Revista de la Facultad de Ciencias
Agrarias. Universidad Nacional de Cuyo. Tomo 57(2). ISSN (en línea) 1853-8665.
Año 2025.
Original article
The Native Dryland PGPR ‘Pseudomonas
42P4’ Promotes Adventitious Rooting in Woody Cuttings of Vitis spp.
La
PGPR nativa de zonas áridas ‘Pseudomonas 42P4’ promueve la fomación de
raíces adventicias en estacas leñosas de Vitis spp.
Maria Gabriela
Gordillo1,
Fanny Colombo3,
1Universidad Nacional de Cuyo. Facultad de Ciencias Agrarias.
Instituto de Biología Agrícola de Mendoza (IBAM-CONICET). Almirante Brown 500.
Chacras de Coria. M5528AHB. Mendoza. Argentina.
2Universidad Nacional de Cuyo. Facultad de Ciencias Agrarias.
Almirante Brown 500. Chacras de Coria. M5528AHB. Mendoza. Argentina.
3Trapiche Winery. Calle Nueva Mayorga s/n. Maipú. Mendoza.
Argentina. CPA: M5513
*cgonzalez@fca.uncu.edu.ar
Abstract
This study
evaluated the effect of two native PGPR strains from an arid region (Mendoza,
Argentina) on the rooting of woody cuttings of Vitis spp. These strains
are known for their growth-promoting capacity, including auxin production.
Dormant V. vinifera cv. Malbec cuttings were grafted onto four
rootstocks - 1103 Paulsen, 110 Richter, 101-14 MGt and SO4. Then, basal ends of
these grafted cuttings and own rooted controls were incubated for 12 h in
solutions of (1) Pseudomonas 42P4 at 107 CFU mL-1,
(2) Enterobacter 64S1 at 107 CFU mL-1,
(3) autoclaved LB medium, (4) water, and (5) a quick-dip immersion of
Indole-3-butyric acid (IBA). After treatment, the cuttings were placed in a
forcing chamber at 28°C and relative humidity ~100% for 21 days. Rooting
parameters and scion-rootstock union percentages were recorded. Pseudomonas 42P4
significantly promoted rooting in Malbec own-rooted cuttings. However, Enterobacter
64S1 had negative or null effects. Furthermore, Pseudomonas 42P4
enhanced rooting in Malbec grafted onto 1103 Paulsen, but not on 101-14 MGt,
110 Richter or SO4. This strain also improved graft union success on SO4, but
did not affect the other rootstocks. These results suggest that a dryland
native strain such as Pseudomonas 42P4 could sustainably enhance the quality of
both own-rooted and grafted grapevine plants in commercial nurseries.
Keywords: PGPR, grapevine
rootstock, Malbec, rooting, graft union, nursery
Resumen
El objetivo de este
estudio fue evaluar el efecto de dos cepas PGPR nativas de zonas áridas
(Mendoza, Argentina) con capacidad promotora del crecimiento (que producen
auxinas) sobre el enraizamiento de estacas leñosas de Vitis spp. La base
de las estacas de Vitis vinifera cv. Malbec, tanto francas como
injertadas sobre cuatro portainjertos:1103 Paulsen, 110 Richter, 101-14 MGt y
SO4, se incubaron durante 12 h en soluciones de 1) Pseudomonas 42P4 y 2)
Enterobacter 64S1 107 UFC mL-1,
3) medio LB autoclavado, 4) agua y 5) una inmersión rápida de ácido
indol-3-butírico (IBA). Posteriormente, las estacas se colocaron en una cámara
de forzadura a 28°C y humedad relativa ~100% durante 21 d. Se midieron
diferentes parámetros de enraizamiento y el porcentaje de unión del injerto.
Los resultados mostraron que Pseudomonas 42P4, pero no Enterobacter 64S1
promovió el enraizamiento, de forma similar a IBA en estacas de Malbec. La cepa
Pseudomonas 42P4 también promovió el enraizamiento de estacas injertadas
sobre 1103 Paulsen, pero no sobre 101-14, 110 Richter y SO4. Además, Pseudomonas
promovió la unión del injerto en SO4, pero no en 110 Richter, 1103 Paulsen
y 101-14. Estos resultados sugieren que una cepa nativa de zonas áridas podría
utilizarse como una herramienta sustentable para mejorar la calidad de plantas
francas e injertadas de vid.
Palabras clave: PGPR, portainjertos
de vid, Malbec, enraizamiento, unión del injerto, vivero
Originales: Recepción: 16/04/2025
- Aceptación: 06/07/2025
Introduction
A significant
portion of worldwide agricultural land (45%) is dryland. Climate change (CC)
threatens agroecosystems, causing detrimental effects on crop productivity (Berdugo
et al., 2020; Burrel et al., 2020). Viticulture
covers 7.3 million hectares of the world, with over 60% of grapes produced in
drylands (OIV
2023; Flexas et al., 2010). Argentina ranks seventh, with the largest cultivated vineyard
area globally. This country mainly develops irrigated viticulture in drylands (OIV
2023, INV 2023).
Grapevines are
clonally propagated through one-year woody cuttings. Grafted or ungrafted woody
cuttings are forced under high relative humidity (RH) and ± 27°C to stimulate
adventitious root formation and scion-rootstock healing (in grafted plants).
External factors (temperature, humidity, substrate aeration) and internal
factors (carbohydrate reserves, hormones, and genotype) influence these processes
(Hartmann
et al., 2014).
Rootstocks exhibit
resistance to pathogens and diseases, and confer tolerance to abiotic stresses
(Hartmann
et al., 2014; Ollat et al., 2016, Keller, 2020, D’Innocenzo et
al., 2024). V. vinifera cultivars are generally grafted onto
American Vitis spp. hybrid rootstocks due to
their resistance to phylloxera (Mudge et al., 2009). Grafting is
essential in most European wine-growing regions. However, outside Europe,
vineyards can be planted with own-rooted V. vinifera plants.
One main problem in
grapevine propagation is the differential rooting capacity of rootstocks.
