Revista de la Facultad de Ciencias
Agrarias. Universidad Nacional de Cuyo. Tomo 57(2). ISSN (en línea) 1853-8665.
Año 2025.
Original article
Effects
of Gibberellic Acid on Flowering Reduction, Fruit Quality and Yield of ‘d’Agen’
Plum (Prunus domestica L.) in Mendoza, Argentina
Efecto
del ácido giberélico sobre la reducción de la floración, calidad de fruta y
rendimiento del ciruelo ‘d’Agen’ (Prunus domestica L.) en Mendoza,
Argentina
Georgina Leoncelli1,
Norma G. Micheloud2,
Hilario Lázaro3,
1Universidad Nacional del Litoral. Facultad de Ciencias Agrarias.
Kreder 2805. (3080), Esperanza. Santa Fe. Argentina.
2ICiAgro Litoral-UNL-CONICET-FCA. Kreder 2805, Esperanza. Santa
Fe. Argentina.
3Estación Experimental Agropecuaria del INTA Rama Caída. El
Vivero s/n. (5600) Rama Caída. Mendoza. Argentina.
*ngarigli@fca.unl.edu.ar
Abstract
In Mendoza, the
primary industrial plum-producing region in Argentina, the ‘d’Agen’ cultivar
represents approximately 90% of the cultivated area. The limited implementation
of fruit thinning has a detrimental effect on final fruit size. The objective
of this study was to determine the timing of flower induction in ’d’Agen’ plum
and to evaluate the response to gibberellic acid (GA) application to reduce
flower density and improve fruit size. Over three growing seasons in San Rafael
(Mendoza), experiments were conducted on plants grafted onto ‘Marianna 2624’,
spaced at 5x3 m and drip irrigated. GA (100 ppm) was applied at four distinct
phenological stages: fruit set, young fruit, fruit near final size,
postharvest, and a control with no GA application. In the first two seasons,
the H phenological stage (fruit set, Baggiolini scale) was identified as the
optimum time for reducing flowering via GA application. In the third season,
increasing GA concentrations (0, 25, 50, 75 and 100 ppm) were evaluated. All
concentrations reduced floral density compared to the control. However, fruit
set was negatively affected by the 75 and 100 ppm treatments. The decline in
flowering (between 60% and 90%) was incompatible with commercial yields. It was
concluded that the optimal time for GA application to reduce floral density in
‘d’Agen’ plum was during phenological stage H. Further research is required to
determine the most effective dose below 25 ppm.
Keywords: chemical thinning, flower induction, fruit load, fruit size, Prunus domestica
Resumen
En Mendoza, la
principal provincia argentina productora de ciruelas para industria, el cv.
‘d’Agen’ representa aproximadamente el 90% de la superficie cultivada. La
limitada aplicación de prácticas de aclareo impacta negativamente en el tamaño
de frutos. El objetivo de este trabajo fue determinar el momento de inducción
floral y evaluar la aplicación de ácido giberélico (GA) para reducir la
densidad floral y mejorar el tamaño de los frutos. Durante tres temporadas, en
San Rafael (Mendoza), se realizaron ensayos con plantas injertadas sobre
‘Marianna 2624’, con espaciamiento de 5x3 m y riego por goteo. GA (100 ppm) se
aplicó en cuatro estadios fenológicos: cuajado de frutos; frutos jóvenes;
frutos próximos al tamaño final; postcosecha; y un control, sin tratamiento de
GA. En las dos primeras temporadas, el estado fenológico H (cuajado de frutos,
Baggiolini) resultó ser el momento óptimo para reducir la floración mediante
aplicaciones de GA. En la siguiente temporada, se evaluaron diferentes
concentraciones de GA (0, 25, 50, 75 y 100 ppm) aplicadas en dicha etapa. Todas
las dosis redujeron la floración en comparación con el control. No obstante,
las dosis de 75 y 100 ppm afectaron negativamente el cuajado de frutos. La
reducción de la floración (entre 60 y 90%) resultó incompatible con
rendimientos comerciales. La etapa fenológica H resultó el momento óptimo de
aplicación de GA con el fin de reducir la densidad floral del ciruelo ‘d’Agen’.
Se requieren investigaciones para evaluar la dosis más efectiva por debajo de
25 ppm.
Palabras clave: carga frutal, inducción floral, raleo químico, tamaño de fruto, Prunus domestica
Originales: Recepción: 08/05/2025-
Aceptación: 23/08/2025
Introduction
World production of
dried plums is estimated at 270,000 metric tons, with the United States, Chile,
France and Argentina as the primary producers and exporters (9). However, the
United States and Chile together account for over 70% of global production. The
predominant cultivar used for dehydration is ’d’Agen’, which accounts for over
98% of worldwide production (16). The increasing
global demand for dried plums can be attributed to the well-documented health
benefits of this fruit (13).
