Revista de la Facultad de Ciencias
Agrarias. Universidad Nacional de Cuyo. Tomo 57(2). ISSN (en línea) 1853-8665.
Año 2025.
Original article
Guava
Leaf Meal (Psidium guajava L.) in Broiler Diets: Effects on Performance,
Nutrient digestibilty, and Intestinal Morphology
Harina
de hojas de guayaba (Psidium guajava L.) en dietas para pollos de
engorde: efectos sobre el rendimiento, la digestibilidad de los nutrientes y la
morfología intestinal
Juan Carlos Blandon
Martínez1*,
Luz Estella Vásquez
David1,
Hader Iván Castaño
Peláez1,
Luis Fernando
Londoño Franco1,
Camilo Soto Londoño1
1Politécnico Colombiano JIC. Facultad de Ciencias Agrarias. Cra
48 No. 7-151 of P19-111A. C. P. 050022. Medellín. Colombia.
Abstract
This study
investigated the effect of guava leaf meal (GLM) as a phytobiotic in broilers,
focusing on its chemical properties and potential physiological benefits. 135
one-day-old male Cobb broilers were randomly allocated to five treatments (nine
replicates per treatment and three birds per replicate): a basal diet with
regulated commercial antibiotic (T1), without regulated commercial antibiotic
or growth promoters (T2), 1% GLM (T3), 1.5% GLM (T4), and 2% GLM (T5) for 38 d.
T2, T3, T4, and T5 reduced feed intake (FI) during the finishing phase (days
20–38, P < 0.0001), but there were no statistical differences in accumulated
feed intake (AFI) between treatments. GLM groups had lower ADG during the
starter phase (days 3-20, P < 0.05), but there were no statistical
differences in accumulated gain. Accumulated feed conversión rate (FCR) was
better in T2 to T5 compared to T1 (P < 0.05). GLM groups (T3, T4 and T5)
showed significantly higher values of nutrient digestibility (P < 0.05).
Duodenum morphology showed that number of villi (P=0.02) and the villus height
(P= 0.03) increased with GLM supplementation with respect to control groups (T1
and T2). In conclusion, GLM-based diets enhanced nutrient digestibility and
improved intestinal architecture, thereby supporting their inclusion in broiler
chicken diets to optimize production efficiency.
Keywords: Intestinal
architecture, phytobiotics, poultry, plant extracts, additives
Resumen
Se estudió el
efecto de la harina de hojas de guayaba como fitobiótico en pollos de engorde,
centrándose en sus propiedades químicas y sus posibles beneficios fisiológicos.
135 pollos Cobb machos de un día de edad se aleatorizaron en 5 tratamientos (9
réplicas por tratamiento y 3 aves por réplica): una dieta basal con antibiótico
comercial (T1), sin antibiótico comercial ni promotores de crecimiento (T2), 1%
GLM (T3), 1,5% GLM (T4) y 2% GLM (T5) durante 38 días. T2, T3, T4 y T5
redujeron el consumo de alimento (CA) en fase de finalización (día 20-38, P ˂
0,0001), pero en consumo de alimento acumulado (CAA), no hubo diferencias
estadísticas entre tratamientos. Los grupos GLM tuvieron menor ganancia media
diaria (GMD) en fase de inicio (día 3-20, P < 0,05), pero en ganancia
acumulada no hubo diferencias estadísticas. La tasa de conversión alimenticia
acumulada (TCA) fue mejor en los grupos T2 a T5 en comparación con T1 (P <
0,05). Los grupos GLM (T3, T4 y T5) mostraron valores significativamente
mayores de digestibilidad de nutrientes (P < 0,05). La morfología del
duodeno mostró que el número de vellosidades (P = 0,02) y altura de
vellosidades (P = 0,03) aumentaron con la adición de GLM en comparación con los
grupos control (T1 y T2). En conclusión, las dietas adicionadas con GLM
mejoraron la digestibilidad de los nutrientes y la arquitectura intestinal, lo
que justifica su inclusión en las dietas de pollos de engorde, para optimizar
la eficiencia productiva.
Palabras clave: Arquitectura
intestinal, fitobióticos, aves de corral, extractos vegetales, aditivos
Originales: Recepción: 24/03/2025 - Aceptación: 11/08/2025
Introduction
Using technological
or food additives is an effective strategy to enhance animal productivity while
providing a natural approach to reducing production costs. Plant extracts, also
known as phytobiotics or phytogenics, have been used for medicinal purposes since
ancient times and are widely employed in traditional and alternative veterinary
medicine (6). In recent years, the use of herbal
medicines and plant-based extracts in livestock production has gained
popularity, driven by concerns over the side effects of conventional drugs,
high input costs, toxic residues in feed, microbial resistance, and the growing
demand for organic and sustainable livestock production systems (16,
20). For this reason, plant-based additives have been widely
investigated as alternatives to antibiotics and growth promoters for use in
animal health and production, since they perform multiple beneficial functions
in the gastrointestinal tract and are less likely to induce the development of
microbial resistance (34).
Guava leaf,
characterized by its unique chemical composition (28), has been
demonstrated to exert various physiological effects in humans and animals. It
serves as an antimicrobial agent (8, 10), provides
health-promoting bioactive compounds (15, 19), offers protective
effects on the gastrointestinal tract (33), and exhibits
potential nutraceutical benefits (13), among other
advantages.
Adding guava leaf
extracts has been shown to enhance productive performance and reduce the
incidence of diarrhea in weaned piglets (33). In broiler
chickens, incorporating guava fruit by-products during the starter phase (7-21
days) improved productive performance and meat quality, with a linear increase
in daily weight gain (DWG) as the by-product inclusion increased. Although no
changes were observed in villus height or crypt depth, the villus
height-to-crypt depth ratio increased with higher levels of the by-product (23). Similarly, diets
containing guava leaf meal combined with olive oil in chickens led to improved
weight gain, better feed conversion rates, reduced fat content in breast and
thigh muscles, and lower total blood lipid levels (22).
