cell viability for lyophilized LAB strain stored at 25°C was analyzed by Mood’s
median test, where the general median value was 0.47 (figure 4).
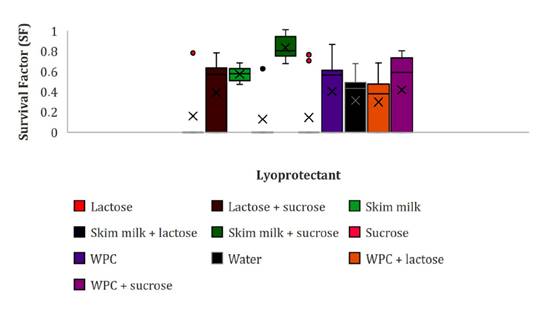
WPC: whey protein
concentrate.
WPC:
concentrado proteico de suero.
Figure
4. Viability (SF) of lyophilized E. gallinarum CRL
1826 during 24 months storage at 25°C.
Figura
4. Viabilidad (SF) de E. gallinarum
CRL 1826 liofilizado y almacenado durante 24 meses a 25°C.
Thus, after 24 months, viability was greater than the median
when using SM and SM-S, with mean SF values of 0.72 and 0.49, respectively. In
those cultures, dried in S, L, and SM-L, cell viability was only detected at
1-month storage (figure
4).
Regarding bacteriocin production (RA), a Mood’s median test analyzed the
freeze-dried cultures in different lyoprotectants and stored at 4 and 25°C
(median: 0.14 and 0.006, respectively). Therefore, during 24 months of storage
at 4°C the best RA values were obtained in WPC (figure 5), while at 25°C
the greatest RA values were detected in SM and SM-S (figure 5).
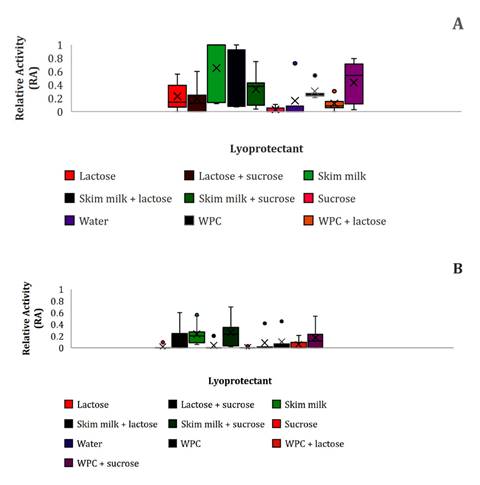
(A):4°C; (B):25°C.
WPC: whey protein concentrate.
(A):4°C;
(B):25°C. WPC: concentrado proteico de suero.
Figure
5. Bacteriocin activity (RA) of lyophilized E.
gallinarum CRL 1826 during 24 months storage.
Figura
5. Actividad bacteriocina (RA) de E.
gallinarum CRL 1826 liofilizado durante 24 meses de almacenamiento.
Growth/resistance
and bacteriocin activity of E. gallinarum CRL 1826 in individual
gastrointestinal conditions
Tests compared pre-lyophilized (PL) and lyophilized (L)
bacterial cultures with the best lyophilisation matrix. Thus, PL cultures of E.
gallinarum CRL 1826 grew ≥ 3 logarithmic units in LAPTg broth pH 5 to 8,
0.1% bile, and 0.01 to 0.2% pancreatin (table 4). In these
conditions, the highest bacteriocin activities (79,600 AU/mL) were detected at
pH 6.8 (control) and pH 7. Likewise, PL cultures resisted all tested conditions
(SF=0.44-0.99) (table
4),
except 6 and 10% bile for 10 min. Moreover, the highest bacteriocin activity
(8,080 AU/mL) was determined with 1% bile for 10 min.
Table 4. Growth
(Log CFU/mL), viability (SF) and bacteriocin activity (AU/mL) of
pre-lyophilized (PL) and lyophilized (L) E. gallinarum CRL 1826 when
subjected to individual gastrointestinal conditions.
Tabla
4. Crecimiento (Log CFU/mL),
viabilidad (SF) y actividad bacteriocina (AU/mL) de E. gallinarum CRL
1826 preliofilizado (PL) y liofilizado (L) cuando se somete a condiciones
gastrointestinales individuales.