Rootstocks 110 Richter (110 R) and Selection Oppenheim 4 (SO4) are recalcitrant
to form adventitious roots. This differs from other widely used grapevine
rootstocks like 1103 Paulsen (1103 P) and 101-14 Millardet et de Grasset
(101-14 MGt), which induce more root development (Keller, 2020). Low rooting
capacity causes significant economic losses to nurseries. Additionally,
scion-rootstock interaction plays a crucial role in rooting. The scion may
affect root dry weight per woody cutting (Tandonnet et al., 2009), as the
scion-rootstock union simultaneously occurs with rooting, depending on cutting
reserves.
Auxins are the main phytohormones in adventitious root
production of woody cuttings (Burnoni et al.,
2022), though their effect varies among genotypes. For example,
indole-3-butyric acid (IBA), a primary synthetic auxin used for grapevine
rooting (Machado et al., 2005),
promotes rooting in woody cutting of 110 R, SO4 and 101-14 MGt (Gordillo
et al., 2022; Satisha et al., 2008).
In contrast, IBA may promote (Satisha et
al., 2008; Daskalakis et al., 2018) or not (Boeno
et al., 2023; Gordillo et al., 2022)
rooting of 1103 P woody cuttings. However, synthetic agrochemicals are
progressively being excluded as organic and agroecological agriculture gains
attention (Centeno et al., 2008).
Plant
Growth-Promoting Rhizobacteria (PGPR) are plant symbiotic bacteria that
colonize the rhizosphere and produce indole acetic acid (IAA)-type auxins (Glick et
al., 2012; Pantoja Guerra et al., 2023). Some PGPR
strains promote rooting of difficult-to-root rootstocks, while others have no
effect (Isçi
et al., 2019; Toffanin et al., 2016). Our group
isolated and characterized two PGPR strains from Mendoza´s arid soils of
Argentina. Pseudomonas 42P4 (42P4) and Enterobacter 64S1 (64S1)
produce IAA (Pérez-Rodríguez
et al., 2020a) and promote seedling growth of Solanum lycopersicum (Pérez-Rodríguez
et al., 2022) and Capsicum annuum seedlings (Lobato
Ureche et al., 2021), alleviate saline stress in tomato (Pérez-Rodríguez
et al., 2022), and increase drought tolerance in Arabidopsis thaliana plants
(Jofré
et al., 2024). Furthermore, Pseudomonas 42P4 enhances tomato growth,
yield and fruit quality under field conditions (Pérez-Rodríguez et al.,
2020b).
Both strains also exhibit biocontrol activity, inhibiting tomato and pepper
crop diseases (unpublished data). Native strains easily adapt to edaphic
conditions, resist local environmental stresses, and are more successful when
inoculated into the plant rhizosphere.
PGPR improve
rooting and survival of young plants of various species. However, few studies
report PGPR´s effect on woody plant production or grapevine woody cuttings,
specifically (Bartolini
et al., 2017; Köse et al., 2003, 2005; Tofanin et al.,
2016).
Currently, exploring sustainable tools is imperative, especially given global
warming threats, particularly challenging in drylands.
Therefore, this
work evaluated the effect of two native PGPR strains from Mendoza’s arid soils,
Pseudomonas 42P4 and Enterobacter 64S1, on adventitious root
production of the Argentinean emblematic cultivar: Malbec, and the four most
widespread rootstocks in global viticulture and Argentine arid areas: SO4, 110
R, 1103 P, and 101-14 MGt (Riaz et al., 2019). These strains,
adapted to arid soils, could constitute a sustainable alternative for synthetic
agrochemicals in grapevine propagation.
Materials
and Methods
Bacterial
Culture
Pseudomonas 42P4 (42P4) and Enterobacter
64S1 (64S1) were collected, isolated and characterized by the Plant
Physiology and Microbiology Group (IBAM- FCA, CONICET-UNCuyo, Mendoza,
Argentina). The partial 16S rRNA sequence of both strains was deposited in
GenBank under accession numbers MT045993.1 and MT047267, respectively (Pérez-Rodríguez
et al., 2020a). Inocula were prepared in 1 L Erlenmeyer Flask with 400 mL of
Luria Broth (LB) culture medium (10 g Peptone, 5 g Yeast Extract, 5 g NaCl in 1
L of bidistilled H2O). Bacteria were cultured in an orbital shaker at 120
rpm and 32°C for 24 h. Strain concentration was estimated by optical density at
540 nm in a spectrophotometer according to Pérez-Rodriguez et al.
(2020a). From these cultures, a dilution to 107 colony-forming units
(CFU) mL–1 was prepared for each
strain in PBS (phosphate buffer saline).
Plant
Material, Inoculation and Rooting Conditions
Two experiments
were conducted in the grapevine nursery of Grupo Peñaflor S.A. (Trapiche
Winery), located in Santa Rosa, Mendoza, Argentina (33°15‘39.4” S 68°07’48.9”
W). The first experiment was conducted during the 2020 season
(September-October) and the second, in 2021. Cuttings (length: 40 cm and
diameter: 9 mm) were collected in winter and stored at 4°C until experiment
initiation.
In the first
experiment, we tested different doses of Pseudomonas 42P4 and Enterobacter
64S1 on the rooting capacity of V. vinifera cv. Malbec woody
cuttings. Before forcing, we applied five treatments: (1) 30-second quick
immersion in 1000 ppm IBA solution (Gordillo et al. 2022); or 12-hour
incubation in solutions of: (2) Pseudomonas 42P4 at 107 CFU mL-1,
(3) Enterobacter 64S1 at 107 CFU
mL-1, (4)
autoclaved LB, and (5) tap water (control treatment) on the base (1.5 cm) of 50
woody cuttings per treatment.
In a second
experiment, we evaluated Pseudomonas 42P4 on the rooting of Malbec
cuttings (with 2 buds and approximately 5 cm long) grafted onto cuttings of
101-14 MGt (V. riparia × V. rupestris), SO4 (V. berlandieri × V.
riparia), 1103 Paulsen, and 110 Richter (V. berlandieri × V. rupestris),
with 5 buds (approximately 35 cm long and 9 mm in diameter). We used an Omega
grafting machine (Fornasier Cesare & C., Italy). Each grafted cutting was
40 cm long. The grafting zone was covered with a commercial initiation wax
(Guerowax Crecimiento 78, Guerola Industries, Spain) to prevent dehydration and
diseases during forcing. Before forcing we applied the following treatments:
(1) a 30-second quick immersion at 750 ppm IBA (Gordillo et al. 2022), and 12-hour
incubations with: (2) Pseudomonas 42P4 at 107 CFU mL-1,
(3) autoclaved LB, and (4) tap water (control) on the base (1.5 cm) of 50
grafted cuttings per treatment.