The
gastrointestinal tract plays a critical role in digestion and nutrient
absorption, essential for proper animal growth and development, ultimately
enhancing productive performance. Specifically, the morphology and histological
structure of the small intestine, particularly the duodenum, are vital. The
duodenal mucosa with its villi and microvilli, facilitates efficient nutrient
assimilation. These structures significantly amplify nutrient absorption,
increasing it by approximately 10 and 20 times, respectively. Moreover, the
quantity and length of villi and microvilli can further expand the intestinal
mucosa’s surface area, enhancing absorption capacity (11,
14, 31).
Research indicates
that guava leaf meal and its extracts, when included in animal diets,
positively influence intestinal morphology, immune response, and productive
parameters. Specifically, these supplements enhance intestinal morphology,
improving nutrient absorption (12, 16, 31). They also
strengthen immune response, reducing microbial load and supporting better
physiological outcomes (8, 10, 13, 18). Additionally,
they contribute to improved productive parameters, such as growth and feed efficiency
(1,
2). However, no prior studies have specifically investigated these
effects in chickens, particularly regarding nutrient digestibility. Therefore,
this study aims to evaluate the impact of adding guava leaf meal to chicken
diets on intestinal morphology, nutrient digestibility, and productive
parameters.
Materials
and methods
The trial was
conducted at the Experimental Farm of the Facultad de Ciencias Agrarias de la
Universidad Politécnico Colombiano JIC (Jaime Isaza Cadavid). The experimental
procedures in this trial were approved by the Ethical Committee Animal Care and
Use of the University under filed protocol number 20610801-202301004049.
Experimental
Design and Diets
A total of 135 male
day-old broiler chickens (Avian Male Cobb) were purchased from a local
authorized distribution company. The broiler chickens were randomly assigned to
five groups in a completely randomized design. Each group consisted of nine
replicate cages, with three birds housed per cage in an appropriate housing
facility. The five dietary treatments consisted of a basal diet with a
regulated commercial antibiotic (Zinc bacitracin 15%, 500 g/t and Halquinol 60,
100g/t) (T1), a basal diet without antibiotics or growth promoters (T2), and
diets added with1% (T3), 1.5% (T4), or 2% (T5) guava leaf meal (GLM) in both
starter (days 3-20) and finisher (days 21-38) phases. The basal diet, a
corn-soybean meal-based commercial crumble feed, was formulated to meet broiler
nutritional requirements per NRC (1994) and purchased from
a recognized local feed company. Diets were isoproteic and isoenergetic, with
their chemical composition analyzed following AOAC (2005) methods, as
presented in table
1.
Table
1. Composition and nutritional value of
basal diets for starter and finisher periods (g.kg-1).
Tabla
1.
Composición y valor nutritivo de las dietas basales para periodo iniciador y
terminador (g.kg-1).
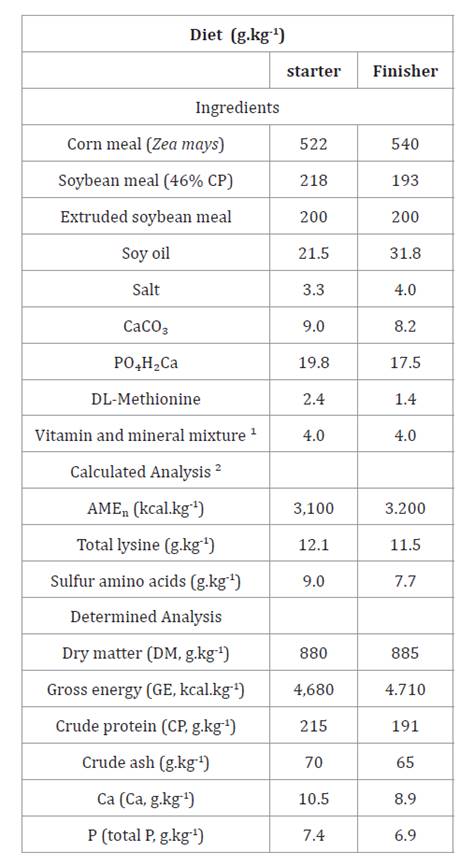
1Provided the following per
kilogram of complete diet: vitamin A, 12,000 IU; vitamin D3, 2,400 IU; vitamin E, 30 mg; vitamin K3,
3 mg; vitamin B1,
2.2 mg; vitamin B2,
8 mg; vitamin B6,
5 mg; vitamin B12,
11 mg; folic acid, 1.5 mg; biotin, 150 mg; calcium pantothenate, 25 mg;
nicotinic acid, 65 mg; Mn, 60 mg; Zn, 40 mg; I, 0.33 mg; Fe, 80 mg; Cu, 8 mg;
Se, 0.15 mg; ethoxyquin, 150 mg. 2FEDNA (2003).
1Se proporcionó lo siguiente por kilogramo de dieta completa:
vitamina A, 12.000 UI; vitamina D3, 2.400 UI; vitamina E, 30 mg;
vitamina K3, 3 mg; vitamina B1, 2,2 mg; vitamina B2,
8 mg; vitamina B6, 5 mg; vitamina B12, 11 mg; ácido
fólico, 1,5 mg; biotina, 150 mg; pantotenato de calcio, 25 mg; ácido nicotínico,
65 mg; Mn, 60 mg; Zn, 40 mg; I, 0,33 mg; Fe, 80 mg; Cu, 8 mg; Se, 0,15 mg;
etoxiquina, 150 mg. 2FEDNA
(2003).
Broiler chickens
were fed a basal diet during the first 2 days of acclimatization. The groups
were provided a basal diet, either added with guava leaf meal (GLM) or unadded
based on the treatment. The birds had ad libitum access to feed and water
throughout the experimental period. Lighting was 24 hours per day during the
first week and 16 hours of light and 8 hours of darkness in subsequent weeks.
All birds were subjected to consistent environmental and management conditions.