In Argentina,
annual dried plum consumption is estimated at 3,500 metric tons (7). The province of
Mendoza is the main contributor to the industry’s plum production, with 10,000
hectares cultivated and 51,317 metric tons of fresh fruit harvested during the
2020/21 season (9). The cultivated
area underwent expansion between 1992 and 2010 (7), with the ‘d’Agen’
group occupying over 90% of the area (9). In the southern
region of the province (departments of San Rafael and General Alvear),
approximately 70% of “d’Agen” plums are cultivated and dehydrated (9), with 95% destined
for export. However, the ‘Oasis Sur’ region is subject to high climatic risk,
characterized by hailstorms and late frosts, resulting in substantial
fluctuations in its annual production (8).
Under typical
conditions, fruit trees often set more fruit than they can support to reach
satisfactory commercial quality (12). In order to
mitigate these effects, fruit thinning is implemented and is considered one of
the most critical cultural practices in fruit tree management (12,
14, 15).
Floral induction
marks the onset of the reproductive phase and, consequently, the initiation of
competition among developing floral organs. Gibberellins have been identified
as inhibitors of floral induction in several fruit tree species (1). A high fruit
load, particularly in plants bearing fruits with numerous seeds capable of
synthesising gibberellin, has been shown to induce alternate bearing patterns (10).
Aiming at
efficacious agronomic intervention and regulation of floral density, knowing
the precise temporal dynamics of floral induction across different crop and
cultivar types turns imperative. The application of gibberellins during floral
induction has been used to modulate flowering density in various crops,
including citrus (1), apple (14) and stone fruit (2,
5, 6, 15). The reduction in floral bud number resulting from the
application of GA decreases the time required for manual fruit thinning in
peach trees (6). This finding was
corroborated in other stone fruit species (5,
10). In the ‘Opal’ plum cultivar, gibberellin application during
stage I of fruit development effectively reduced floral induction and the next
year’s crop load (11).
In European plum (Prunus
domestica L.), fruit load can be managed through manual, mechanical or
chemical thinning techniques. These practices are used to achieve marketable
fruit size and to mitigate alternate bearing (11). However, manual
thinning is applied in only approximately 10% of plum orchards in Mendoza, as
it is not considered cost-effective (9). The price
received by farmers is strongly dependent on fruit size, with annual decrease in
the international prices of smaller fruits (49–62 fresh units per kg) (7).
Particularly
considering ‘d’Agen’ plums, there is scarce experimental data on the use of
chemical thinning to reduce crop load and improve fruit quality and
profitability (11). Moreover,
knowledge is limited regarding the timing of floral induction and the effect of
GA on flowering reduction and fruit size improvement in this cultivar. The use
of gibberellins has been identified as a potentially cost-effective strategy
for crop load regulation and supporting agronomic decision-making under the
environmental and production conditions of the Oasis Sur region in Mendoza.
The objective of
this study was to determine the timing of floral induction and to evaluate the
optimal concentration of gibberellic acid for reducing flower density and crop
load, thereby improving the commercial fruit size in ‘d’Agen’ plums.
Materials
and methods
The trial was
conducted in a commercial plum orchard in the San Rafael department, Mendoza
province (34°06’S, 68°33’W, 750 m above sea level). It spanned three
consecutive growing seasons, from November 2018 to February 2021. On 5 October
2020, the occurrence of frost led to partial damage as the crop was at the
flowering to fruit set phenological stages. These stages are particularly
susceptible to frost injury in temperate fruit orchards (4).
European plum trees
(Prunus domestica L.) of the ‘d’Agen’ cultivar, 12 years old, grafted
onto ‘Mariana 2624’ (Prunus cerasifera x Prunus munsoniana) were
used. The trees were cultivated in loamy soil, with drip irrigation and
protected from hail damage by nets. They were selected based on their
uniformity in canopy size and trunk diameter, and were trained in a narrow vase
system, with a spacing of 5x3 m. Trunk cross-sectional area (TCSA) was measured
at the beginning of each growing season.
Determination
of Floral Induction Timing (2018/2019 season)
Gibberellic acid
(GA) treatments (Gibberellin KA; S. Ando y Cía. S.A.) were applied at a
concentration of 100 mg l-1 (ppm), with the solution
pH adjusted to 5.5 using 1 M acetic acid. Manual spraying was performed until
runoff, with an average application volume of 2.3 liters per plant.
Applications were conducted in mid-morning at four phenological stages
according to the Baggiolini scale (8), resulting in five
treatment groups: T1, H stage (fruit set, 02/11/2018); T2, I stage (young
fruits, 20/11/18); T3, J stage (fruit near final size, 02/01/19); T4,
postharvest (one week after harvest, 15/02/19). A control group (T0) received
no GA treatment.