Subsequently, in
both experiments, cuttings were horizontally placed in plastic boxes with peat
(KEKKILÄ professional, https://www.kekkilaprofessional.com/). Then, cuttings
were forced and maintained for 21 days at 28°C and 100% RH.
The experimental
design was a completely randomized design, considering each cutting as an
experimental unit.
Morphological
Parameters
Treatment effect
was evaluated by callus and rooting percentages, followed by root number and
biomass (g) per cutting. Rooting percentage is the percentage of cuttings that
produced at least one root. Callus percentage was visually determined as the
proportion of the cutting base occupied by callus (0-25, 25-50, 50-75, and
75-100%). Root biomass was determined as dry weight (DW) of all adventitious
roots from a cutting, oven-dried at 60°C to constant weight. In grafted
cuttings, the percentage of scion-rootstock union was visually determined as
well (0-25, 25-50, 50-75, and 75-100%).
Statistical
Analysis
Statistical
analyses were performed using the InfoStat P 2020v software (Di
Rienzo et al., 2020). Rooting, callus and graft union percentages, along with root
number and biomass, were analysed with Generalized Linear Models. A binomial
distribution was used for the first three parameters and the Poisson
distribution for the fourth. Root biomass was first tested for ANOVA assumptions
(using Shapiro-Wilks test for normality and Levene test for homoscedasticity).
Different letters indicate significant differences among treatments according
to the post-hoc test DGC (α = 0.05). Data visualization was conducted in R (R Core
Team, 2024) and the ggplot2 package (Wickham, 2016).
Results
Own-rooted
Malbec cuttings
Basal callus
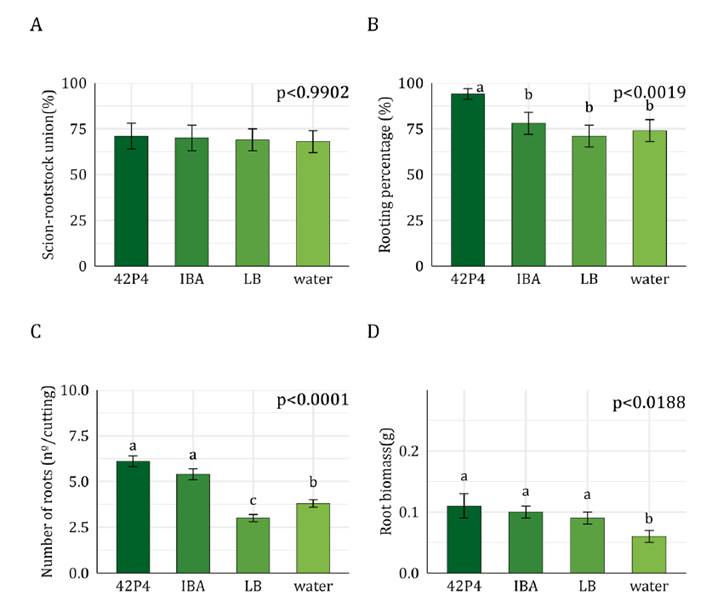
percentage on Malbec was similar among treatments (>75%, p > 0.05) (figure 1A). Pseudomonas 42P4
and IBA 1000 ppm increased rooting percentage (>80%), compared to autoclaved
LB and water treatments (70%). However, Enterobacter 64S1 decreased
rooting percentage (25%) compared to water (figure 1B). The number of
roots per cutting was similar in cuttings incubated with 42P4 and IBA (~ 6
roots per cutting), and higher than in the remaining treatments (~ 4 roots per
cutting) (figure
1C).
Root biomass per cutting was 30% higher in cuttings incubated in IBA than in
42P4. The remaining treatments yielded lower biomass (figure 1D). Pseudomonas 42P4
promoted three of the four rooting parameters compared to the water control:
rooting percentage, number of roots per cutting, and root biomass. In contrast,
the native Enterobacter 64S1 strain did not promote any evaluated
parameter.
Based on these results, we assessed the ability of the native Pseudomonas
42P4 strain (but not Enterobacter) to promote rooting and graft
union of Malbec cuttings grafted onto four grapevine rootstocks.
A)
Callus percentage, B) Rooting percentage, C) Number of roots per cutting, and
D) Root biomass per cutting.
Values
correspond to adjusted means ± SEM (n=50). Data were analysed with General or
Generalized Mixed Linear Models. Different letters indicate significant
differences among treatments according to the post-hoc test DGC (α = 0.05).
42P4:
Pseudomonas 42P4, 64S1: Enterobacter 64S1, IBA: Indole-3-butyric
acid, LB: Luria Broth culture medium, water: tap water.
A)
Porcentaje de callo, B) Porcentaje de enraizamiento, C) Número de raíces por
estaca y D) Biomasa de raíces por estaca.
Los
valores corresponden a las medias ajustadas ± EE (n=50). Los datos fueron
analizados mediante Modelos Lineales Mixtos Generales o Generalizados. Letras
diferentes indican diferencias significativas entre tratamientos según el test
post-hoc DGC (α = 0,05).
42P4: Pseudomonas
42P4, 64S1: Enterobacter 64S1, IBA: ácido indol-3-butírico, LB:
medio de cultivo Luria Broth, water: agua de red.
Figure
1. Own-rooted Malbec cuttings.
Figura 1. Estacas
de Malbec a pie franco.