GLM
Collection
To prepare guava
leaf meal (GLM), leaves were collected from the northern region of Antioquia in
the Colombian tropics, located at 1,100 meters above sea level with an average
temperature of 26°C. The leaves were cleaned, dried in a forced-air oven, and ground
into a fine powder using a laboratory mill for incorporation into experimental
diets. The composition of GLM is presented in table 2.
Table 2.
Chemical Composition of Guava Leaf Meal (GLM) (g.100-1) on a Dry Matter Basis.
Tabla
2. Composición química (g.100-1) de la harina de hoja de guayaba en
base a materia seca.
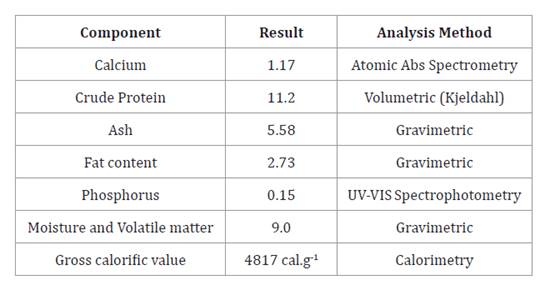
Results
are expressed on a dry matter basis. Crude protein content was calculated using
a conversión factor of 6.25. Results apply only to the analyzed simple (Sample
Code: 78376, Request: 12247).
Los
resultados se expresan en materia seca. El contenido de proteína cruda se
calculó utilizando un factor de conversión de 6,25. Los resultados corresponden
únicamente a la muestra analizada (Código de muestra: 78376, Solicitud: 12247).
Growth
Performance
Initial body weight (BWi) was determined at the beginning of the
experiment. Body weight was recorded weekly for each cage and subsequently
analyzed by period (starter: days 3-20; finisher: days 21-38). In addition, the
feed offered and the feed refused were weighed daily. The recorded data was
used to calculate average daily gain (ADG, g.chick-1),
feed intake (FI, g.chick-1),
feed conversion ratio (FCR, FI.WG-1)
and weight gain (WG, g.chick-1)
for the starter, finisher and total periods.
Digestibility
To assess nutrient
digestibility, feed intake was recorded, and total excreta were collected on
days 34 and 35. Excreta from each experimental diet were quantitatively
collected daily from five cages per treatment. Excreta samples were
homogenized, and subsamples were taken per cage. Both feed and excreta samples
were dried in a forced-air oven at 103°C to constant weight to determine dry
matter (DM) content, following AOAC method 925.09 (4). Ash content was
determined by incinerating the samples in a muffle furnace at 525°C for 7
hours, according to AOAC method 923.03 (4). Organic matter
(OM) was calculated as:
OM (%) = 100 - %
Ash
Crude protein (CP)
was determined using the Kjeldahl method, multiplying nitrogen content by 6.25,
as per AOAC method 984.13 (4). Calcium (Ca) and
phosphorus (P) were analyzed by atomic absorption spectrophotometry for Ca
(AOAC method 927.02) and colorimetry for P (AOAC method 965.17) (4). Apparent
digestibility for each nutrient (dry matter, organic matter, crude protein,
calcium, and phosphorus) was calculated using the formula:
Experimental
Sampling and Intestinal Morphology
On day 38, six
birds per treatment were randomly selected from two replicate cages (three
birds per cage) to ensure representative sampling. The birds were transported
to the slaughterhouse and euthanized by cervical dislocation. Slaughter weight
was recorded for each bird. A 1 cm segment of the medial duodenum was collected
from each bird and immediately fixed in 10% neutral buffered formalin for
preservation. Tissue samples were processed using a rotary microtome to obtain
5 μm sections, stained with hematoxylin and eosin (H&E) following standard
histological procedures.
The intestinal
mucosa was examined under a light microscope with a Moticam® digital camera at
4× and 10× magnifications. Morphometric analysis focused on the duodenal villi
and crypts. Villus height was measured in microns from the basal edge (at the
junction with the crypt) to the apical edge. Crypt depth was determined by
measuring the distance from the base of the crypt to the villus-crypt junction.
The number of villi per visual field (villi/visual field ratio) was quantified
by counting the total number of intact villi within a standardized field of
view at 4× magnification, ensuring consistent measurements across samples. All
measurements were performed using calibrated image analysis software coupled
with the Moticam® system.
Model
and Statistical Analysis
The experiment was
conducted using a completely randomized design with nine replicates per
treatment. For performance parameters, the experimental unit was defined as a
cage containing three birds, while for intestinal morphology measurements, six
birds per treatment were sampled from two replicate cages (three birds per
cage). The statistical model used for analysis was:
Yij = μ + Ti + eij
where
Yij = the observed
dependent variable
μ = the overall
mean
Ti i = the fixed
effect of the ith treatment
eij = the random error
term.
Data were analyzed using the General Linear Model (GLM)
procedure (PROC GLM) in SAS (version 9.4) (2017).
Differences among treatment means were evaluated using analysis of variance
(ANOVA), followed by Tukey test for comparisons when significant effects were
detected (P < 0.05).