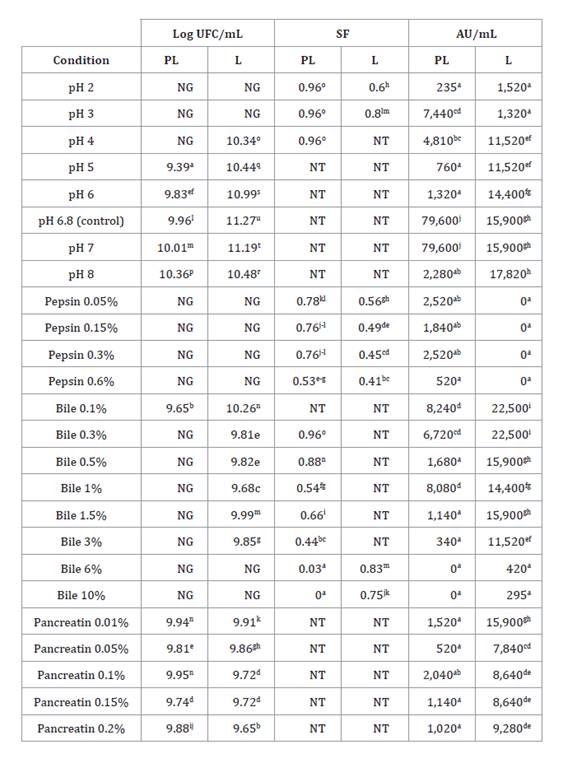
a-b: indicates significant
differences (p>0.05); NG: not grown; NT: not tested.
a-b: indica
diferencias significativas (p>0,05); NG: sin crecimiento; NT: no
testeado.
Lyophilized cultures (L) grew in a greater number of conditions.
For example, LAPTg broth at pH 4, and 0.3-3% bile. Moreover, the highest
bacteriocin activities (22,500 AU/mL) were detected with 0.1 and 0.3% bile (table 4). Also, L cultures
resisted acid pH (2 and 3), 6 and 10% bile, and all pepsin concentrations. In
these conditions, the highest bacteriocin activity (1,520 AU/mL) was determined
at pH 2 for 18 min and was not detected with pepsin (table 4). Therefore, L
cultures showed the greatest mean growth value (10.17 Log CFU/mL) and
resistance (SF=0.83) significantly higher than for PL (8.50 Log CFU/mL and
SF=0.73, respectively) (LSD Fisher, p>0.05).
Viability
and beneficial properties of E. gallinarum CRL 1826 in a simulated
gastrointestinal digestion model
Overall, LAB strain viability decreased during the simulated
gastrointestinal digestion process. The L cultures showed higher viability
values (mean SF=0.92) than PL (mean SF=0.82). Both PL and L cultures showed no
significant differences in viability between stages b to d (Phase 1: pH 7.4 to
2.0 + pepsin 0.6%, 90 min). Moreover, the lowest viability values were observed
at stages f to h (Phase 3: pH 8 + 0.3% bile + 0.1% pancreatin; 30, 60, and 90
min), where no significant differences were determined (p>0.05) (table 5).
Table 5. Viability
(SF) and beneficial properties of pre-lyophilized (PL) and lyophilized (L) E.
gallinarum CRL 1826 in a simulated gastrointestinal digestion model.
Tabla
5. Viabilidad (SF) y propiedades
benéficas de E. gallinarum CRL 1826 preliofilizado (PL) y liofilizado
(L) en un modelo de digestión gastrointestinal simulado.
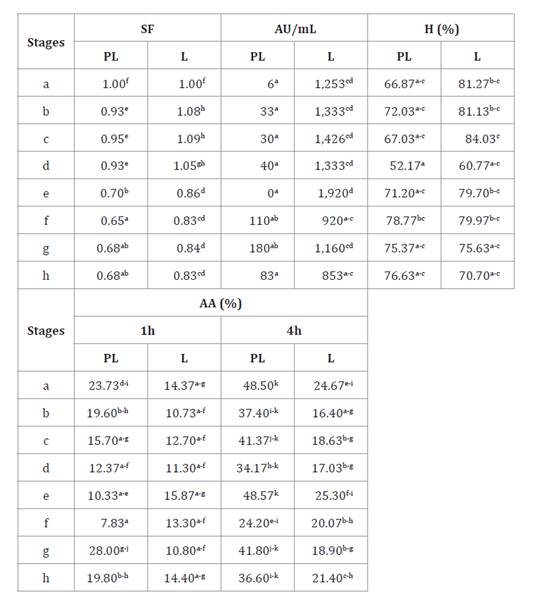
Stages: a-d: gradual
pH decrease of PBS solution (7.4 to 2) + pepsin 0.6%, 0, 30, 60, and 90 min; e:
PBS solution pH 8 + 1% bile; f-g: PBS solution pH 8 containing 0.3% bile + 0.1%
pancreatin, 30, 60, and 90 min. a-b: indicates significant differences (p>0.05).
AU/mL: bacteriocin activity; H:
hydrophobicity; AA: autoaggregation.
Etapas: a-d:
disminución gradual del pH de solución PBS (7,4 a 2) + pepsina 0,6%, 0, 30, 60
y 90 min; e: solución PBS pH 8 + bilis 1%; f-g: solución PBS pH 8 conteniendo
bilis 0,3% + pancreatina 0,1%, 30, 60 y 90 min. a-b: indica
diferencias significativas (p>0,05).
AU/mL: actividad bacteriocina; H:
hidrofobicidad; AA: auto-agregación.