Grafted
Cuttings
1103 Paulsen
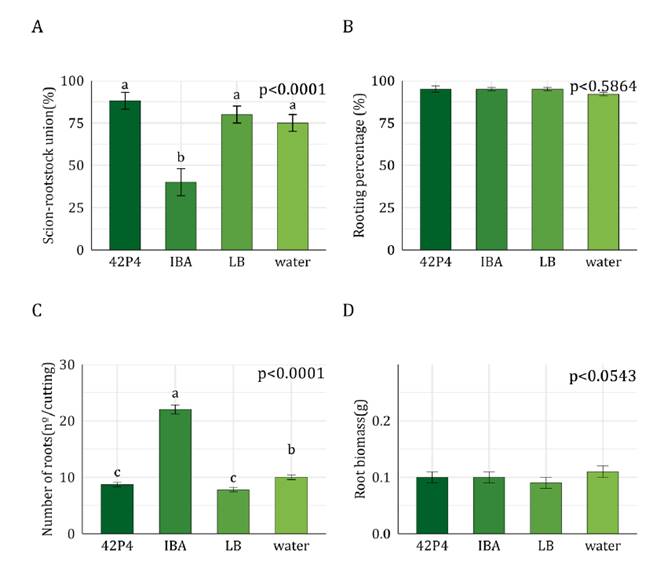
The graft union percentage (~75%) between Malbec and 1103 P
rootstock, and callus percentage (~100%, p > 0.05, data not shown) were
unaffected by treatments (figure 2A).
Rooting percentage was higher (~90%) in cuttings incubated with Pseudomonas 42P4
(figure
2B) than in other treatments (~75%). The number of roots per cutting
was duplicated in 42P4 and IBA treatments than in the controls (water and LB) (figure
2C). However, root biomass was 10% higher in cuttings incubated
with 42P4 and IBA compared to the other treatments (figure 2D).
Pseudomonas 42P4 promoted a 15% increase in rooting percentage compared
to IBA, while matching root number and biomass results to this hormone
treatment.
A)
Scion-rootstock union percentage, B) Rooting percentage, C) Number of roots per
cutting, and D) Root biomass per cutting. Values correspond to adjusted means ±
SEM (n=50). Data were analysed with General or Generalized Linear Models.
Different letters indicate significant differences between treatments according
to the post-hoc test DGC (α = 0.05). 42P4: Pseudomonas 42P4, 64S1: Enterobacter
64S1, IBA: Indole-3-butyric acid, LB: Luria Broth culture medium, water:
tap water.
A) Porcentaje de
unión entre la púa y el portainjerto, B) Porcentaje de enraizamiento, C) Número
de raíces por estaca y D) Biomasa de raíces por estaca. Los valores
corresponden a las medias ajustadas ± EE (n=50). Los datos fueron analizados
mediante Modelos Lineales Mixtos Generales o Generalizados. Letras diferentes
indican diferencias significativas entre tratamientos según el test post-hoc
DGC (α = 0,05). 42P4: Pseudomonas 42P4, 64S1: Enterobacter 64S1,
IBA: ácido indol-3-butírico, LB: medio de cultivo Luria Broth, water: agua de
red.
Figure
2. Rootstock 1103 Paulsen.
Figura 2. Portainjerto
1103 Paulsen.
101-14 MGt
The scion-rootstock union percentage between Malbec and 101-14
MGt rootstock decreased by 25% when cuttings were incubated with IBA compared
to the other treatments (figure 3A).
Callus percentage (~100%, p>0.05) (data not shown), rooting percentage
(~100%), and root biomass of 101-14 MGt were not affected by the treatments (figure
3B and 3D). The IBA treatment increased the number of roots per cutting
(20 roots per cutting) compared to the water control (10 roots per cutting) (figure
3C), while 42P4 and LB treatments decreased this variable to 7
roots per cutting compared to the water control. Inoculation of 101-14
rootstock cuttings with the 42P4 native strain did not promote rooting or graft
union.
A)
Scion-rootstock union percentage, B) Rooting percentage, C) Number of roots per
cutting, and D) Root biomass per cutting. Values correspond to adjusted means ±
SEM (n=50). Data were analyzed with General or Generalized Linear Models.
Different letters indicate significant differences between treatments according
to the post-hoc test DGC (α = 0.05). 42P4: Pseudomonas 42P4, 64S1: Enterobacter
64S1, IBA: Indole-3-butyric acid, LB: Luria Broth culture medium, water:
tap water.
A) Porcentaje de
unión entre la púa y el portainjerto, B) Porcentaje de enraizamiento, C) Número
de raíces por estaca y D) Biomasa de raíces por estaca. Los valores
corresponden a las medias ajustadas ± EE (n=50). Los datos fueron analizados
mediante Modelos Lineales Mixtos Generales o Generalizados. Letras diferentes
indican diferencias significativas entre tratamientos según el test post-hoc
DGC (α = 0,05). 42P4: Pseudomonas 42P4, 64S1: Enterobacter 64S1,
IBA: ácido indol-3-butírico, LB: medio de cultivo Luria Broth, water: agua de
red.
Figure
3. Rootstock 101-14 MGt.
Figura 3. Portainjerto
101-14 MGt.
110 Richter
Graft union percentage between Malbec and 110 R rootstock was
higher in autoclaved LB compared to the other treatments (figure 4A).
However, callus percentage (~100%, p > 0.05, data not shown), rooting
percentage (~75%), and root biomass per cutting were similar among treatments (figure
4B and 4D). Root number per cutting was higher in cuttings incubated with
IBA (4 roots per cutting) than in other treatments (2.5 roots per cutting) (figure
4C). Pseudomonas 42P4 did not promote rooting or graft
union of 110 R.
A)
Scion-rootstock union percentage, B) Rooting percentage, C) Number of roots per
cutting, and D) Root biomass per cutting. Values correspond to adjusted means ±
SEM, n=50. Data were analysed with General or Generalized Linear Models.
Different letters indicate significant differences between treatments according
to the post-hoc test DGC (α = 0.05). 42P4: Pseudomonas 42P4, 64S1: Enterobacter
64S1, IBA: Indole-3-butyric acid, LB: Luria Broth culture medium, water:
tap water.