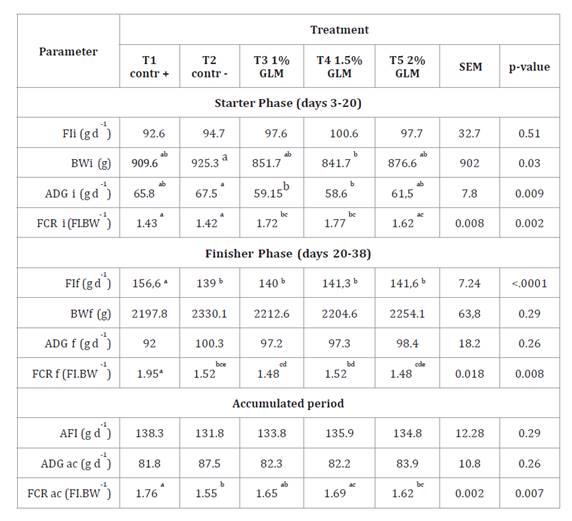
Results
Growth
performance
All broiler chickens fed with the experimental diets remained
healthy throughout the study, with no observed adverse symptoms or signs of
disease. Table
3 summarizes the growth performance parameters across starter
(days 3-20), finisher (days 20-38), and accumulated phases for broiler chickens
fed diets supplemented with guava leaf meal (GLM). Feed intake (FI) in the
finisher phase was significantly reduced in treatments T2 (negative control),
T3 (1% GLM), T4 (1.5% GLM), and T5 (2% GLM) compared to T1 (positive control
with commercial antibiotic) (P < 0.0001). However, accumulated feed intake
(AFI) showed no significant differences across treatments (P = 0.29). Average
daily gain (ADG) in the starter phase was lower in T3 and T4 than T2 (P =
0.009), with T1 and T5 showing intermediate values. In contrast, no significant
differences were observed in ADG during the finisher phase (P = 0.26) or
accumulated ADG (P = 0.26). The accumulated feed conversion ratio (FCR) was
significantly improved in T2, T3, T4, and T5 compared to T1 (P =0.007), with T2
and T5 exhibiting the lowest FCR values (1.55 and 1.62, respectively).
Table 3.
Effect of additing guava leaf meal (GLM) in the diet of broiler chickens on the
growth performance during starter, finisher and accumulated phases.
Tabla 3.
Efecto de la adición de harina de hoja de guayaba (GLM) en la dieta de pollos
de engorde sobre parámetros productivos en las fases de crecimiento,
finalización y acumulado.
Nutrient
Digestibility
Table 4 shows apparent
whole-tract digestibility of nutrients. Supplementation with GLM, particularly
at 1.5% (T4) and 2% (T5), significantly enhanced crude protein (CP)
digestibility compared to T1, T2, and T3 (P = 0.0002). Specifically, T4 and T5
achieved CP digestibility values of 0.52 and 0.45, respectively, compared to
0.32 (T1), 0.30 (T2), and 0.35 (T3), indicating that 1% GLM (T3) did not
improve CP digestibility relative to the control groups. Organic matter (OM)
digestibility was significantly higher in T4 (0.64) compared to T2 (0.52) (P =
0.045), with T1, T3, and T5 showing intermediate values. Dry matter (DM)
digestibility was also improved in T4 (0.58) compared to T1, T2, and T3 (P =
0.003), and was statistically similar to T5 (0.52). Calcium (Ca) digestibility
was highest in T3 (0.68) (P < 0.0001), followed by T2 and T4, with T1 and T5
exhibiting the lowest values. Phosphorus (P) digestibility was significantly
enhanced with increasing GLM supplementation, peaking in T5 (0.56), followed by
T4 (0.40) and T3 (0.37) (P < 0.0001), compared to T1 (0.19) and T2 (0.23).
Table 4. Effect
of adding guava leaf meal (GLM) in the diet of broiler chickens on nutrient
digestibility.
Tabla 4.
Efecto de la adición de harina de hoja de guayaba (GLM) en la dieta de pollos
de engorde sobre la digestibilidad de los nutrientes.
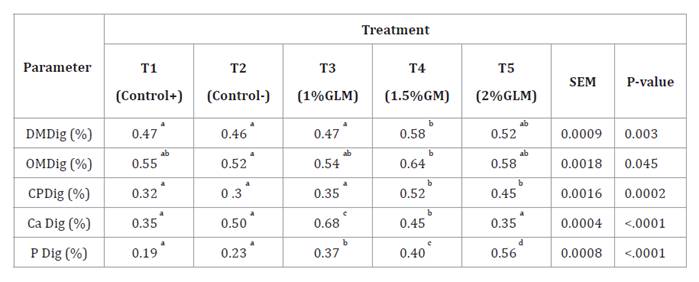
Digestibility
results are presented as means of five replicates per treatment. In the same
row, values with no superscript or the same superscript indicate no significant
difference (P > 0.05), while different superscripts indicate significant
differences (P < 0.05). DM Dig: Dry Matter Digestibility; OM Dig: Organic
Matter Digestibility; CP Dig: Crude Protein Digestibility; Ca Dig: Calcium
Digestibility; P Dig: Phosphorus Digestibility. T1: Positive control with
commercial antibiotic; T2: Negative control without antibiotic or growth
promoters; T3: 1% GLM; T4: 1.5% GLM; T5: 2% GLM.
Los resultados
de digestibilidad se presentan como medias de cinco réplicas por tratamiento.
En la misma fila, los valores sin superíndice o con el mismo superíndice
indican que no hay diferencia significativa (P > 0,05), mientras que
diferentes superíndices indican diferencias significativas (P < 0,05). Dig.
MS: Digestibilidad de la materia seca; Dig. MO: Digestibilidad de la materia
orgánica; Dig. PC: Digestibilidad de la proteína cruda; Dig. Ca: Digestibilidad
del calcio; Dig. P: Digestibilidad del fósforo. T1: Control positivo con
antibiótico comercial; T2: Control negativo sin antibiótico ni promotores de
crecimiento; T3: 1 % GLM; T4: 1,5 % GLM; T5: 2% GLM.