The highest bacteriocin activity (1,920 AU/mL) was observed in L
cultures at stage “e” with significant differences at stages f and h (table 5). However, PL
cultures presented lower activity during whole digestion, increasing slightly
between stages f and h without significant differences (table 5). Concerning
surface properties, hydrophobicity values did not show significant differences
for PL or L cultures (table
5).
The highest value was observed in L cultures (84.03%) at stage “c”, remaining
above 52.17% during the assay. Moreover, auto-aggregation (AA) mean values in
PL cultures (28.12%) were significantly higher than those observed for L
cultures (16.62%), independently of sampling stage. The AA values were always
higher at 4 h (table
5).
Overall, opposite stages (a and h) exhibited no significant differences in any
culture. The highest value (48.57%) was observed in PL cultures at stage “e” at
4 h, while the lowest value (7.83%) was detected in PL cultures at stage “f” at
1 h (table
5).
Discussion
Designing a
probiotic product involves safety considerations (resistance to
chemotherapeutics and absence of virulence factors), technological aspects
(lyophilization and spray drying), and physiological studies (tolerance to host
conditions such as pH, digestive enzymes, and bile) (2).
In high-scale
aquaculture, chemotherapeutics could lead to resistance among pathogenic
bacteria (52). Therefore, probiotics represent an
alternative to chemotherapeutics reducing disease outbreaks (18).
Enterococcus genus presents both
beneficial and technological properties for constituting probiotic products.
However, this genus is not universally considered Generally Regarded As Safe
(GRAS) given virulence factors and vancomycin resistance genes (2). Evaluating
sensitivity and resistance to various antimicrobials, including vancomycin, is
regulated (15). Moreover, microorganisms should not contain
antimicrobial resistance genes that could be horizontally transferred to
members of the autochthonous microbiota or potential pathogens (37).
According to their
beneficial properties, E. gallinarum CRL 1826 from bullfrog was selected
as a probiotic candidate for ranaculture (31,
33). The LAB strain showed antibiotic sensitivity, except for
ceftazidime, related to the intrinsic resistance of Enterococcus to
cephalosporins (12). Metronidazole
sensitivity explains the antagonistic activity mainly on anaerobic bacteria (29).
Nowadays,
vancomycin resistance genes in Enterococcus are associated with three
well-defined phenotypes, according resistance degrees, induction, and transfer.
Likewise, only the vanC gene is chromosomal, constitutive, and
non-transferable (6). Enterococcus
gallinarum CRL 1826 could be accepted as a safe strain since its vancomycin
sensitiveness (MIC=4-32 mg/L), and only present vanC gene.
Considering that E.
gallinarum is associated with infections in aquaculture (44), and that the esp
gene was revealed in E. gallinarum from ready-to-eat seafood (21), we demonstrated
the absence of virulence factors genes of the CRL 1826 strain supporting their
GRAS characteristics.
Concerning
antiseptics, E. gallinarum CRL 1826, RLS-related pathogens (C.
freundii, P. aeruginosa), and L. monocytogenes resulted to be
highly resistant to doses used in bullfrog hatcheries. Thus, we could
hypothesize that the bioaugmentation with this LAB strain in hatchery
conditions would eliminate the pathogens and potential pathogens by competitive
exclusion or antibiosis (11, 34, 38).
Technologically,
freeze-drying preserves cell viability and beneficial properties during
probiotic product design, storage, and transport (32). Lyoprotectants,
including sugars, amino acids, and proteinaceous compounds often mitigate
cellular damage during freeze-drying (32).
Cocci generally
exhibit greater resistance to lyophilization compared to bacilli. This can be
explained by its low surface/volume ratio, as demonstrated in E. faecium,
Streptococcus thermophilus, L. lactis subsp. lactis,
and L. lactis subsp. cremoris strains
(24). However, the
behavior towards lyophilization is related to strain characteristics, such as
an E. faecium strain (potential probiotic isolated from oysters) with
low viability recovery in skim milk (36.2%) (25), while Romyasamit
et al. (2022) demonstrated 96% of viability recovery for the same
lyoprotectant in two E. faecium strains with probiotic potential for
functional food, medicine, and feed industries. Enterococcus gallinarum CRL
1826 showed 93% viability recovery in skim milk and intrinsic resistance to
lyophilization (water).
Regarding
bacteriocin activity maintenance, we demonstrated a diminution of relative
activity after lyophilization. However, antimicrobial titer values could be
suitable for RLS-related pathogens and foodborne bacteria control (33).
As relative indices
to initial lyophilization stages and storage, we defined survival and
bacteriocin activity parameters (SF, RA). These parameters allowed data
analysis independently of the initial values of viability and bacteriocin
activity. Similar criteria were applied by Vera Pingitore et al. (2012) who studied a
bacteriocin produced by Lactobacillus salivarius and evaluated specific
activity after lyophilization and storage. Our study demonstrated a
non-correlation between bacterial viability and bacteriocin activity,
indicating that factors were differently affected by freeze-drying conditions.