A) Porcentaje de
unión entre la púa y el portainjerto, B) Porcentaje de enraizamiento, C) Número
de raíces por estaca y D) Biomasa de raíces por estaca. Los valores
corresponden a las medias ajustadas ± EE (n=50). Los datos fueron analizados
mediante Modelos Lineales Mixtos Generales o Generalizados. Letras diferentes
indican diferencias significativas entre tratamientos según el test post-hoc
DGC (α = 0,05). 42P4: Pseudomonas 42P4, 64S1: Enterobacter 64S1,
IBA: ácido indol-3-butírico, LB: medio de cultivo Luria Broth, water: agua de
red.
Figure
4. Rootstock 110 Richter.
Figura 4. Portainjerto
110 Richter.
SO4
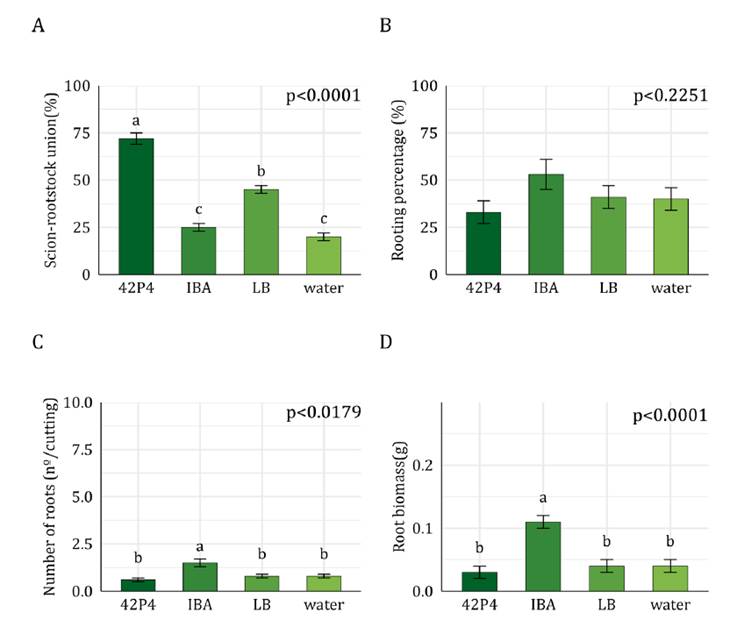
Union percentage between Malbec and SO4 was higher in cuttings
incubated with 42P4 compared to the other treatments (50% higher than IBA and water,
and 25% higher than the LB treatment) (figure 5A).
Callus percentage (~80%, p>0.05) and rooting percentage (40%) of the SO4
rootstock were similar among treatments (figure 5B).
Root number and biomass per cutting were higher in cuttings incubated with IBA
than in other treatments (figure 5C and 5D).
Pseudomonas 42P4 did not promote rooting but increased scion-rootstock
union percentages.
A)
Scion-rootstock union percentage, B) Rooting percentage, C) Number of roots per
cutting, and D) Root biomass per cutting. Values correspond to adjusted means ±
SEM (n=50). Data were analysed with General or Generalized Linear Models.
Different letters indicate significant differences between treatments according
to the post-hoc test DGC (α = 0.05). 42P4: Pseudomonas 42P4, 64S1: Enterobacter
64S1, IBA: Indole-3-butyric acid, LB: Luria Broth culture medium, water:
tap water.
A) Porcentaje de
unión entre la púa y el portainjerto, B) Porcentaje de enraizamiento, C) Número
de raíces por estaca y D) Biomasa de raíces por estaca. Los valores
corresponden a las medias ajustadas ± EE (n=50). Los datos fueron analizados
mediante Modelos Lineales Mixtos Generales o Generalizados. Letras diferentes
indican diferencias significativas entre tratamientos según el test post-hoc
DGC (α = 0,05). 42P4: Pseudomonas 42P4, 64S1: Enterobacter 64S1,
IBA: ácido indol-3-butírico, LB: medio de cultivo Luria Broth, water: agua de
red.
Figure
5. Rootstock SO4.
Figura 5. Portainjerto
SO4.
Discussion
This study is the
first to report applications of native PGPR strains from drylands in grapevine
propagation. We found that a Pseudomonas PGPR can improve rooting in
vine woody cuttings. We evaluated the ability of two native PGPR from arid
zones, Pseudomonas 42P4 and Enterobacter 64S1, to stimulate
rooting of ungrafted and grafted Vitis woody cuttings. Concerning
Malbec’s rooting, Pseudomonas 42P4 improved rooting compared to the
control, matching IBA results (figure 1, and figure
6).
Chart
sectors show the evaluated variables: rooting percentage, number of roots, and
root biomass per cutting. Green indicates significant promotion of the
parameter compared to the water control. Red indicates lower values, and yellow
indicates no significant differences among means. 42P4: Pseudomonas 42P4,
64S1: Enterobacter 64S1, IBA: indole-3-butiric acid, water: tap water.
Los
sectores del gráfico muestran las variables evaluadas: porcentaje de
enraizamiento, número de raíces y biomasa de raíces por estaca. El color verde
indica que el tratamiento promovió significativamente el parámetro en
comparación con el control con agua; el color rojo indica una disminución en
los valores observados, y el color amarillo indica que no hubo diferencias
significativas entre las medias. 42P4: Pseudomonas 42P4, 64S1: Enterobacter
64S1, IBA: ácido indol-3-butírico, wáter: agua de red.
Figure
6. Malbec. Coloured rings represent the treatments
(water, IBA, 42P4, and 64S1).
Figura
6. Malbec. El gráfico representa a los
tratamientos (agua, IBA, 42P4 y 64S1) como anillos.
Conversely, Enterobacter
64S1 failed to promote rooting of Malbec cuttings. Concerning grafted
material, Pseudomonas 42P4’s promoted rooting and graft union in a
rootstock-dependent fashion, enhancing rooting in 1103 Paulsen, but not
affecting the other rootstocks (figure 2, figure
3,
figure
4,
figure
5
and figure
7).
Optimal auxin concentrations stimulate adventitious rooting,
while higher concentrations are inhibitory (Garay-Arroyo et
al., 2014). The root-promoting effect of Pseudomonas 42P4 on
Malbec own-rooted woody cuttings could be attributed to IAA production by this
bacterium (Perez-Rodriguez et al., 2020a).
