Revista de la Facultad de Ciencias
Agrarias. Universidad Nacional de Cuyo. En prensa. ISSN (en línea) 1853-8665.
Original article
Impact
of fire on the genetic variability of a natural population of Stylosanthes
hippocampoides (Fabaceae) in Corrientes, Argentina
Efecto
de los incendios sobre la variabilidad genética de una población natural de Stylosanthes
hippocampoides (Fabaceae) en Corrientes, Argentina
Julieta Berenice
Arcangeli1,
1Instituto de Botánica del Nordeste (IBONE, UNNE -CONICET).
Sargento Juan Bautista Cabral 2131. 3402BKG. Casilla de Correo 209. Corrientes.
Argentina.
2Universidad Nacional del Nordeste (FACENA - UNNE). Facultad de
Ciencias Exactas y Naturales y Agrimensura. Avenida Libertad 5470. Campus
Deodoro Roca. Corrientes. Argentina.
*celestesilvestri@gmail.com
Abstract
Stylosanthes
hippocampoides, a native legume forage species, was first studied by our
research team in 2016 to assess genetic variability in natural populations in
northeastern Argentina. Recurrent fires in the following years raised concerns
about their effects on population genetics. This study aimed to evaluate and
compare the genetic variability and structure of S. hippocampoides populations
before and after fire events, and to infer whether such disturbances influence
genetic diversity. Using Geographic Information System tools, we selected one
fire-affected population (ASI) and one unaffected population (TC). Genetic
analyses were conducted using inter-simple sequence repeat markers, and pre-
and post-fire genetic statistics were compared. Greater genetic variability was
observed between populations than within them. The TC population exhibited
higher intra-population genetic diversity than the ASI population. Genetic
variability decreased in ASI post-fire, while TC slightly increased. However,
neither change was statistically significant. Genetic structure analysis
consistently grouped individuals by population, regardless of fire exposure.
These findings provide a foundation for future research on Stylosanthes,
incorporating additional populations with varied fire histories and examining
post-fire recovery processes.
Keywords: Stylo, NEA, ISSR,
wildfires, forage legumes
Resumen
Stylosanthes
hippocampoides, una leguminosa forrajera nativa, fue estudiada por primera vez
por nuestro equipo de investigación en 2016 para evaluar la variabilidad
genética en poblaciones naturales del noreste de Argentina. Los incendios
recurrentes en los años siguientes generaron interrogantes sobre sus efectos en
la genética de las poblaciones. Este estudio tuvo como objetivo evaluar y
comparar la variabilidad y la estructura genética de poblaciones de S.
hippocampoides antes y después de los eventos de incendio, e inferir si
tales perturbaciones influyen en la diversidad genética. Utilizando
herramientas del Sistema de Información Geográfica, seleccionamos una población
afectada por incendios (ASI) y otra no afectada (TC). Se realizaron análisis
genéticos utilizando marcadores moleculares de inter secuencias simples
repetidas, y se compararon las estadísticas genéticas anteriores y posteriores
a los incendios. Se observó mayor variabilidad genética entre poblaciones que
dentro de ellas. La población TC mostró una mayor variabilidad genética
intrapoblacional que la población ASI. En ASI, la variabilidad genética
disminuyó tras el incendio, mientras que TC mostró un ligero aumento; sin
embargo, ninguno de los cambios fue estadísticamente significativo. El análisis
de estructuración genética agrupó a los individuos por poblaciones, independientemente
de la exposición al fuego. Estos resultados proporcionan una base para futuras
investigaciones sobre Stylosanthes, incorporando poblaciones adicionales
con historias de incendios variadas y examinando los procesos de recuperación
post-incendio.
Palabras clave: Stylo, NEA, ISSR,
fuego, leguminosas forrajeras
Originales: Recepción: 07/03/2025
- Aceptación: 30/05/2025
Introduction
Ecological
disturbances, whether natural or anthropogenic, can alter key demographic and
biological processes, ultimately affecting the distribution of genetic
diversity within populations (4). Genetic
variability influences all levels of biodiversity, from individual fitness and
population viability to species adaptability to environmental change and even
speciation. Consequently, the interaction between disturbances and genetic
variability carries significant ecological and evolutionary implications (4, 19). Understanding how genetic variability
fluctuates in populations exposed to recurrent disturbances is crucial for
effective biodiversity conservation and management.
Since mid-2019,
northeastern Argentina (NEA) has experienced negative monthly precipitation
anomalies, leading to prolonged droughts (33).
This situation, combined with fire use in agricultural practices as a
vegetation management tool, created favorable conditions for wildfires (35). Notably, recurrent fires in the province of
Corrientes affected 12% of its total area (30).