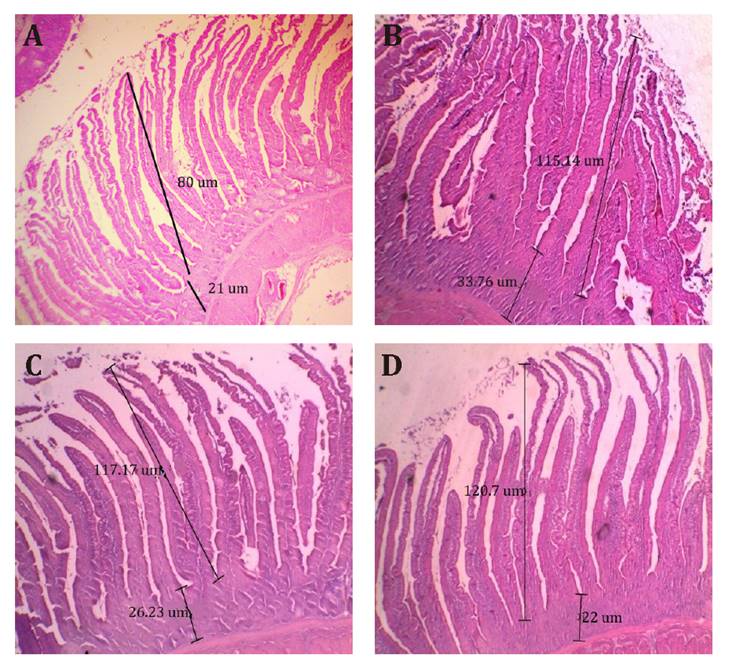
Duodenum
Morphology
The effects of GLM
inclusion on the duodenum morphology are detailed in table 5 and illustrated in
figure
1.
The number of villi per visual field (Villi/Vis field) was significantly higher
in T5 (27 villi) compared to T2 (16.3 villi) (P = 0.02), with T1, T3, and T4
showing intermediate values. Villus height was significantly increased in T3,
T4, and T5 (115.1 - 120.7 ţm) compared to T2 (80 ţm) (P = 0.031), and was
comparable to T1 (116.8 ţm). Crypt depth did not differ significantly among
treatments (P = 0.22). Figure
1
illustrates the morphological differences in the small intestine, with T2 (negative
control, figure
1A)
exhibiting the shortest villi and T4 and T5 (figures 1C and 1D) showing enhanced
villus height, supporting improved nutrient absorption capacity. These findings
suggest that GLM supplementation, particularly at 1.5% and 2%, enhances
intestinal morphology, potentially contributing to improved nutrient
digestibility.
Table 5.
Effect of adding guava leaf meal (GLM) in the diet of broiler chickens on
duodenum morphology.
Tabla 5.
Efecto de la adición de harina de hoja de guayaba (GLM) en la dieta de pollos
de engorde sobre la morfología del duodeno.
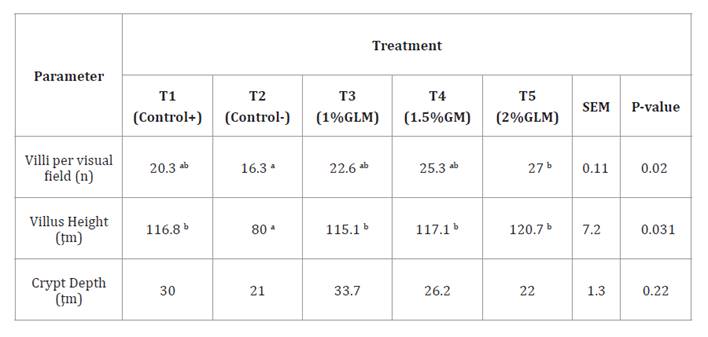
Duodenum
morphology results are presented as means of two replicates (six birds per
treatment). In the same row, values with no superscript or the same superscript
indicate no significant difference (P > 0.05), while different superscripts
indicate significant differences (P < 0.05). T1: Positive control with
commercial antibiotic; T2: Negative control without antibiotic or growth
promoters; T3: 1% GLM; T4: 1.5% GLM; T5: 2% GLM.
Los resultados
de la morfología del duodeno se presentan como medias de dos réplicas (seis
aves por tratamiento). En la misma fila, los valores sin superíndice o con el
mismo superíndice indican que no hay diferencia significativa (P > 0,05),
mientras que los superíndices diferentes indican diferencias significativas (P
< 0,05). T1: Control positivo con antibiótico comercial; T2: Control
negativo sin antibiótico ni promotores de crecimiento; T3: 1% GLM; T4: 1,5%
GLM; T5: 2% GLM.
Images
were obtained by hematoxylin and eosin staining and observed under 40x
magnification. A: Negative control (basal diet without GLM or commercial
antibiotic). B: T3 (diet with 1% GLM). C: T4 (diet with 1.5% GLM). D: T5 (diet
with 2% GLM). The scale represents 200 μm. Long lines indicate villi height,
while short lines indicate crypt depth. Average villi height and crypt depth
values are shown in table 5.
Las imágenes se
obtuvieron mediante tinción con hematoxilina y eosina y se observaron con un
aumento de 40x. A: Control negativo (dieta basal sin GLM ni antibiótico
comercial). B: T3 (dieta con 1% de GLM). C: T4 (dieta con 1,5% de GLM). D: T5
(dieta con 2% de GLM). La escala representa 200 μm. Las líneas largas indican
la altura de las vellosidades, mientras que las cortas indican la profundidad
de las criptas. Los valores promedio de la altura de las vellosidades y la
profundidad de las criptas se muestran en la tabla
5.
Figure
1. Small intestine (medial duodenum) morphology in broiler
supplemented with guava leaf meal (GLM).
Figura 1. Morfología del intestino delgado (duodeno medial) en pollos
de engorde suplementados con harina de hoja de guayaba (GLM).
Discussion
In the starter
phase, feed intake (FIi) showed no significant differences across treatments
(P=0.51), indicating that guava leaf meal (GLM) inclusion at 1% (T3), 1.5%
(T4), and 2% (T5) did not affect early feed consumption compared to the
positive (T1) and negative (T2) controls. However, body weight (BWi) and
average daily gain (ADGi) varied significantly (P=0.03 and P=0.009,
respectively). The negative control (T2) exhibited the highest ADGi (67.5 g),
followed by T1 (65.8 g), while T3 and T4 showed lower values (59.15 g and 58.6
g, respectively), suggesting that GLM at 1% and 1.5% may not enhance early
growth compared to T2. In contrast, T5 (2% GLM) displayed an intermediate ADGi
(61.5 g), indicating a potential dose-dependent effect (2,
3, 26). Feed conversion ratio (FCRi) was significantly less efficient
in T3 and T4 (1.72 and 1.77, respectively) compared to T1 and T2 (1.43 and
1.42, P=0.002). This suggests that lower GLM doses may reduce feed efficiency
in the starter phase, possibly due to palatability issues or mild
antinutritional effects (26).