However, Jawan
et al. (2022) verified a correlation between these two factors when analyzed
as absolute values.
Despite
lyoprotectants enhance survival during lyophilization, they may not provide
protection during storage (9). Thus, the CRL
1826 strain viability decreased gradually and a total loss was observed at 25°C
in L, S, and SM-L after one month of storage.
Skim milk+sucrose
was the best lyoprotectant for E. gallinarum CRL 1826 strain during
storage. In ranaculture, L. plantarum CRL 1606
lyophilized in SM-S showed high viability recovery at 4 and 25°C up to 18
months’ storage (31). The protein
matrix added to sugars increased viability recovery concerning the individual
components. Using SM would represent an extra source of proteins and
carbohydrates for bullfrog feeding, considering that balanced feed used in
hatcheries provides 40% of proteins (fish meal, meat meal, and milk powder).
Enterococcus
gallinarum CRL 1826 kept its bacteriocin activity after lyophilization in
SM and SM-S during 24 months’ storage at 4 and 25°C. These results are in
agreement with Jawan
et al. (2022) who demonstrated that Lactococcus lactis Gh1, isolated
from an Iranian traditional flavor enhancer, maintained the ability to produce
bacteriocin with various lyoprotectants during 2 months of storage at 4°C.
Adult bullfrog
specimens have a developed gastrointestinal tract (GIT) that represents one
entry route for RLS-related pathogens (35). Considering that
probiotics must be applied in bullfrog hatcheries during life cycle and that
these microorganisms must resist host conditions and reach the intestine for
adhesion and colonization, we evaluated GIT restrictive conditions on viability
and maintenance of beneficial properties of E. gallinarum CRL 1826. All
studies were performed by considering physiological concentration ranges (47) in sequence,
temperature, and time.
The pH affects
growth factors transport across the cell membrane of LAB and antagonistic
efficacy (14). In PL cultures of E. gallinarum CRL
1826 exposed for 18 min at pH 2, viability recovery was similar to initial
values (SF=0.96), whereas PL cultures of Enterococcus avium, E.
pseudoavium, and E. raffinosus (from carp) treated at pH 2.5 up to 5
h showed viability diminution around 2 log units (43).
Bacteriocins
constitute an important competitive advantage for microorganisms, particularly
interesting for bioinputs (40). The highest
bacteriocin production by E. gallinarum CRL 1826 was detected at pH 6.8
and 7, and the lowest at pH 2. Bacteriocins are stable over a wide pH range,
with high activity at neutral and basic pH (14). Thus, PL cultures
of Pediococcus pentosaceus 1101, from fermented meat products, showed
the highest bacteriocin activity at pH 5.5 and 7 (14).
Considering that
pH/time interaction and pepsin presence could affect bacterial survival (26), we observed that
pepsin diminished E. gallinarum CRL 1826 growth of PL and L cultures,
probably given by enzymatic action since viability values were lower than those
obtained at pH 2 (table
4)
(LSD Fisher, p>0.05).
Bile significantly
affects protein and DNA and participates in the emulsification of fats and cell
membranes (49). Our bile
concentration ranged from 0.1 to 10%. Thus, PL and L cultures of E.
gallinarum CRL 1826 maintained viability up to 90 min in 0.5% bile. In this
sense, different behaviors have been reported for PL cultures of aquaculture
enterococci. Some resisted up to 3 h (40) while others
perished after 4 and 24 h (5, 27) with 0.3% bile. Pereira
et al. (2018) measured OD 630 nm, and reported that PL cultures of L.
plantarum strains, isolated from bullfrog hatcheries, tolerated 5% bile for
24 h. Nevertheless, PL cultures of E. gallinarum CRL 1826 resisted 0.5%
bile for 90 min, while L cultures resisted 10% bile for 10 min.
Pancreatin from
porcine pancreas contains proteases, lipase, and amylase, that cleave specific
molecules for their assimilation (10). This enzyme
complex could affect E. gallinarum CRL 1826 and, consequently, its
probiotic effect. However, we observed that PL and L cultures of the LAB strain
grew in all concentration ranges (0.01-0.2%) tested.
When E.
gallinarum CRL 1826 was subjected to simulated gastrointestinal digestion,
we observed that L cultures over-performed (growth/resistance, bacteriocin
activity, and surface properties maintenance) than PL cultures. This behavior
could relate to a protective effect of the freeze-drying matrix (SM-S) that
would interfere with gastrointestinal conditions as reported for a L. rhamnosus
strain (28). Moreover, some
authors postulate an adaptation mechanism of probiotics called
“cross-protection”, occurring when microorganisms are pre-adapted and gain
greater tolerance towards different types of stress than the original (1,
16). In our experimental conditions, lyophilization of E.
gallinarum CRL 1826 could act as a pre-adaptation process improving
bacterial survival to simulated gastrointestinal digestion.