Stylosanthes Sw. (Fabaceae)
comprises approximately 50 species adapted to tropical and subtropical regions
of the Americas, ranging from the southern United States to Argentina. These
species are valued for their use as forage (18),
as well as for soil recovery (41) and
ecological restoration (36). Eight Stylosanthes
species are native to Argentina, most of which are found in the NEA (40). As part of a project to conserve native
forage legumes in the NEA, our research team evaluated the genetic variability
of Stylosanthes hippocampoides Mohlenbr. populations
in 2016 (34). These populations were
later affected by fires (30), providing
an opportunity to examine potential genetic impacts.
Genetic consequences of fire on plant populations are diverse
and context-dependent, influenced by the scale and frequency of fire events,
biological traits and adaptive strategies of the species involved (3). For instance, individuals of some species can
survive fire and resprout, resulting in minimal effects on genetic variability.
In contrast, other species depend on the formation of seed banks and the
genetic variability they preserve for post-fire regeneration (37). Moreover, fires can fragment previously
continuous populations, creating barriers that isolate subpopulations. Such
fragmentation is expected to reduce gene flow and increase genetic
differentiation among populations (9).
In this context,
this study analyses the genetic variability and structure of a natural
population of S. hippocampouides affected by fires, comparing it with a
control population not exposed to such events. This would allow inferring
whether these disturbances influence population genetic variability and
structure. Considering the extent of recent fires in the region, the small
population sizes of S. hippocampoides, and the time elapsed since such
events, we hypothesize that the post-fire ASI population exhibits reduced
genetic variability compared to pre-fire levels, likely due to a bottleneck
effect. Additionally, we expect the genetic structure of the ASI population to
remain unchanged, as the elapsed time may be insufficient for spatial genetic
subdivision to occur.
Materials
and methods
Stylosanthes
hippocampoides is a perennial herb or sub-shrub with a prostrate to semi-erect
growth habit, native to grasslands and savannas of Argentina, Brazil, Bolivia,
Paraguay, and Uruguay (40). Its recorded
lifespan is up to five years, with an estimated generation time of 6-12 months (23). Stylosanthes species are
self-pollinating or predominantly self-pollinating (29).
Populations
evaluated in this study were selected by overlaying geolocations of the
populations assessed in 2016 by the research team (34)
with fire-affected areas in Corrientes province, identified using historical
satellite images from the FIRMS database (15).
A fire was recorded at the ASI population site (27°27’58” S; 57°28’12” W) on
September 30, 2020, with additional events occurring 0.96 km and 0.24 km away
in July 2019. Based on this, the ASI population was selected. As a control, we
selected a population within the Private Natural Reserve “Paraje Tres Cerros”
(29°6’18” S; 56°56’10” W), which was confirmed to be unaffected by fire (figure 1).
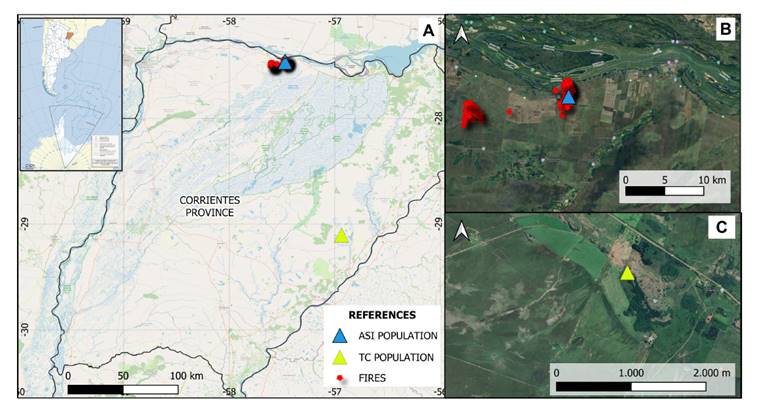
Figure
1. Location of S. hippocampoides populations
under study and fire hotspots within a 15 km radius of the ASI population
location in July 2019 and September 2020 (A). Satellite zoom of the location of
the ASI population (B) and the TC population (C).
Figura
1. Localización de las poblaciones de S.
hippocampoides, y focos de incendio en un radio de 15 km de la localización
de la población ASI en julio de 2019 y septiembre de 2020 (A). Zoom satelital
de la ubicación de la población ASI (B) y de la población TC (C).
The ASI site lies
on a broad roadside shoulder along National Route 12 (km 1171), about 700
meters from the Santa Isabel stream, subject to human disturbance due to
routine roadside maintenance. In contrast, the TC population is located within
the protected reserve, on the northern slope of Nazareno Hill. This area,
characterized by dry air, high solar radiation, and sparse herbaceous
vegetation, is free from human disturbance.
Sample collection
was conducted in February-March 2022. Following Silvestri
et al. (2020), we sampled approximately 10 - 15 adult individuals
from each population, ensuring at least 3 meters between individuals. Total DNA
was extracted from 200 mg of dry leaves from 10-11 individuals per population,
following the protocol of Doyle and Doyle (1987).