In the finisher
phase, feed intake (FIf) was significantly higher in T1 (156.6 g/d) compared to
T2, T3, T4, and T5 (139-141.6 g/d, P<0.0001), indicating that GLM inclusion
reduced feed consumption. Despite this, final body weight (BWf) and average
daily gain (ADGf) showed no significant differences across treatments (P=0.29
and P=0.26, respectively), suggesting that GLM maintained overall growth
despite lower feed intake. Notably, FCR in the finisher phase (FCRf) improved
significantly in T3, T4, and T5 (1.48-1.52) compared to T1 (1.95, P=0.008),
with T2 showing an intermediate value (1.52). These results align with Langerudi
et al. (2022), who reported improved FCR with guava leaf essential oil (5
mg/kg), and suggest that GLM enhances feed efficiency in later growth stages,
likely due to improved nutrient utilization (1,
30).
Over the entire
growth cycle, accumulated feed intake (AFI) and accumulated average daily gain
(ADGac) showed no significant differences (P=0.29 and P=0.26, respectively),
indicating that GLM did not affect overall feed consumption or weight gain.
However, accumulated FCR (FCRac) was significantly better in T2, T3, T4, and T5
(1.55-1.69) compared to T1 (1.76, P=0.007), reinforcing the role of GLM in
improving feed efficiency without compromising final body weight. These findings
differ from Mahmoud
et al. (2013), who reported significant improvements in body weight, daily
gain, and FCR with 1% dried guava leaves, and Adeyemi et al. (2022), who noted
differences in cumulative feed intake with 0.25-0.5% GLM. These discrepancies
may arise from variations in GLM dosage, bird genetics, or environmental
conditions (8, 27).
The GLM doses
(1-2%) used in this study, compared to lower doses (0.25-0.5%) in Adeyemi
et al. (2022), may explain the lack of consistent growth performance
improvements. High GLM doses may introduce antinutritional factors, such as
tannins or phenolic compounds, which can bind proteins and minerals, reducing
bioavailability and negatively impacting early growth (5,
20). For example, tannins form complexes with dietary proteins,
impairing digestion and absorption (20). The numerically
higher FIi in T3 and T4 (97.6 and 100.6 g/d, respectively) compared to T1 (92.6
g/d) and T2 (94.7 g/d) may reflect reduced palatability or mild antinutritional
effects. However, T5 (2% GLM) showed comparable FIi (97.7 g/d), suggesting that
higher doses may not exacerbate these effects, possibly due to adaptive
responses in gut microbiota or enzyme activity (24,
25). Excessive phenolic compounds at higher doses could also
inhibit digestive enzymes or disrupt gut microbiota balance, as noted in
studies on phytogenic additives (26).
Nutrient
digestibility was significantly enhanced by GLM inclusion. Dry matter
digestibility (DM Dig) was highest in T4 (0.58) compared to T1, T2, and T3
(0.46-0.47, P=0.003), with T5 (0.52) showing an intermediate value. Organic
matter digestibility (OM Dig) followed a similar trend, with T4 (0.64)
outperforming T2 (0.52, P=0.045). Crude protein digestibility (CP Dig) was
markedly improved in T4 and T5 (0.52 and 0.45, respectively) compared to T1,
T2, and T3 (0.30-0.35, P=0.0002). Calcium digestibility (Ca Dig) was highest in
T3 (0.68) and significantly lower in T1 and T5 (0.35, P<0.0001), while
phosphorus digestibility (P Dig) showed a dose-dependent increase, with T5
(0.56) outperforming all other treatments (0.19-0.40, P<0.0001). These
improvements likely stem from the bioactive compounds of GLM, such as
polyphenols, flavonoids, and essential oils, which stimulate digestive enzyme
secretion, enhance bile acid synthesis, and modulate gut microbiota (25,
32, 35, 36). For instance, flavonoids promote villus development,
increasing absorptive surface area, as evidenced by increased villi height in
T3, T4, and T5 (115.1-120.7 μm) compared to T2 (80 μm, P=0.031). The
dose-dependent increase in P Dig suggests that higher GLM levels (2%) enhance
phosphorus absorption, possibly through improved phytase activity or reduced
antinutritional interference (3). The variability
in Ca Dig, with T3 showing the highest value, may reflect complex interactions
between GLM bioactives and mineral metabolism, warranting further investigation
(35).
Improved nutrient digestibility in GLM-supplemented groups
contributed to enhanced FCR in the finisher and accumulated phases (P=0.008 and
P=0.007, respectively), despite no significant differences in final body
weight. This suggests that GLM enables broilers to achieve comparable growth
with reduced feed intake, potentially lowering production costs (1,
30). GLM supplementation also improved intestinal morphology, with
T5 showing higher villi counts (27 villi/visual field) and villi heights
(115.1-120.7 μm) compared to T2 (16.3 villi/visual field and 80 μm, P=0.02 and
P=0.031, respectively). Notably, T5 (2% GLM) achieved villi height and count
comparable to or numerically surpassing T1 (positive control with antibiotics),
suggesting that GLM can replicate the beneficial effects of antibiotics on gut
health (1, 7, 18).
Similar crypt depth in T5 (22 μm) and T2 (21 μm) indicates that GLM maintains
mucosal integrity. These findings are consistent with what was reported by Wang
et al. (2024), on the effect of GLM on intestinal structure.