Determination
of the Gibberellin doses (2019/2020 season)
In this study, GA
applications were performed on 1 November 2019 at stage H (fruit set). Five
treatments were established based on GA concentration: Control (C, 0 ppm); T1
(25 ppm); T2 (50 ppm); T3 (75 ppm); T4 (100 ppm). Manual fruit thinning was not
applied in any of the treatments.
To monitor the evolution of plant parameters in both trials,
four branches of similar size were selected per tree during the winter period.
These were distributed in the four quadrants of the canopy at a uniform height
of approximately 1.5 m above ground level. The diameter of each branch at the
point of insertion and its total length were measured. Reproductive structures
(flowers and fruits) on the selected branches were counted weekly throughout
the spring. Data were expressed as the number of flowers and fruits per unit of
branch cross-sectional area (cm2).
The relative fruit drop rate (RFDR) was calculated for each interval between
observation dates (13).
Harvesting began
once the fruits reached a minimum soluble solids content of 22°Brix, as
measured using an Arcano DBR0045nD digital refractometer, and a pulp firmness
of 3-4 lb in-2,
as determined using a Turoni FT327 penetrometer equipped with an 8 mm diameter
tip. The total weight and number of fruits per plant were recorded for
subsequent analysis. Results were also expressed as the number of fruits per
unit of trunk cross-sectional area (TCSA). A sub-sample of 50 fruits per plant
was used to measure individual fruit diameter using a SCHWYZ digital caliper.
Commercial fruit size was determined by randomly selecting three 1 kg samples
per tree and counting the number of fruits in each. Fruit size classification
followed the fresh weight standard of the Plum Exporters Committee of Mendoza
(CECIM, unpublished data), which defined three categories: large fruits (<
34 fruits kg-1),
medium fruits (35-48 fruits kg-1),
and small fruits (49-62 fruits kg-1).
In both experiments,
a completely randomized block experimental design was used, with five
replications per treatment, totaling 25 plants per trial. Irrigation (tree row)
was used as a blocking factor. The experimental unit was the individual tree,
while the observation unit comprised the selected branches. Data were then
analyzed using analysis of variance (ANOVA) and means were compared using the
DGC test at a 5% significance level. Statistical analyses were performed using
the INFOSTAT software (3). A general linear
and mixed model was used to analyze variables such as flowering density, fruit
set, and fruit yield. Conversely, variables related to the dynamics of flower
and fruit abscission, including the relative fruit abscission rate, were treated
as repeated measures over time and analyzed with a general mixed model.
Additionally, regression analysis was conducted to evaluate the relationships
between crop load and individual fruit weight, as well as between floral
density, fruit set, and fruit yield.
Results
and discussion
Determination
of Floral Induction Timing
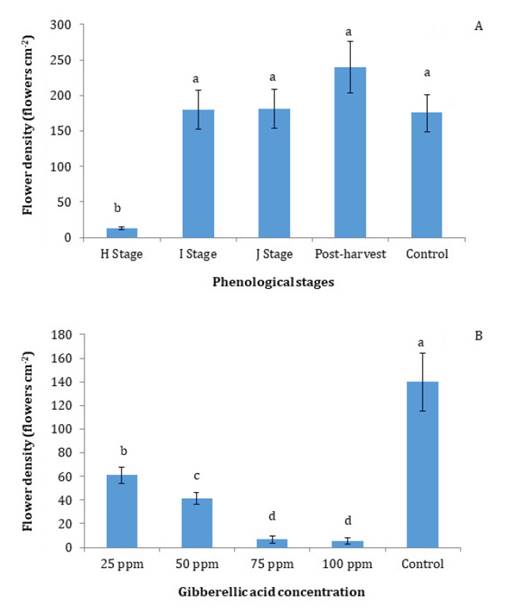
In the control
treatment, flowering density of ‘d’Agen’ plum reached approximately 180 flowers
per cm2 of branch cross-section
area. The application of GA at 100 ppm during the phenological stage H, which
corresponds with the fruit-setting period, five weeks after full bloom,
resulted in a 90% reduction in the flowering density. In contrast, no effect on
flower density was observed when GA was applied in the phenological stages I,
J, and post-harvest (figure
1A).
Different
letters on bars indicate significant differences, DGC test (P <
0.05). Vertical bars indicate standard error. Data correspond to the first year
of the trial. H Stage: fruit set; I Stage: young fruits; J Stage: fruit near
final size; Postharvest: one week after harvest; Control: without treatment.
Letras
diferentes sobre las barras indican diferencias significativas, test DGC (P <
0.05). Barras verticales indican el error estándar. Los datos corresponden al
primer año del experimento. Estado H: cuajado de frutos; Estado I: frutos
jóvenes; Estado J: frutos próximos al tamaño final; Poscosecha: una semana
posterior a la cosecha; Control: sin tratamiento.