DNA integrity was confirmed by electrophoresis on 0.8% agarose gel in 1× TAE
buffer stained with ethidium bromide (10 mg/ml), and concentration was
determined by spectrophotometry. Nuclear DNA was amplified using PCR reactions
with the same ISSR primers and conditions described by Silvestri
et al. (2020). Amplified products were separated on 2% agarose gels
in 1× TAE buffer at 60 V for 2 h and stained with ethidium bromide (10 mg/ml).
Bands were scored as present or absent to generate a binary matrix based on
homologous DNA fragments. Genetic diversity within populations was estimated
using the total number of bands, number of private bands, percentage of
polymorphic loci, number of different alleles, number of effective alleles,
expected heterozygosity, and Shannon’s information index. These indices were
compared between pre-fire and post-fire periods for each population.
To assess whether differences in genetic variability were
statistically significant, we compared the Shannon index and expected heterozygosity
using previously published data (34).
Given the non-normal distribution of the data, the non-parametric
Wilcoxon-Mann-Whitney test was applied (42).
Analyses were performed in the R Studio environment (28).
Total genetic differentiation was analyzed at the species level, population,
and by population combined with collection period using analysis of molecular
variance (AMOVA) (11). Nei’s distance and
genetic identity indexes (21) were
calculated between individuals. Based on genetic distance values, Principal
Coordinate Analysis (PCoA) was used to visualize the distribution of genetic
variation within and among populations. All statistical analyses and diversity
indices were computed using GenAlEx 6.5 (24).
Genetic differentiation within and between populations was further assessed
using Bayesian clustering in STRUCTURE 2.3.4 (26),
applying the admixture model with correlated allele frequencies. The analysis
used a burn-in of 50,000 iterations followed by 100,000 Markov chain Monte
Carlo (MCMC) replications (12). The
algorithm was run 10 times for each number of genetic clusters (K), ranging
from 1 to 6. The optimal number of clusters (ΔK) was determined following the
method of Evanno et al. (2005).
Results
Genetic
variability before and after fire
A total of 55 loci
were scored in S. hippocampoides, of which 62.27 % were polymorphic. At
the species level, expected heterozygosity (He) was 0.25, and the Shannon index
(I) was 0.37. Intrapopulation genetic diversity was lower in the ASI population
compared to TC population.
Based on the same populations and molecular markers, comparisons
between pre-fire (2016) and post-fire (2022) periods showed generally similar
values. In the ASI population, I, He, and PLP values decreased from 0.20 to
0.17, from 0.13 to 0.11, and from 39.26% to 30.91%, respectively. Conversely,
in the TC population, these indices increased: I from 0.17 to 0.23, He from
0.11 to 0.15, and PLP from 33.33% to 38.18%. The Na values increased in both
populations, from 0.90 to 1.11 in ASI and 0.74 to 1.24 in TC. The Ne values
slightly decreased in ASI (from 1.22 to 1.19) and increased in TC (from 1.20 to
1.27). The TNB decreased in both populations, from 69 to 44 in ASI, and from 56
to 47 in TC. The NPB remained constant in ASI (8 bands) but increased in TC
from 1 to 11 bands (table 1). However, differences in I and
He between pre- and post-fire periods for each population were not
statistically significant according to the Wilcoxon-Mann-Whitney test (42) (ASI: w = 3997, p = 0.3393; TC:
w = 3452, p = 0.374).
Table 1. Descriptive
statistics of genetic variability of each population before (pre-fires, year
2016) and after fire events (post-fires, year 2022).
Tabla 1. Comparación
de los estadísticos descriptivos de la variabilidad genética de cada población
antes (pre incendios, año 2016) y después de los incendios (post incendios, año
2022).
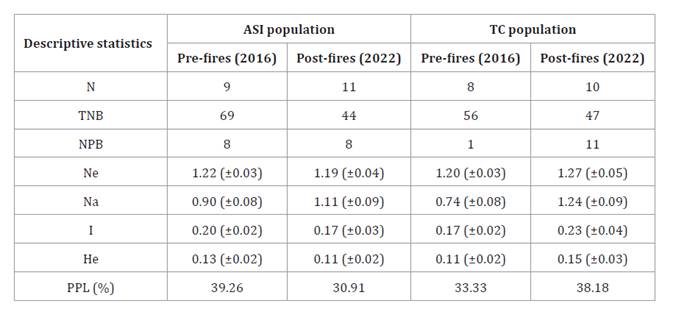
N:
Number of individuals, TNB: total number of bands, NPB: number of private
bands, Ne: number of effective alleles, Na: number of different alleles, I:
Shannon’s information index, He: expected heterozygosity, PPL: percentage of
polymorphic loci, ±: standard deviation. The genetic data of the populations
from the pre-fire period were obtained from Silvestri
et al. (2020).