Finally, PL and L
cultures of E. gallinarum CRL 1826 maintained hydrophobicity and
auto-aggregation when subjected to the simulated gastrointestinal digestion
model that would enhance its capability of gut colonization.
Conclusion
Enterococcus gallinarum CRL 1826 exhibits promising
characteristics as a probiotic for bullfrog hatcheries, offering viability and
bacteriocin activity even after lyophilization and under simulated
gastrointestinal conditions. These properties, combined with its safety
profile, make it a potential solution for preventing RLS outbreaks and
controlling foodborne bacteria, with potential benefits for bullfrog growth
performance.
Acknowledgements,
financial support, and full disclosure
Financial support
from Consejo de Investigaciones de la Universidad Nacional de Tucumán (PIUNT
D-528) and Agencia Nacional de Promoción Científica y Tecnológica (PICT
2017-2244) are gratefully acknowledged.
The authors declare that they have no known competing financial
interests or personal relationships that could have appeared to influence the
work reported in this paper.
1. Anglenius, H.;
Mäkivuokko, H.; Ahonen, I.; Forssten, S. D.; Wacklin, P.; Mättö, J.; Lahtinen,
S.; Lehtoranta, L.; Ouwehand, A. C. 2023. In vitro screen of
lactobacilli strains for gastrointestinal and vaginal benefits. Microorganisms.
11(2): 329. https://doi. org/10.3390/microorganisms11020329
2. Barzegar, H.;
Alizadeh Behbahani, B.; Falah, F. 2021. Safety, probiotic properties,
antimicrobial activity, and technological performance of Lactobacillus strains
isolated from Iranian raw milk cheeses. Food Sci. Nutr. 9(8): 4094-4107.
https://doi.org/10.1002/fsn3.2365
3. Berg, G.;
Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M. C. C.; Charles, T.; Chen,
X.; Cocolin, L.; Eversole, K.; Herrero Corral, G.; Kazou, M.; Kinkel, L.;
Lange, L.; Lima, N.; Loy, A.; Macklin, J. A.; Maguin, E.; Mauchline, T.;
McClure, R.; Mitter, B.; Ryan, M.; Sarand, I.; Smidt, H.; Schelkle, B; Roume, H.;
Kiran, G. S.; Selvin, J.; Soares Correa de Souza, R.; van Overbeek, L; Singh,
B. K.; Wagner, M.; Walsh, A.; Sessitsch, A.; Schloter, M. 2020. Microbiome
definition re-visited: old concepts and new challenges. Microbiome. 8: 1-22.
https://doi.org/10.1186/s40168- 020-00875-0
4. Bühler, M. I.;
Sánchez Toranzo, G.; Zaltz, S. 2000. La ranicultura: una alternativa
productiva. Top. Graph. Argentina.
5. Cai, X.; Wen, J.
S.; Long, H.; Ren, W.; Zhang, X.; Huang, A. Y.; Xie, Z. Y. 2022. The probiotic
effects, dose, and duration of lactic acid bacteria on disease resistance in Litopenaeus
vannamei. Aquac. Rep. 26: 101299.
https://doi.org/10.1016/j.aqrep.2022.101299
6. CDC.
Vancomycin-Resistant Enterococci (VRE) and the Clinical Laboratory HAI CDC.
2020. https:// www.cdc.gov/hai/settings/lab/vreclinical-laboratory.html.
7. Clinical and
Laboratory Standards Institute. Methods for antimicrobial dilution and disk
susceptibility testing of infrequently isolated or fastidious bacteria 3rd ed. CLSI guideline M45.
2015. Clinical and Laboratory Standards Institute. Wayne. PA. USA.
8. Clinical and
Laboratory Standards Institute. Performance standards for antimicrobial
susceptibility testing, 32nd ed.
CLSI guideline M100. 2022. Wayne. PA. USA.
9. Crowe, J. H.;
Crowe, L. M.; Carpenter, J. F.; Wistrom, C. A. 1987. Stabilization of dry
phospholipid-bilayers and proteins by sugars. Biochem. J. 242(1): 1-10.
https://doi.org/10.1042/ bj2420001
10. Dehkordi, R. A.
F.; Karimi, I.; Karimi, B.; Eshkaftaki, R. G.; Abtahi, R.; Mohammadi, H. 2023.
Effect of pancreatin on acute pancreatitis resulting from L-arginine
administration in mice, a morpho-histopathological and biochemical study. Braz.