Conclusions
GLM inclusion at 1-2% did not significantly enhance growth
performance but significantly improved nutrient digestibility and feed
efficiency, particularly in the finisher and accumulated phases. The
dose-dependent effects on digestibility and gut morphology suggest that GLM’s
bioactive compounds enhance nutrient absorption and maintain intestinal
integrity. These findings support further research to identify key bioactive
compounds, evaluate interactions with dietary components, and determine optimal
inclusion levels. Additionally, GLM’s ability to replicate antibiotic effects
on gut health positions it as a promising alternative to synthetic growth
promoters, reducing reliance on antibiotics.
1.
Abang, F. B.; Echeonwu, I. E.; Amu, M. U. 2023. Effect of graded levels of
guava (Psidium guajava L.) leaf meal on productive performance and meat
organoleptic properties of chicken. Online Journal of Animal and Feed Research.
13(1): 73-78.
2.
Adeyemi, K. D., Agboola, K.; Quadri R. O.; Kelani, A. M.; Ahmed El‐Imam, A. M.; Ishola, H. 2022. Influence
of Dietary Supplementation of Guava Leaf, Oxytetracycline, and Tert‐Butylhydroxytoluene on Growth
Performance, Gut Microbial Population, Immune Status, Carcass, and Meat Quality
in Broiler Chickens. Iranian Journal of Applied Animal Science. 12(2): 329-339.
3.
Amad, A. A.; Männer, K.; Wendler, K. R.; Neumann, K.; Zentek, J. 2011. Effects
of a phytogenic feed additive on growth performance and ileal nutrient
digestibility in broiler chickens. Poult Sci. 90(12): 2811-6. doi: 10.3382/ps.2011-01515
4.
Association of Official Analytical Chemists (AOAC). 2005. Official Methods of
Analysis of AOAC International.
5.
Buyse, K.; Delezie, E.; Goethals, L.; Van Noten, N.; Ducatelle, R.; Janssens,
G. P. J.; Lourenço, M. 2021. Chestnut tannins in broiler diets: performance,
nutrient digestibility, and meat quality. Poult Sci. 100(12):101479. doi: 10.1016/j.psj.2021.101479
6.
Chechani, B.; Roat, P.; Hada, S.; Yadav, D. K.; Kumari, N. 2024. Psidium
guajava: An Insight into Ethnomedicinal Uses, Phytochemistry, and
Pharmacology. Comb Chem High Throughput Screen. 27(1):2-39. doi:
10.2174/1386207326666230426093315
7.
Diaz-Sanchez, S.; D’Souza, D.; Biswas, D.; Hanning, I. 2015. Botanical
alternatives to antibiotics for use in organic poultry production. Poult Sci.
94(6): 1419-30. doi: 10.3382/ps/pev014.
8.
Dos Santos, A.; Da Silva, A. S.; Galli, G. M.; Paglia, E. B.; Dacoreggio, M.
V.; Kempka, A. P.; Souza, C. F.; Baldissera, M. D.; Rosa, G.; Boiago, M. M.;
Paiano, D. 2020. Addition of yellow strawberry guava leaf extract in the diet
of laying hens had antimicrobial and antioxidant effect capable of improving
egg quality. Biocatalysis and Agricultural Biotechnology. 29: 101788.
https://doi.org/10.1016/j.bcab.2020.101788
9.
FEDNA. 2003. Tablas FEDNA de composición y valor nutritivo de alimentos para la
fabricación de piensos compuestos. 2° ed. Fundación Española para el Desarrollo
de la Nutrición Animal. Madrid. España.
10.
Geidam, Y. A.; Ambali, A. G.; Onyeyili, P. A.; Tijjani, M. B.; Gambo, H. I.;
Gulani, I. A. 2015. Antibacterial efficacy of ethyl acetate fraction of Psidium
guajava leaf aqueous extract on experimental Escherichia coli (O78)
infection in chickens. Veterinary World. 8(3): 358-362. doi:
10.14202/vetworld.2015.358-362
11.
Geneser, F. 1997. Histología. 2° Ed. Editorial Médica Panamericana S. A. 768 p.
12.
Gheisar, M. M.; Kim, I. H. 2018. Phytobiotics in poultry and swine nutrition -
a review. Italian Journal of Animal Science. 17(1): 92-99. DOI:
10.1080/1828051X.2017.1350120
13.
Gupta, M.; Wali, A.; Anjali, Gupta, S.; Annepu, S. K. 2018. Nutraceutical
Potential of Guava. In: Mérillon, J. M.; Ramawat, K. (eds) Bioactive Molecules
in Food. Reference Series in Phytochemistry. Springer. Cham.
https://doi.org/10.1007/978-3-319-54528-8_85-1
14.
Jiang, S.; Mohammed, A. A.; Jacobs, J. A.; Cramer, T. A.; Cheng, H. W. 2020.
Effect of synbiotics on thyroid hormones, intestinal histomorphology, and heat
shock protein 70 expression in broiler chickens reared under cyclic heat stress.
Poultry Science. 99(1): 142-150. https:// doi.org/10.3382/ps/pez571
15.
Kumar, M.; Tomar, M.; Amarowicz, R.; Saurabh, V.; Nair, M. S.; Maheshwari, C.;
Sasi, M.; Prajapati, U.; Hasan, M.; Singh, S.; Changan, S.; Prajapat, R. K.;
Berwal, M. K.; Satankar, V. 2021. Guava (Psidium guajava L.) Leaves:
Nutritional Composition, Phytochemical Profile, and Health- Promoting
Bioactivities. Foods. 10(4): 752. doi:
10.3390/foods10040752
16.
Kuralkar, P.; Kuralkar, S. V. 2021. Role of herbal products in animal production
- An updated review. Journal of Ethnopharmacology. 278:114246. doi:
10.1016/j.jep.2021.114246
17. Langerudi, M. T.; Youssefi, M. R.;
Tabari, M. A. 2022. Ameliorative effect of Psidium guajava essential oil
supplemented feed on chicken experimental coccidiosis. Tropical Animal Health
and Production. 54(2): 120. doi:
10.1007/s11250-022-03117-7
18.