N: Número de
individuos, TNB: número total de bandas, NPB: número de bandas privadas, Ne:
número de alelos efectivos, Na: Número de alelos diferentes, I: Índice de
Shannon; He: Heterocigosis esperada, PPL: Porcentaje de Loci Polimórficos, ±:
Desviación estándar. Los datos genéticos de las poblaciones del periodo
pre-incendios fueron obtenidos de Silvestri
et al. (2020).
Genetic
structure analysis
AMOVA results
showed that genetic variation was higher among populations than within them,
when grouping by population alone and by population with collection period (table 2).
Table 2. Hierarchical
analysis of the genetic variability distribution estimated by AMOVA.
Tabla
2. Análisis jerárquico de la
distribución de la variabilidad genética estimada por AMOVA.
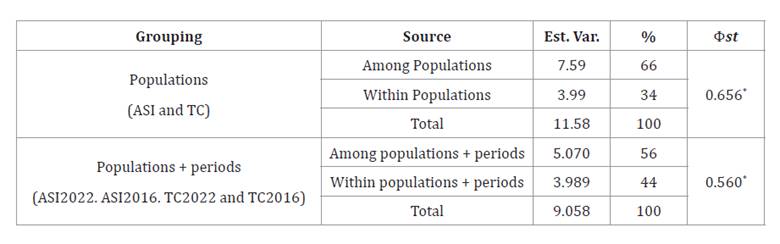
Est.
Var: Estimated Variance component, %: Percentage of total variance, Φst=
Phi value for each cluster, *p < 0.05.
Est.
var= Componente de la varianza, %= Porcentaje de la varianza total, Φst=
Valor de phi para cada agrupación, *significativo p < 0,05.
Genetic distances
between ASI2016 and ASI2022 and between TC2016 and TC2022 were 0.05 and 0.01,
respectively, with corresponding genetic identity values of 0.95 and 0.998.
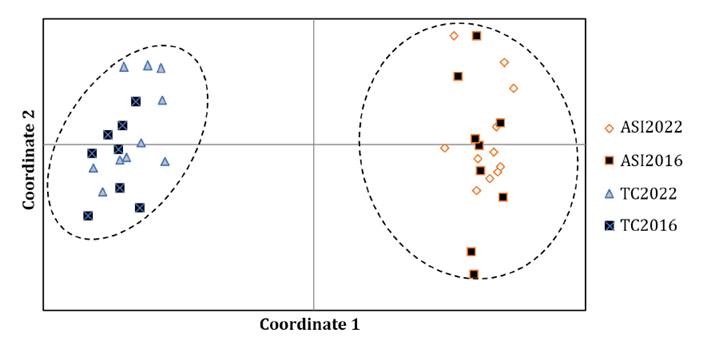
PCoA based on individual genetic distances revealed that the first three
coordinates accounted for 61.71% of total variation, with coordinates 1 and 2
explaining the largest proportion (56.73%). Coordinate 1 separates ASI and TC
individuals. ASI individuals showed greater dispersion than those from TC.
However, individuals within each population could not be grouped by collection
period (pre- and post-fire) (figure 2).
Figure
2.
Principal Coordinate Analysis (PCoA) according to Nei’s genetic distance
between individuals of the ASI2022, ASI20116, TC2022 and TC2016 populations of S.
hippocampoides.
Figura
2. Análisis de Coordenadas Principales
(PCoA) de acuerdo a la distancia genética de Nei entre individuos de las
poblaciones ASI2022, ASI20116, TC2022 and TC2016 de S. hippocampoides.
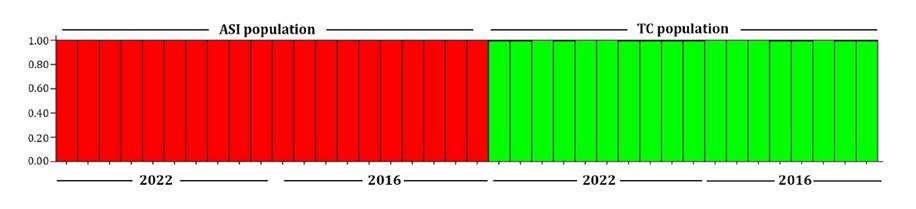
Bayesian clustering identified two groups with the highest ΔK
observed at K = 2. Individuals showed high membership coefficients to a
single group corresponding to their geographic population, ASI (combined from
pre- and post-fire individuals) and the TC (also including individuals from
both periods) (figure 3).
Figure
3. Bayesian clusters inferred by STRUCTURE of the
populations under study of S. hippocampoides. Each individual is
represented by a single vertical bar. Bars are partitioned into K = 2
components that represent each individual’s proportional assignment to one of
the genetic clusters. The populations and the collection period are indicated
above and below the figure, respectively.
Figura
3. Agrupaciones Bayesianas inferidas
por STRUCTURE de las poblaciones en estudio de S. hippocampoides. Cada
individuo está representado por una barra vertical. Las barras están divididas
en K = 2 componentes que representan la proporción del genoma de cada individuo
asignada a un grupo genético. Las poblaciones y los periodos de colección se
indican por encima y por debajo del gráfico, respectivamente.