J. Pharm. Sci. 59: e21494. https:// doi.org/10.1590/s2175-97902023e21494
11. Densmore, C.
L.; Green, D. E. 2007. Diseases of amphibians. ILAR J. 48(3): 235-254.
https://doi. org/10.1093/ilar.48.3.235
12. De Oliveira, D.
M.; Forde, B. M.; Kidd, T. J.; Harris, P. N.; Schembri, M. A.; Beatson, S. A.;
Paterson, D. L.; Walker, M. J. 2020. Antimicrobial resistance in ESKAPE
pathogens. Microbiol. Rev. 33(3): e00181-19.
https://doi.org/10.1128/cmr.00181-19
13. Di Rienzo, J. A.; Casanoves, F.; Balzarini, M. G.; Gonzalez,
L.; Tablada, M. R. C. W.; Robledo, C. W. 2020. InfoStat versión 2020. Centro de Transferencia
InfoStat, FCA, Universidad Nacional de Córdoba, Argentina.
URL http://www. infostat. com.
ar
14.
Escobar-Sánchez, M.; Carrasco-Navarro, U.; Juárez-Castelán, C.; Lozano-Aguirre,
L.; Pérez-Chabela, M. L.; Ponce-Alquicira, E. 2022. Probiotic properties and
proteomic analysis of Pediococcus pentosaceus 1101. Foods. 12(1): 46.
https://doi.org/10.3390/ foods12010046
15. European Food
Safety Authority Panel on Additives and Products or Substances used in Animal
Feed (FEEDAP). 2018.efsa.2018.5206
16. Fiocco, D.;
Longo, A.; Arena, M. P.; Russo, P.; Spano, G.; Capozzi, V. 2020. How probiotics
face food stress: They get by with a little help. Crit. Rev. Food Sci. Nutr.
60(9): 1552-1580. https:// doi.org/10.1080/10408398.2019.1580673
17. Garza, M.; Mohan,
C. V.; Rahman, M.; Wieland, B.; Häsler B. 2019. The role of infectious disease
impact in informing decision-making for animal health management in aquaculture
systems in Bangladesh. Prev. Vet. Med. 167: 202-213. https://doi.org/10.1016/j.
prevetmed.2018.03.004
18. Gloor, G. B.;
Reid, G. 2016. Compositional analysis: a valid approach to analyze microbiome
high-throughput sequencing data. Can. J. Microbiol. 62(8): 692-703.
https://doi.org/10.1139/ cjm-2015-0821
19. Hadfield, C.
A.; Whitaker, B. R. 2005. Amphibian emergency medicine and care. Seminars in
avian and exotic pet medicine. 14(2): 79-89.
https://doi.org/10.1053/j.saep.2005.04.003
20. Hidano, A.;
Yamamoto, T.; Hayama, Y.; Muroga, N.; Kobayashi. S.; Nishida, T.; Tsutsui, T.
2015. Unraveling antimicrobial resistance genes and phenotype patterns among Enterococcus
faecalis isolated from retail chicken products in Japan. PloS One. 10(3):
e0121189. https:// doi.org/10.1371/ journal.pone.0121189
21. Igbinosa, E.
O.; Beshiru, A. 2019. Antimicrobial resistance, virulence determinants, and
biofilm formation of Enterococcus species from ready-to-eat seafood.
Front. Microbiol. 10: 728. https://doi.org/ 10.3389/fmicb.2019.00728
22. Indriani, S.;
Sae Leaw, T.; Benjakul, S.; Quan, T. H.; Karnjanapratum, S.; Nalinanon, S.
2022. Impact of different ultrasound-assisted processes for preparation of
collagen hydrolysates from Asian bullfrog skin on characteristics and
antioxidative properties. Ultrason. Sonochem. 89: 106163. https://doi.org/
10.1016/j.ultsonch.2022.106163
23. Jawan, R.;
Abbasiliasi, S.; Tan, J. S.; Kapri, M. R.; Mustafa, S.; Halim, M. 2022.
Influence of type and concentration of lyoprotectants storage temperature and
storage duration on cell viability and antibacterial activity of freeze-dried
lactic acid bacterium Lactococcus lactis Gh1. Dry. Technol. 40(9):
1774-1790. https://doi.org/ 10.1080/07373937.2021.1874968
24. Kandil, S.; El
Soda, M. 2015. Influence of freezing and freeze drying on intracellular
enzymatic activity and autolytic properties of some lactic acid bacterial
strains. Adv. Microbiol. 5: 371-382. https://doi.org/ 10.4236/aim.2015.56039
25. Kang, C. H.;
Gu, T.; So, J. S. 2018. Possible probiotic lactic acid bacteria isolated from
oysters (Crassostrea gigas). Probiotics Antimicrob. Proteins. 10(4):
728-739. https://doi.org/ 10.1007/s12602-017-9315-5
26. Ko, H. I.;
Jeong, C. H.; Hong, S. W.; Eun, J. B.; Kim, T. 2022. Optimizing conditions in
the acid tolerance test for potential probiotics using response surface
methodology. Microb. Spectrum. 10(4): e01625-22.
https://doi.org/10.1128/spectrum.01625-22
27. Maji, U. J.;
Mohanty, S.; Mahapatra, A. S.; Maiti, N. K. 2016. Diversity and probiotic
potentials of putative lactic acid bacteria for application in freshwater
aquaculture. Turkish J. Fish Aquat. Sci. 16: 805-818.