Each
chart represents a treatment (water, IBA, 42P4) as rings. Chart sectors show
the evaluated variables rooting percentage, number of roots, and root biomass
per cutting. Green indicates significant promotion of the parameter compared to
the water treatment; red indicates lower values, and yellow indicates no
significant differences among means. 42P4: Pseudomonas 42P4, IBA:
indole-3-butiric acid, water: tap water.
Cada gráfico
representa los tratamientos (agua, IBA, 42P4) como anillos. Los sectores del gráfico
muestran las variables evaluadas: porcentaje de enraizamiento, número de raíces
y biomasa de raíces por estaca. El color verde indica que el tratamiento
promovió significativamente el parámetro en comparación con el tratamiento con
agua; el color rojo indica una disminución en los valores observados, y el
color amarillo indica que no hubo diferencias significativas entre las medias.
42P4: Pseudomonas 42P4, IBA: ácido indol-3-butírico, wáter: agua de red.
Figure
7. Rootstocks A) 1103 Paulsen, B) 101-14 MGt, C) 110
Richter and D) SO4.
Figura 7. Portainjertos
A) 1103 Paulsen, B) 101-14 MGt, C) 110 Richter y D) SO4.
However, as Enterobacter
produces an in vitro higher concentration of auxins than Pseudomonas
42P4 (six times more) (Perez-Rodriguez et al.,
2020a),
an excessive concentration of IAA may have led to an inhibitory effect.
Considering this, we had previously tested lower Enterobacter dilutions
(104, 105
and 106 CFU mL-1)
and observed no rooting promotion. Another plausible explanation is the plant
recognizing Enterobacter as a pathogen. However, this would indicate a
species-specific response. In this regard, we previously reported Enterobacter
promoted growth in pepper and tomato (Perez-Rodriguez et al.,
2020a; Lobato Ureche et al., 2021).
Rooting capacity
varied with rootstock (Keller 2020; Ollat et al., 2016). We found that
101-14 MGt showed the highest rooting compared with the control treatment,
followed by 1103 P and 110R, while SO4 had the lowest rooting (figure 2, figure 3, figure 4, and figure 5). SO4 (Berlandieri-Riparia
family), 1103 P (Berlandieri-Rupestris family), and 110R (Berlandieri-Rupestris
family) were expected to have lower rooting capacity than 101-14 MGt (Riparia-Rupestris
family), because they are hybrids of V. berlandieri, an American
difficult-to-root Vitis species (Keller et al., 2020; Riaz et
al., 2019). Cuttings of 1103 P incubated with Pseudomonas 42P4
showed increased rooting compared to the control. Additionally, rooting
percentage was 15% higher than with the synthetic rooting agent IBA, which only
promoted two of the four assessed parameters. However, contrasting reports
exist regarding IBA’s effectiveness in 1103 P (Boeno et al., 2023;
Daskalakis et al., 2018; Gordillo et al., 2022; Satisha et al.,
2008).
Rooting of 110 R, 101-14, and SO4 cuttings inoculated with Pseudomonas showed
no significant improvements compared to the control. Isçi et
al. (2019) evaluated a commercial bacteria consortium on Ramsey rootstock
after nursery forcing, successfully increasing rooting percentage and root DW
compared to IBA. Rootstock response to Pseudomonas 42P4 and IBA
treatments may depend on genetic diversity and the presence of inhibitors (Wilson
and Van Staden, 1990).
Scion-rootstock
union percentage in SO4 cuttings inoculated with Pseudomonas 42P4 was
threefold higher than in water or with IBA (75% vs. 25%). This result aligns
with Köse
et al. (2005), who reported that Pseudomonas BA8 promoted graft union
of the Italia and Beyaz Çavuş scions onto 41B and 5BB rootstocks. Similarly, Toffanin
et al. (2016) evaluated the effect of inoculating nine rootstock cuttings
with Azospirillum brasilense Sp245 and found improved union only in 1103
P grafted onto Sangiovese. Likewise, A. brasilense Sp245 only improved
the union of Colorino grafted onto 420A (Bartolini et al., 2017). Our native Pseudomonas’
failure to improve graft union in three out of the four evaluated
rootstocks could be explained by impaired polar auxin transport. Auxins may not
have moved towards the rootstock-scion union, but rather accumulate at the
cutting’s basal end, probably given its genetic origin. V. riparia and V.
berlandieri develop callus on both extremities, while V. rupestris predominantly
forms it on the upper extremity (Galet, 1993).
Considering the Vitis genus cuttings lack preformed root
primordia, adventitious root formation is a prerequisite for successful cutting
propagation (Hartmann et al., 2014).
Once cuttings are removed from the plant (wounding), a series of wound
responses occur, and de novo adventitious root generation proceeds. De
novo adventitious rooting involves four stages: cell dedifferentiation
(possibly medullary rays in grapevine), cell proliferation, development and
organization of root primordia, and growth of root primordia (Hartmann
et al., 2014). In early rooting stages, high auxin concentrations either
from buds or exogenous sources like IAA are necessary for dedifferentiation and
cell proliferation (Hartmann et al., 2014; Jarvis,
1986). Under our conditions, the native Pseudomonas synthetised
and exuded IAA into the medium where bacteria had grown and cuttings were
incubated. Although we do not know whether the strains can colonise cuttings
endophytically or epiphytically, we believe bacteria may have produced IAA in
the medium, promoting rooting. Currently, IBA and NAA are the most commonly
used auxins in nurseries (Waite et al., 2014).
Synthetic growth regulators often excessively used, can be sufficiently
replaced by organic products for plant rooting, at least for some species and
varieties (Atak et al., 2024).
However, further investigation should consider the mechanisms underlying
bacterium-plant interaction between Pseudomonas 42P4 and Vitis spp.
and long-term effects.
Conclusions
Pseudomonas 42P4, but not Enterobacter 64S1
promoted rooting (rooting percentage, root number and biomass per cutting) of
Malbec cuttings reaching similar values as IBA 1000 ppm. Furthermore, this
strain increased 1103 P rooting (rooting percentage, root number and biomass
per cutting), compared to the water treatment, but. This was not observed in
101-14 MGt, 110 R or SO4. In 1103 P, this strain increased rooting percentage,
exceeding IBA treatment. Pseudomonas 42P4 also increased SO4 graft union
threefold compared to IBA and water. The use of this bacterium, native to arid
soils, enhances rooting parameters of ungrafted and grafted V. vinifera cv.