Discussion
Our results show
high genetic variability in S. hippocampoides, relative to values
reported for this species using ISSR markers (33)
and random amplified polymorphic DNA markers (17).
Higher genetic diversity has been observed in other Stylosanthes species
using ISSR markers (1, 6). These
variations may result from differences in sampling and inherent genetic
variability among species. For instance, Silvestri et
al. (2020) analyzed eight populations, while we included only two. Kazan et al. (1993) used four individuals per
population, whereas we analyzed ≥10 individuals per population. AMOVA results
indicated that genetic variation is greater among populations than within
populations, consistent with previous studies on Stylosanthes species
using ISSR (1, 6, 34) and other molecular
markers (5, 31, 39). This is expected
given the predominant self-fertilizing reproduction of most Stylosanthes species.
The total number of bands (TNB) in both populations was lower than in the
pre-fire evaluation, while the number of private bands (NPB) increased in the
TC population and remained stable in the ASI population. These differences may
be due to variations in the number of populations analyzed and differences in
band interpretation between studies. We assessed two geographically distant
populations, whereas Silvestri et al. (2020)
included eight populations, two of which were from the “Tres Cerros” Natural
Reserve. Since only one of these populations is included in our current
analysis, the increase in NPB in the TC population can be attributed to the
absence of nearby populations in both evaluations, whereas the NPB in the ASI
population remained unchanged.
Following natural
or artificial fire events, population genetic variability may decrease,
increase, or remain unchanged. A decrease is the least commonly reported
outcome (43), while increases or
stability are more frequent (3, 27, 32).
In our study, the ASI population showed no statistically significant changes in
genetic diversity indices after fire. Genetic diversity often requires multiple
generations to show significant shifts, depending on the intensity and
frequency of disturbances and the degree of population fragmentation (2).
The maintenance of
genetic variability may result from adaptations that mitigate fire negative
effects. Like many Fabaceae species, Stylosanthes seeds have hard,
impermeable coats that require scarification at 80°C or exposure to surfaces
reaching 120°C to break dormancy and enable germination (7, 13). This trait supports the formation of
persistent seed banks below the soil surface, which can store genetic material
from recent and earlier pre-fire generations. Seed bank formation has been
documented in S. hippocampoides (23),
and considering its generation time and the interval between our evaluations,
this mechanism is a plausible explanation for the observed genetic stability.
Such a strategy enables post-fire regeneration from multi-generational seed
banks rather than a few surviving individuals (3).
Additionally, Stylosanthes species are known for drought tolerance and
high colonization capacity (18), traits
that promote rapid recovery after fire events.
The ASI population,
located along a roadside subject to frequent burning and mowing, may be adapted
to such disturbances. Banks et al. (2013)
suggest that populations in frequently disturbed areas may rely on these
conditions for persistence, as disturbance can enhance resource availability
and shape community composition. In this context, the ASI population may
exhibit fire adaptation, with a strong colonizing response enabled by
heat-softened seeds and reduced competition. This interpretation aligns with Gardener (1980), who found that Stylosanthes cultivars
from arid environments were more fire-resistant than those from humid coastal
areas.
Bayesian clustering
analysis showed a high degree of individual assignment to a single cluster,
corresponding to their respective geographic populations. This pattern, along
with the separation of populations along different axes in the PCoA, supports
the observed genetic differentiation. The presence of private bands in both
populations further indicates strong genetic identity. These findings suggest
that the two sampled sites represent genetically structured and distinct
populations. However, no signs of fragmentation were detected in the genetic
structure of the fire-affected ASI population. Fire effects on genetic
structure are often spatially heterogeneous (22),
and seed bank depletion may vary depending on fire intensity and frequency (25, 38). Such dynamics can influence demographic
connectivity and affect the distribution of genetic diversity within and among
populations. Despite this, few studies have compared the genetic composition of
seed banks and adult populations in fire-prone ecosystems (3, 9, 25). Gardener (1980)
found that most Stylosanthes cultivars possessed sufficient seed
reserves for regeneration, with the number of seedlings in each regenerating
line correlating with seed quantity in the soil. Nevertheless, direct
comparisons between the genetic profiles of pre-fire adults and post-fire
seedlings remain scarce, making it difficult to determine whether seed banks
fully preserve the genetic composition of earlier generations. The lack of
changes in genetic structure in the ASI population may be explained by several
biological traits of the species, such as its predominantly self-fertilizing
reproduction, which limits within-population genetic variability, a short
generation time that allows a rapid regeneration; a large and persistent seed
bank; and the relatively brief time between fire events and post-fire sampling,
which may not have been sufficient for genetic subdivision to emerge.
Given the
increasing frequency and intensity of fires across the natural range of S.
hippocampoides in recent years (14, 20, 30),
future research should expand on this study by including more populations with
contrasting fire histories. Determining whether post-fire individuals originate
from soil seed banks or surviving adults would also be valuable. Such
information is essential for informing germplasm collection and designing
effective conservation strategies.