https://doi.org/10.4194/1303-2712-v16_4_07
28. Melchior, S.;
Marino, M.; Innocente, N.; Calligaris, S.; Nicoli, M. C. 2020. Effect of
different biopolymer-based structured systems on the survival of probiotic
strains during storage and in vitro digestion. J. Sci. Food Agric. 100:
3902-3909. https://doi.org/10.1002/ jsfa.10432
29. Mendes, S. D.
N. C.; Esteves, C. M.; Mendes, J. A. V.; Feres, M.; Figueiredo, N.; de Miranda,
T. S.; Shibli, J. A.; Figueiredo, L. C. 2023. Systemic antibiotics and
chlorhexidine associated with periodontal therapy: Microbiological effect on
intraoral surfaces and saliva. Antibiotics. 12(5): 847. https://doi.org/
10.3390/antibiotics12050847
30. Minitab, LLC.
2018. Statistical (versión 2018) [Software]. www.minitab.com
31. Montel Mendoza,
G.; Pasteris, S. E.; Ale, C. E.; Otero, M. C.; Bühler, M. I.; Nader Macías, M.
E. 2012. Cultivable microbiota of Lithobates catesbeianus and advances
in the selection of lactic acid bacteria as biological control agents in
raniculture. Res. Vet. Sci. 93: 1160-1167. https:// doi.org/
10.1016/j.rvsc.2012.05.007
32. Montel Mendoza,
G.; Pasteris, S. E.; Otero, M. C., Nader Macías, M. E. F. 2014. Survival and
beneficial properties of lactic acid bacteria from raniculture subjected to
freeze-drying and storage. J. Appl. Microbiol. 116: 157-166. https://doi.org/
10.1111/jam.12359
33. Montel Mendoza,
G.; Ale, C. E.; Nader Macías, M. E. F.; Pasteris, S. E. 2015. Characterization
of a bacteriocin produced by Enterococcus gallinarum CRL 1826 isolated
from captive bullfrog: evaluation of its mode of action against Listeria
monocytogenes and Gram-negatives. J. Bioprocess Biotech. 5: 250-256.
https://doi.org/ 10.4172/2155-9821.1000250
34. Niederle, M. V.; Bosch, J.; Ale, C. E.; Nader Macías, M. E.;
Aristimuno Ficoseco, C.; Toledo, L. F.; Valenzuela, Sánchez, A.; Soto Azat, C.;
Pasteris, S. E. 2019. Skin-associated lactic acid bacteria from North American
bullfrogs as potential control agents of Batrachochytrium dendrobatidis.
PLoS One. 14(9): e0223020. https://doi.org/ 10.1371/journal. pone.0223020
35. Pasteris, S.
E.; Bühler, M. I.; Nader Macías, M. E. 2006. Microbiological and histological
studies in farmed-bullfrog (Rana catesbeiana) displaying red-leg
syndrome. Aquaculture. 251: 11-18. https://doi.org/
10.1016/j.aquaculture.2005.05.007
36. Pasteris, S.
E.; Roig Babot, G.; Otero, M. C.; Nader Macías, M. E. 2009. Beneficial
properties of lactic acid bacteria isolated from a Rana castesbeiana hatchery.
Aquac. Res. 40: 1605-1615. https://doi.org/10.1111/j.1365-2109.2009.0226.x
37. Pasteris, S.
E.; Vera Pingitore, E.; Roig Babot, G.; Otero, M. C.; Bühler, M. I.; Nader
Macías, M. E. 2009. Characterization of the beneficial properties of
lactobacilli isolated from bullfrog (Rana catesbeiana) hatchery. Antonie
van Leeuwenhoek. 95: 375-385. https://doi. org/10.1007/s10482-009-9329-4
38. Pasteris, S.
E.; Montel Mendoza, G.; Llanos, R. J.; Pucci Alcaide, F.; Nader Macías, M. E.
F. 2017. Preliminary assessment of in vivo safety of potentially
probiotic lactic acid bacteria for American bullfrog culture. Aquac. Res.
48(5): 2157-2172. https://doi.org/ 10.1111/ are.13053
39. Pereira, S. A.;
Jerônimo, G. T.; Marchiori, N. C.; Oliveira, H. M.; Jesus, G. F. A.; Schmidt,
E. C.; Bouzon, Z. L.; Vieira, F. N.; Martins, M. L.; Mouriño, J. L. P. 2018.
Tadpoles fed supplemented diet with probiotic bacterium isolated from the
intestinal tract of bullfrog Lithobates catesbeianus: Haematology, cell
activity and electron microscopy. Microb. Pathog. 114: 255-263. https://
doi.org/10.1016/j.micpath.2017.11.033
40. Pereira, W. A.;
Mendonça, C. M. N.; Urquiza, A. V.; Marteinsson, V.; LeBlanc, J. G.; Cotter, P.