Liu, M.; Zhou, J.; Li, Y.; Ding, Y.; Lian, J.; Dong, Q.; Qu, Q.; Lv, W.; Guo,
S. 2023. Effects of dietary polyherbal mixtures on growth performance, antioxidant
capacity, immune function and jejunal health of yellow-feathered broilers.
Poultry Science. 102: 102714.
19.
Luo, Y.; Peng, B.; Wei, W.; Tian, X.; Wu, Z. 2019. Antioxidant and
Anti-Diabetic Activities of Polysaccharides from Guava Leaves. Molecules.
24(7): 1343. doi: 10.3390/ molecules24071343
20.
Mahfuz, S.; Shang, Q.; Piao, X. 2021. Phenolic compounds as natural feed
additives in poultry and swine diets: a review. J Animal Sci Biotechnol. 12:
48. https://doi.org/10.1186/s40104- 021-00565-3
21.
Mahmoud, R. El-Sayed, Ibrahim, Doaa, Badawi, M. El-Sayed. 2013. Effect of
supplementation of broiler diets with Guava Leaves and/or Olive Oil on growth,
meat composition, blood metabolites and immune response. Benha Vet. Med. J.
25(2): 23‐32.
22.
National Research Council. 1994. Nutrient Requirements of Poultry. The National
Academies Press. https://doi.org/10.17226/2114
23.
Oliveira, M.; Mello, H.; Mascarenhas, A.; Arnhold, E.; Conceição, E.; Martins,
J.; Junior, A. 2018. Antioxidant effect of the guava by product in the diet of
broilers in the starter phase. Revista Brasileira de Zootecnia. 47.
10.1590/rbz4720160290
24.
Parra Ferrín, D.; Cusme Lucas, G.; Talledo Solórzano, V.; Loor Gorozabel, B.;
Pazmiño Castro, A.; Cuenca-Nevárez, G. J. 2023. Efficacy of zinc lactate and Lactobacillus
bulgaricus on nutrition and health of broiler chickens. Revista de la
Facultad de Ciencias Agrarias. Universidad Nacional de Cuyo. Mendoza.
Argentina. 55(2): 120-128. DOI: https://doi.org/10.48162/ rev.39.114
25.
Pliego, A. B.; Tavakoli, M.; Khusro, A.; Seidavi, A.; Elghandour, M. M.; Salem,
A. Z.; Márquez-Molina, O.; Rene Rivas Caceres, R. 2020. Beneficial and adverse
effects of medicinal plants as feed supplements in poultry nutrition: A review.
Animal Biotechnology. 33(2): 369-391.
26.
Rafeeq, M.; Bilal, R. M.; Alagawany, M.; Batool, F.; Yameen, K.; Farag, M. R.,
Ali, S.; Elnesr, S. & El-Shall, N. A. 2022. The use of some herbal plants
as effective alternatives to antibiotic growth enhancers in poultry nutrition,
World’s Poultry Science Journal. 78(4): 1067-1085. DOI:
10.1080/00439339.2022.2108362
27.
Rahman, Z.; Siddique, M. N.; Khatun, M. A.; Kamruzzamen, M. 2013. Effect of
guava (Psidium guajava) leaf meal on production performance and
antimicrobial sensitivity in commercial broiler. Journal of Natural Products.
6: 177-187.
28.
Ryu, B.; Cho, H. M.; Zhang, M.; Lee, B. W.; Doan, T. P.; Park, E. J.; Lee, H.
J.; Oh, W. K. 2021. Meroterpenoids from the leaves of Psidium guajava (guava)
cultivated in Korea using MS/MS-based molecular networking. Phytochemistry.
186:112723. doi: 10.1016/j. phytochem.2021.112723
29.
SAS® Institute Inc. 2017. Statistical Analysis Systems Institute. SAS/STAT
User’s Guide. Version 14. 3rd ed. Cary, NC: Autor.
30.
Singh, J.; Gaikwad, D. S. 2020. Phytogenic Feed Additives in Animal Nutrition.
In: Singh, J., Yadav, A. (eds) Natural Bioactive Products in Sustainable
Agriculture. Springer. Singapore. https:// doi.org/10.1007/978-981-15-3024-113
31.
Song, J.; Xiao, K.; Ke, Y. L.; Jiao, L. F.; Hu, C. H.; Diao, Q. Y.; Shi, B.;
Zou, X. T. 2014. Effect of a probiotic mixture on intestinal microflora,
morphology, and barrier integrity of broilers subjected to heat stress. Poultry
Science. 93: 581-588.
32.
Sugiharto, S.; Ranjitkar, S. 2019. Recent advances in fermented feeds towards
improved broiler chicken performance, gastrointestinal tract microecology and
immune responses: A review. Animal Nutrition. 5(1): 1-10.
https://doi.org/10.1016/j.aninu.2018.11.001
33.
Wang, D.; Zhou, L.; Zhou, H.; Hu, H.; Hou, G. 2021. Chemical composition and
protective effect of guava (Psidium guajava L.) leaf extract on piglet
intestines. Journal of the Science of Food and Agriculture. 101(7): 2767-2778. doi: 10.1002/jsfa.10904
34.
Wang, J.; Deng, L.; Chen, M.; Che, Y.; Li, L.; Zhu, L.; Chen, G.; Feng, T.
2024. Phytogenic feed additives as natural antibiotic alternatives in animal
health and production: A review of the literature of the last decade. Animal
Nutrition. 17: 244-264. doi:
10.1016/j.aninu.2024.01.012
35.
Windisch, W.; Schedle, K.; Plitzner, C.; Kroismayr, A. 2008. Use of phytogenic
products as feed additives for swine and poultry. J Anim Sci. 86(14 Suppl):
E140-8. doi: 10.2527/jas.2007-0459
36. Yitbarek, M. B. 2015. Phytogenics as
feed additives in poultry: A review on their effects on gut health.
International Journal of extensive research. 3: 49-60.