Conclusion
Our results showed no statistically significant changes in the
genetic variability indices of the ASI population after fire, nor evidence of
fragmentation or alterations in its genetic structure. However, as only one
fire-affected population was analyzed, our capacity to fully assess the
species’ response to fire is limited. This study should therefore be viewed as
a preliminary step. Future research should include additional populations with
contrasting fire histories and examine whether post-fire individuals originate
from soil seed banks or surviving adults. Given the increasing frequency and
intensity of fires in recent years in Argentina, driven by human activities and
intensified by climate change, such studies are crucial for developing
effective conservation strategies for native species.
Acknowledgments
This work was supported by the Secretaría General de Ciencia y
Técnica of the Universidad Nacional del Nordeste (Special Grant for Strategic
Research in the Context of Drought and Wildfires in the Province of Corrientes.
Research Group: Germplasm of Leguminous Species of Productive Interest:
Conservation, Characterization, and Pre-breeding) and by PICT-2021-I-INVI-00202
(Agencia Nacional de Promoción Científica y Tecnológica, ANPCyT).
1. Alzate-Marin, A.
L.; Costa-Silva, C.; Rivas, P. M. S.; Bonifacio-Anacleto, F.; Santos, L. G.;
Moraes Filho, R. M. D.; Martinez, C. A. 2019. Diagnostic fingerprints ISSR/SSR
for tropical leguminous species Stylosanthes capitata and Stylosanthes
macrocephala. Sci. Agr. 77(3): e20180252.
https://doi.org/10.1590/1678-992X-2018-0252
2. André, T.;
Lemes, M. R.; Grogan, J.; Gribel, R. 2008. Post-logging loss of genetic
diversity in a mahogany (Swietenia macrophylla King, Meliaceae)
population in Brazilian Amazonia. For. Ecol. Manag. 255: 340-345.
https://doi.org/10.1016/j.foreco.2007.09.055
3. Ayre, D. J.;
Ottewell, K. M.; Krauss, S. L.; Whelan, R. J. 2009. Genetic structure of
seedling cohorts following repeated wildfires in the fire‐sensitive shrub Persoonia
mollis ssp. nectens. J. Ecol. 97(4):
752-760. https://doi.org/10.1111/j.1365-2745.2009.01516.x
4. Banks, S. C.;
Cary, G. J.; Smith, A. L.; Davies, I. D.; Driscoll, D. A.; Gill, A. M.;
Lindenmayer, D. B.; Peakall, R. 2013. How does ecological disturbance influence
genetic diversity? Trends Ecol Evol. 28(11): 670-679.
https://doi.org/10.1016/j.tree.2013.08.005
5. Barros, A. M.;
Faleiro; F. G.; Shiratsuchi, L. S.; Pereira de Andrade, R.; Britto Lopes, G. K.
2005. Variabilidade genética e ecológica de Stylosanthes macrocephala determinadas
por RAPD e SIG. Pesqui. Agropecu. Bras. 40: 899-909. https://doi.org/10.1590/S0100-
204X2005000900010
6. Costa, J. C.;
Fracetto, G. G. M.; Fracetto, F. G. C.; Souza, T. C.; Santos, M. V. F.; Lira
Júnior, M. A. 2018. Genetic diversity in natural populations of Stylosanthes
scabra using ISSR markers. Genet. Mol. Res. 17(1): gmr18219.
https://doi.org/10.4238/gmr16039866
7. Dalzotto, D.;
Sharry, S.; Piñuel, L.; Boeri, P. 2025. Challenges in germination of Neltuma
caldenia in semi-arid regions: optimization of germination protocols,
influence of saline stress and seed quality. Revista de la Fcultad de Ciencias
Agrarias. Universidad Nacional de Cuyo. Mendoza. Argentina. 57(1): 67-79. DOI:
https://doi.org/10.48162/rev.39.152
8. Doyle, J. J.;
Doyle, J. L. 1987. A rapid DNA isolation procedure for small quantities of
fresh leaf tissue. Phytochemistry. 19(1): 11-15.
9. England, P. R.;
Usher, A. V.; Whelan, R. J; Ayre, D. J. 2002. Microsatellite diversity and
genetic structure of fragmented populations of the rare, fire‐dependent shrub Grevillea
macleayana. Mol. Ecol. 11(6): 967-977.
https://doi.org/10.1046/j.1365-294X.2002.01500.x
10. Evanno, G.;
Regnaut, S.; Goudet, J. 2005. Detecting the number of clusters of individuals
using the software STRUCTURE: A simulation study. Mol. Ecol. 14(8): 2611–2620.
https://doi. org/10.1111/j.1365-294X.2005.02553.x
11. Excoffier, L.;
Smouse, P. E.; Quattro, J. M. 1992. Analysis of molecular variance inferred
from metric distances among DNA haplotypes: Application to human mitochondrial
DNA restriction data. Genetics. 131(2): 479-491. https://doi.org/10.1093/genetics/131.2.479
12. Falush, D.;
Stephens, M.; Pritchard, J. K. 2003. Inference of population structure using
multilocus genotype data: Linked loci and correlated allele frequencies.