Malbec cuttings. This capacity of Pseudomonas 42P4 presents a promising
sustainable alternative for improving grapevine production in commercial nurseries.
Acknowledgements
The authors would
like to thank Diana Segura, Micaela Perez-Rodriguez, Martín López, Walter Tulle
and Carlos Blanquer for their assistance with morphological measurements.
This study was
supported through funding from Fondo para la Investigación Científica y
Tecnológica de Argentina (FONCYT, PICT 2020-3618 to ACC); Universidad Nacional
de Cuyo (SIIP-UNCUYO M021-T1 to CVG and ACC), Fundación Williams (to ACC) and
CONICET (PIP-KA1 11220210100195CO to ACC and CVG).
ACC and CVG are CONICET researchers and UNCUYO Professors, MGG
is a PhD fellow from CONICET and FC is a member of Trapiche winery staff.
Atak, A. (2024).
Climate change and adaptive strategies on viticulture (Vitis spp.). Open
Agriculture, 9, 1-16. https://doi.org/10.1515/opag-2022-0258
Bartolini, S.,
Carrozza, G., Scalabrelli, G., & Toffanin, A. (2017). Effectiveness of Azospirillum
brasilense Sp245 on young plants of Vitis vinifera L. Open Life
Sciences, 12, 365-372. https://doi. org/10.1515/biol-2017-0042
Berdugo, M.,
Delgado-Baquerizo, M., Soliveres, S., Hernandez-Clemente, R., Zhao, Y., Gaitan,
JJ, Gross, N., Saiz, H., Maire, V., & Lehmann, A. (2020). Global ecosystem
thresholds driven by aridity. Science, 367, 787-790.
https://doi.org/10.1126/science.aay5958
Boeno, D., &
Zuffellato-Ribas, KC. (2023). A quantitative assessment of factors affecting
the rooting of grapevine rootstocks (Vitis vinifera L.). Acta
Scientiarum Agronomy, 45. https://doi.
org/10.4025/actasciagron.v45i1.57987
Brunoni, F.,
Vielba, JN, & Sanchez, C. (2022). Plant growth regulators in tree rooting. Plants,
11(6), 805. https://doi.org/10.3390/plants11060805
Burrel, AL, Evans,
JP, & De Kauwe, MG. (2020). Anthropogenic climate change has driven over 5
million km2 of drylands
towards desertification. Nature Communications, 11, 3853.
https://doi. org/10.1038/s41467-020-17710-7
Centeno, A., &
Gómez del Campo, M. (2008). Effect of root-promoting products in the
propagation of organic olive (Olea europaea L. cv. Cornicabra). HortScience,
43(7), 2066-2069. https:// doi.org/10.21273/HORTSCI.43.7.2066
Daskalakis, I.,
Biniari, K., Bouza, D., & Stavrakaki, M. (2018). The effect that
indolebutyric acid (IBA) and position of cane segment have on the rooting of
cuttings from grapevine rootstocks and from Cabernet Franc (Vitis vinifera L.)
under conditions of a hydroponic culture system. Scientia Horticulturae,
227, 79-84. https://doi.org/10.1016/j.scienta.2017.09.024
D’Innocenzo, SH,
Escoriaza, G, Diaz, ME. (2024). First report of the causal agent of vine crown
gall in Mendoza, Argentina. Revista de la Facultad de Ciencias Agrarias.
Universidad Nacional de Cuyo. Mendoza. Argentina. 56(2): 87-96. DOI:
https://doi.org/10.48162/rev.39.139
Di Rienzo, JA,
Casanoves, F., Balzarini, MG, Gonzalez, L., Tablada, M., & Robledo, CW.
(2020). InfoStat 2020/P. https://www.infostat.com.ar/
Flexas, J., Galmés,
J., Gallé, A., Gulías, J., Pou, A., Ribas-Carbó, M., Tomas, M., & Medrano,
H. (2010). Improving water use efficiency in grapevines: Potential
physiological targets for biotechnological improvement. Australian Journal
of Grape and Wine Research, 16, 106 121.
https://doi.org/10.1111/j.1755-0238.2009.00057.x
Galet, P. (1993). Précis
de viticulture. Dehan Ed.
Garay-Arroyo, A.,
de La Paz Sánchez, M., García-Ponce, B., Álvarez-Buylla, ER & Gutiérrez, C.
(2014). La homeostasis de las auxinas y su importancia. Revista de Educación
Bioquímica, 33(1), 13-22.
Glick, BR. (2012).
Plant growth-promoting bacteria: Mechanisms and applications. Scientifica,
2012, 963401. https://doi.org/10.6064/2012/963401
Gordillo, MG,
Cohen, AC, Roge, M., Belmonte, M., & González, CV. (2022). Effect of
quick-dip with increasing doses of IBA on rooting of five grapevine rootstocks
grafted with ‘Cabernet Sauvignon’. Vitis, 61(3), 147-152.
http://dx.doi.org/10.5073/vitis.2022.61.147-152
Hartmann, HT,
Kester, DE, Davies, FT, Jr., & Geneve, RL. (2014). Plant propagation:
Principles and practices (8th ed.).
Pearson.
Instituto Nacional
de Vitivinicultura. (2023). Superficie cultivada con viñedos en Argentina.
https:// www.argentina.gob.ar/inv/vinos/estadisticas
Isçi, B., Kacar,
E., & Altindisli, A. (2019). Effects of IBA and plant growth-promoting
rhizobacteria (PGPR) on rooting of ramsey american grapevine rootstock. Applied
Ecology and Environmental Research, 17(2), 4693-4705.
http://dx.doi.org/10.15666/aeer/1702_46934705
Jarvis, BC. (1986). Endogenous control of adventitious rooting
in non-woody species. En M. B. Jackson (Ed.), New root formation in plants
and cuttings (p. 137-162). Martinus Nijhoff Publishers.
https://doi.org/10.1007/978-94-009-4358-2_6
Jofré, MF, Mammana,
S., Pérez-Rodríguez, MM, Silva, MF, Gómez, F., & Cohen, AC. (2024). Native
rhizobacteria improve drought tolerance in tomato plants by increasing endogenous
melatonin levels and photosynthetic efficiency. Scientia Horticulturae, 329,
112984. http://dx.doi.org/10.1016/j.scienta.2024.112984
Keller, M. (2020). The
science of grapevines (3rd ed.).