D.; Villalobos, E. F.; Romero, J.; Oliveira, R. P. 2022. Use of probiotic
bacteria and bacteriocins as an alternative to antibiotics in aquaculture.
Microorganisms. 10(9): 1705. https://doi. org/10.3390/microorganisms10091705
41. Pospiech, A.;
Neumann, B. 1995. A versatile quick-prep of genomic DNA from Gram-positive
bacteria. Trends Genet. 11: 217-218.
42. Romyasamit, C.;
Saengsuwan, P.; Boonserm, P.; Thamjarongwong, B.; Singkhamanan, K. 2022.
Optimization of cryoprotectants for freeze-dried potential probiotic Enterococcus
faecalis and evaluation of its storage stability. Dry. Technol. 40(11):
2283-2292. https://doi.org/1 0.1080/07373937.2021.1931294
43. Sahoo, T. K.;
Jena, P. K.; Nagar, N.; Patel, A. K.; Seshadri, S. 2015. In vitro evaluation
of probiotic properties of lactic acid bacteria from the gut of Labeo rohita
and Catla catla. Probiotics Antimicrob. Proteins. 7(2): 126-136.
https://doi.org/10.1007/s12602-015-9184-8
44. Salama, S. A.
E. H. 2022. Bacterial pathogens causing the blue crab (Callinectes sapidus)
mortality at Suez Canal (El-Temsah Lake) in Ismailia Governorate. Egypt J.
Aquat. Biol. Fish. 26(2): 151-168. https://doi.org/10.21608/ejabf.2022.226167
45. Sambrook, J.;
Gething, M. J. 1989. Protein structure Chaperones paperones. Nature. 342(6247):
224-225.
46. Silla, A. J.;
Calatayud, N. E.; Trudeau, V. L. 2021. Amphibian reproductive technologies:
approaches and welfare considerations. Conserv. Physiol. 9(1): coab011.
https://doi.org/10.1093/ conphys/coab011
47. Stevens, E. C.;
Hume, I. D. 1995. Digestion of carbohydrate, lipids and protein. In Comparative
Physiology of the Vertebrate Digestive System. 152-171. Cambridge Academic
Press 2nd ed.
48. Stone, G. G.;
Newell, P.; Bradford, P. A. 2018. In vitro activity of
ceftazidime-avibactam against isolates from patients in a phase 3 clinical
trial for treatment of complicated intra-abdominal infections. Antimicrob.
Agents. Chemother. 62(7): e02584-17. https://doi.org/10.1128/ aac.02584-17
49. Szopa, K.;
Szajnar, K.; Pawlos, M.; Znamirowska-Piotrowska, A. 2023. Probiotic fermented
goat’s and sheep’s milk: effect of type and dose of collagen on survival of
four strains of probiotic bacteria during simulated in vitro digestion
conditions. Nutrients. 15(14): 3241. doi:10.3390/
nu15143241
50. Teixeira, R.
D.; Mello, S. C. P.; dos Santos, C. A. L. 2001. The world market for frog legs.
Food and Agriculture Organization of the United Nations, GLOBEFISH. Fishery
Industries Division. 68: 1-44.
51. Timmons, P. B.;
Hewage, C. M. 2021. Conformation and membrane interaction studies of the potent
antimicrobial and anticancer peptide palustrin-Ca. Sci. Rep. 11(1): 22468.
https://doi. org/10.1038/s41598-021-01769-3
52. Tong, Q.; Cui,
L. Y.; Bie, J.; Han, X. Y.; Hu, Z. F.; Wang, H. B.; Zhang, J. T. 2021. Changes
in the gut microbiota diversity of brown frogs (Rana dybowskii) after an
antibiotic bath. BMC Vet. Res. 17(1): 1-13.
https://doi.org/10.1186/s12917-021-03044-z
53. Vera Pingitore,
E.; Bru, E.; Nader Macías, M. E. 2012. Effect of lyophilization and storage
temperature on the activity of salivaricin CRL 1328, a potential bioactive
ingredient of a urogenital probiotic product. J. Gen. Appl. Microbiol. 58(2):
71-81. https://doi.org/10.2323/ jgam.58.71
54. Wang, H.; Zhou, N.; Zhang, R.; Wu, Y.; Zhang, R.; Zhang, S.
2014. Identification and localization of gastrointestinal hormones in the skin
of the bullfrog Rana catesbeiana during periods of activity and
hibernation. Acta Histochem. 116(8): 1418-1426. https://doi.org/10.1016/j.
acthis.2014.09.005
55. Yang, P.; Zheng, Y.; Zou, X.; Sun, Y.; Liu, Y. 2023.
Comparative transcriptomic analysis of gene expression profiles in the liver
and spleen of American bullfrog (Lithobates catesbeianus) in response to
Citrobacter freundii infection. J. World Aquac. Soc. 55(1): 1-20.
https://doi. org/10.1111/jwas.12999