Genetics. 164(4): 1567-1587. https://doi.org/10.1093/genetics/164.4.1567
13. Fidelis, A.;
Daibes, L. F.; Martins, A. R. 2016. To resist or to germinate? The effect of
fire on legume seeds in Brazilian subtropical grasslands. Acta Bot. Bras. 30:
147-151. https://doi. org/10.1590/0102-33062015abb0187
14. Fidelis, A.; Zirondi, H. L. 2021. And after fire, the
Cerrado flowers: a review of post-fire flowering in a tropical savanna. Flora
280:151849. https://doi.org/10.1016/j.flora.2021.151849
15. FIRMS-NASA’s.
Fire Information for Resource Management System. 2020. https://firms.modaps.
eosdis.nasa.gov/map/#d:24hrs;@0.0,0.0,3z, (Verified 1
July 2020).
16. Gardener, C. J.
1980. Tolerance of perennating Stylosanthes plants to fire. Aust. J. Exp.
Agric. Anim. Husb. 20(106): 587-593. https://doi.org/10.1071/EA9800587
17. Kazan, K.;
Manners, J. M.; Cameron, D. F. 1993. Genetic relationships and variation in the
Stylosanthes guianensis species complex assessed by random amplified
polymorphic DNA. Genome. 36: 43-49. https://doi.org/10.1139/g93-006
18. Maass, B.;
Sawkins, L. 2004. History, relationships and diversity among Stylosanthes species
of commercial significance. In Chakraborty, S. (Ed). High-yielding
anthracnose-resistant Stylosanthes for agricultural systems. CSIRO
Publishing. 12-26.
19. MacDougall, A.
S.; McCann, K. S.; Gellner, G.; Turkington, R. 2013 Diversity loss with
persistent human disturbance increases vulnerability to ecosystem collapse.
Nature. 494(7435): 86-89. https://doi.org/10.1038/nature11869
20. Naval
Fernández, M. C.; Albornoz, J. V.; Bellis, L. M., Baldini, C.; Arcamone, J. R.;
Silvetti, L. E.; Argarañaz, J. P. 2023 Megaincendios 2020 en Córdoba:
Incidencia del fuego en áreas de valor ecológico y socioeconómico. Ecol.
Austral 33: 136-151. http://dx.doi.org/10.25260/ EA.23.33.1.0.2120
21. Nei, M. 1973.
Analysis of gene diversity in subdivided populations. Proc. Natl. Acad. Sci.
70: 3321-3323. https://doi.org/10.1073/pnas.70.12.3321
22. Ooi, M. K. J.;
Whelan, R. J.; Auld, T. D. 2006. Persistence of obligate-seeding species at the
population scale: effects of fire intensity, fire patchiness and long fire-free
intervals. Int. J. Wildland Fire 15: 261-269. https://doi.org/10.1071/WF05024
23. Orr, D. M.
2008. Grazing management influences the dynamics of populations of Stylosanthes
hippocampoides (Oxley fine stem stylo). Trop. Grassl. 42(4): 193-201.
24. Peakall, R.;
Smouse, P. E. 2006. GENALEX 6: Genetic analysis in Excel. Population genetic
software for teaching and research. Mol. Ecol. Not. 6(1): 288-295.
https://doi.org/10.1111/j.1471- 8286.2005.01155.x
25. Premoli, A. C.;
Kitzberger, T. 2005. Regeneration mode affects spatial genetic structure of Nothofagus
dombeyi forests. Mol. Ecol. 14: 2319-2329. https://doi.org/10.1111/j.1365-
294x.2005.02629.x
26. Pritchard, J.
K.; Stephens, M.; Donnelly, P. 2000. Inference of population structure using
multilocus genotype data. Genetics. 155(2): 945-959.
https://doi.org/10.1093/genetics/155.2.945
27. Rajora, O. P.;
Pluhar, S. A. 2003. Genetic diversity impacts of forest fires, forest
harvesting, and alternative reforestation practices in black spruce (Picea
mariana). Theor. Appl. Genet. 106(7): 1203-1212.
https://doi.org/10.1007/s00122-002-1169-9
28. R Core Team.
2020. R: A language and environment for statistical computing. R Foundation for
Statistical Computing v 04.2 [software]. http://www.R-project.org/
29. Santos García,
M. O.; Resende, R. M. S.; Chiari, L.; Zucchi, M. I.; de Souza, A. P. 2011.
Mating systems in tropical forages: Stylosanthes capitata Vog. and Stylosanthes guianensis (Aulbl.) Sw. Euphytica.