Elsevier. https://doi.org/10.1016/B978-0-12- 816365-8.00001-4
Köse, C., Güleryüz,
M., Şahin, F., & Demirtaş, İ. (2003). Effects of some plant growth
promoting rhizobacteria (PGPR) on rooting of grapevine rootstocks. Acta
Agrobotanica, 56(1), 47-52. https://doi.org/10.5586/aa.2003.005
Köse, C., Güleryüz,
M., Şahin, F., & Demirtaş, İ. (2005). Effects of some plant growth
promoting rhizobacteria (PGPR) on graft union of grapevine. Journal of
Sustainable Agriculture, 26(2), 139-147.
https://doi.org/10.1300/J064v26n02_10
Lobato Ureche, MA,
Perez-Rodriguez, MM, Ortiz, R., Monasterio, RP, & Cohen, AC. (2021).
Rhizobacteria improve the germination and modify the phenolic compound profile
of pepper (Capsicum annuum L.). Rhizosphere, 18, 100334. https://doi.org/10.1016/j. rhisph.2021.100334
Machado, MP, Mayer,
JLS, Ritter, M., & Biasi, LA. (2005). Indole butyric acid on rooting
ability of semihardwood cutting of grapevine rootstock ‘VR 043-43’ (Vitis
vinifera x Vitis rotundifolia). Revista Brasileira de
Fruticultura, 27(3), 476-479.
https://doi.org/10.1590/S0100-29452005000300032
Mudge, K., Janick,
J., Scofield, S., & Goldschmidt, EE. (2009). A history of grafting. Horticultural
Reviews, 35, 437-483. https://doi.org/10.1002/9780470593776.CH9
OIV (International
Organization of Vine and Wine). (2023). State of the world vine and wine
sector. https://www.oiv.int/node
Ollat, N.,
Bordenave, L., Tandonnet, JP, Boursiquot, JM, & Marguerit, E. (2016).
Grapevine rootstocks: Origins and perspectives. Acta Horticulturae, 1136,
11-22. https://doi.org/10.17660/ ActaHortic.2016.1136.2
Pantoja Guerra, M.,
Valero Valero, N., & Ramírez, CA. (2023). Total auxin level in the
soil-plant system as a modulating factor for the effectiveness of PGPR inocula:
A review. Chemical and Biological Technologies in Agriculture, 10,
6. https://doi.org/10.1186/s40538-022-00370-8
Pérez-Rodríguez,
MM, Piccoli, P., Anzuay, MS., Baraldi, R., Neri, L., Taurian, T., Lobato
Ureche, MA, Segura, DM, & Cohen, AC. (2020a). Native bacteria isolated from
roots and rhizosphere of Solanum lycopersicum L. increase tomato
seedling growth under a reduced fertilization regime. Scientific Reports,
10, 1-14. https://doi.org/10.1038/s41598-020-72507-4
Pérez-Rodríguez,
MM, Pontin, M., Lipinski, V., Botini, R., Piccoli, P., & Cohen, AC.
(2020b). Pseudomonas fluorescens and Azospirillum brasilense increase
yield and fruit quality of tomato under field conditions. Journal of Soil
Science and Plant Nutrition, 20(3), 1614-1624. https://doi.
org/10.1007/s42729-020-00233-x
Pérez-Rodríguez,
MM, Pontin, M., Piccoli, P., Lobato Ureche, MA, Gordillo, MG, Funes Pinter, I.,
& Cohen, AC. (2022). Halotolerant native bacteria Enterobacter 64S1
and Pseudomonas 42P4 alleviate saline stress in tomato plants. Physiologia
Plantarum, 174(1), 13472. https://doi. org/10.1111/ppl.13742
R Core Team.
(2024). R: A language and environment for statistical computing. R
Foundation for Statistical Computing. https://www.R-project.org/
Riaz, S., Pap, D.,
Uretsky, J., Laucou, V., Boursiquot, JM, & Kocsis, L. (2019). Genetic
diversity and parentage analysis of grape rootstocks. Theoretical and
Applied Genetics, 132(6), 1847-1860. https://
doi.org/10.1007/s00122-019-03320-5
Satisha, J., &
Asdule, PG. (2008). Rooting behaviour of grape rootstocks in relation to IBA
concentration and biochemical constituents of mother vines. Acta
Horticulturae, 785, 121-125. https://
doi.org/10.17660/ActaHortic.2008.785.14
Tandonnet, JP,
Cookson, SJ, Vivin, P., & Ollat, N. (2009). Scion genotype controls biomass
allocation and root development in grafted grapevine: Scion/rootstock
interactions in grapevine. Australian Journal of Grape and Wine Research,
16(1), 290-300. https://doi.org/10.1111/ j.1755-0238.2009.00090.x
Toffanin, A.,
D’Onofrio, C., Carroza, GP, & Scalabrelli, G. (2016). Use of beneficial
bacteria Azospirillum brasilense Sp245 on grapevine rootstocks grafted
with ‘Sangiovese’. Acta Horticulturae, 1136, 24.
https://doi.org/10.17660/ActaHortic.2016.1136.24
Waite, H.,
Whitelaw-Weckert, M., & Torley, P. (2014). Grapevine propagation:
Principles and methods for the production of high-quality grapevine planting
material. New Zealand Journal of Crop and Horticultural Science, 43(2),
144-161. https://doi.org/10.1080/01140671.201 4.978340
Wickham, H. (2016).
ggplot2: Elegant graphics for data analysis. Springer-Verlag.
https://ggplot2. tidyverse.org
Wilson, PJ, & Van Staden, J. (1990). Rhizocaline, rooting
co-factors, and the concept of promoters and inhibitors of adventitious rooting
- A review. Annals of Botany, 66(4), 479-490. https://doi.
org/10.1093/oxfordjournals.aob.a088051