178: 185-193. https://doi.org/10.1093/aobpla/pls001
30. Saucedo, G. I.;
Perucca, A. R.; Kurtz, D. B. 2023. Las causas de los incendios de principios
del año 2022 en la provincia de Corrientes. Ecol Aust. 33(1): 273-284. https://doi.org/10.25260/
EA.23.33.1.0.2020
31. Sawkins, M. C.;
Maass, B. L.; Pengelly, C.; Newburry, H. J.; Ford-Lloyd, B. V.; Maxted, N.;
Smith, R. 2001. Geographical patterns of genetic variation in two species of Stylosanthes
Sw. using amplified fragment length polymorphism. Mol. Ecol. 10: 1947-1958.
https://doi. org/10.1046/j.0962-1083.2001.01347.x
32.
Segarra-Moragues, J. G.; Ojeda, F. 2010. Postfire response and genetic
diversity in Erica coccinea: Connecting population dynamics and
diversification in a biodiversity hotspot. Evolution 64(12): 3511-3524.
https://doi.org/10.1111/j.1558-5646.2010.01064.x
33. Servicio
Meteorológico Argentino. 2020. Informe especial por déficit de lluvias en la
región noreste de Argentina. https://www.smn.gob.ar/boletines/informe-especialsequ%C3%ADa-en-el-nea
(Fecha de consulta: 15 de abril 2020).
34. Silvestri, M.
C.; Acuña, C. A.; Moreno, E. M. S.; Garcia, A. V.; Vanni, R. O.; Lavia, G. I.
2020. Patterns of genetic diversity and potential ecological niches of Stylosanthes
species from northeastern Argentina. Crop Sci. 60: 1436-1449.
https://doi.org/10.1002/csc2.20117
35. Smichowski, H.;
Montiel, M. R.; Romero, V.; Kowalewski, M.; Contreras, F. I. 2021. Evaluación
de incendios en áreas periurbanas de la ciudad de Corrientes (Argentina)
durante la sequía extrema del año 2020. Pap. Geogr. 67: 151-167.
https://doi.org/10.6018/geografia.486441
36. Starr, C. R.;
Corrêa, R. S.; Filgueiras, T. D.; Hay, J. D.; dos Santos, P. F. 2013. Plant
colonization in a gravel mine revegetated with Stylosanthes spp. in a Neotropical savanna. Land. Ecol. Eng. 9: 189-201.
https://doi.org/10.1007/s11355-012-0196-1
37. Steinitz, O.;
Shohami, D.; Ben-Shlomo, R.; Nathan, R. 2012. Genetic consequences of fire to
natural populations. Isr. J. Ecol. Evol. 58: 205-220.
https://doi.org/10.1560/IJEE.58.2-3.205
38. Uchiyama, K.; Goto, S.; Tsuda, Y.; Takahashi, Y.; Ide, Y.
2006. Genetic diversity and genetic structure of adult and buried seed
populations of Betula maximowicziana in mixed and post-fire stands. For.
Ecol. Manag. 237: 119-126. https://doi.org/10.1016/j.foreco.2006.09.037
39. Vander Stappen,
J.; Van Campenhout, S.; Gama Lopez, S.; Volckaert, G. 1998. Sequencing of the
internal transcribed spacer region ITS1 as a molecular tool detecting variation
in the Stylosanthes guianensis species complex. Theor. Appl. Genet. 96:
869-877. https://doi. org/10.1007/s001220050814
40. Vanni, R. O.
2017 The genus Stylosanthes (Leguminosae - Dalbergiaeae) in South
America. Bol. Soc. Argent. Bot. 52(3): 549-585. https://doi.org/10.31055/1851.2372.v52.n3.18033
41. Velásquez
Ramírez, M. G.; del Castillo Torres, D.; Guerrero Barrantes, J. A.; Vásquez
Bardales, J.; Thomas, E.; Cusi Auca, E.; Gushiken, M. C.; Muñoz Díaz, B.;
Russo, R.; Corvera Gomringer, R. 2021. Soil recovery of alluvial gold mine
spoils in the Peruvian Amazon using Stylosanthes guianensis, a promising
cover crop. Land Degrad. Dev. 32(18): 5143-5153. https://doi.
org/10.1002/ldr.4118
42. Wilcoxon F.
1950. Some rapid approximate statistical procedures. Ann. N. Y. Acad. Sci.
52(6): 808-814. https://doi.org/10.1111/j.1749-6632.1950.tb53974.x
43. Worth, J. R.; Jordan, G. J.; Marthick, J. R.; Sakaguchi, S.;
Colhoun, E. A.; Williamson, G. J.; Motomi, I.; Bowman, D. M. 2017. Fire is a
major driver of patterns of genetic diversity in two co‐occurring Tasmanian
palaeoendemic conifers. J. Biogeogr. 44(6): 1254-1267. https://doi.
org/10.1111/jbi.12919