Figure
1. Floral density per unit of branch cross-sectional
area (flowers cm-2)
of ‘d’Agen’ plums in response to: (A) application of gibberellic acid (GA; 100
ppm) at different phenological stages of the previous growing season, and (B)
different GA concentrations applied during stage H of the previous growing
season.
Figura
1. Densidad floral por unidad de área
de sección transversal de rama (flores cm-2) del ciruelo ‘d’Agen’ en respuesta a:
(A) aplicación de ácido giberélico (AG; 100 ppm) en diferentes estados
fenológicos de la estación de crecimiento previa, y (B) diferentes
concentraciones de AG aplicado en el estado H de la estación de crecimiento
previa.
The results
indicate that the phenological stage H corresponds to the period of floral
induction for the ‘d’Agen’ plums. This finding is consistent with previous
observations in the European plum, cv. ‘Opal’, where the application of GA five
weeks after full bloom was identified as the most efficacious timing for the
reduction of flowering (11). Later
applications of GA were ineffective, suggesting that floral induction had
already occurred and that the buds were at a more advanced stage of floral
differentiation. This finding is consistent with the established understanding
that gibberellins are only effective when applied before or during the floral
induction period (6).
In the second-year trial, ‘d’Agen’ plum exhibited high
sensitivity to all GA concentrations applied at phenological stage H. Even the
lowest dose (25 ppm GA) resulted in a significant reduction in flowering
density, with a decrease of approximately 60% (figure 1B).
Floral density declined exponentially with increasing GA doses, from 140
flowers cm-2 of branch cross-sectional
area in the control to less than 10 flowers cm-2 in the 75 and 100 ppm GA
treatments. Significant differences in flowering density were observed among
the different GA concentrations, except between the 75 and 100 ppm treatments (figure
1B). The flowering response to the 100 ppm GA application at stage
H was comparable in both years of the study (figure 1A).
The response of ‘d’Agen’ plum to increasing GA concentration is
consistent with findings reported in other fruit-tree crops. In Japanese plums,
the application of 75 and 100 ppm GA, 106 days after full bloom, resulted in a
75-90% reduction in floral density (1).
Similarly, GA application 60 days after full bloom in peach trees reduced
flower number and minimized the time required for manual fruit thinning in
peach trees (6).
Furthermore, the time required for final thinning was inversely correlated with
GA concentration. In nectarines, cultivars ‘May Fire’ and ‘May Glo’ exhibited a
25-40% reduction in flowering following the application of 118 ppm GA, while
the cultivar ‘Zincal’ showed a reduction of up to 65% (2).
Comparable results have been reported in apricot and cherry trees, where 100
ppm GA effectively reduced flower density in the following season (10).
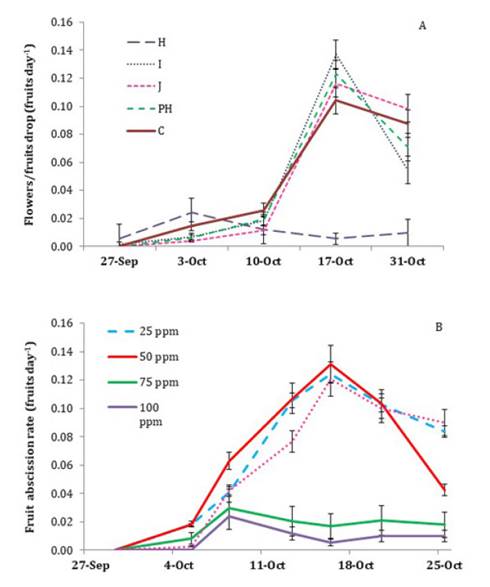
Abscission
of Reproductive Structures
In the control group,
the rate of flower and fruit drop increased markedly after 10 October and
remained high until the end of the month. A similar trend was observed in the
GA treatments at stages I, J, and post-harvest. However, GA application at
stage H showed a one-week advance in flower and fruit drop compared to the
other treatments (figure
2A).
In the second year of the study, the effect of different GA
concentrations at stage H was assessed (figure 2B).
The persistence of reproductive structures and their abscission rate in the 25
and 50 ppm GA treatments exhibited a similar trend to that of the control,
while the 75 ppm GA dose resembled that of the 100 ppm GA treatment. The
highest abscission rate was observed on 17 October, while in the 75 and 100 ppm
GA treatments, the highest abscission rate was recorded one week earlier (7 to
9 October) (figure 2B), as was previously
described for the application of 100 ppm GA at stage H during the first year of
the study (figure
2A).
Vertical
bars correspond to standard error. Data correspond to the first year of the
experiment. H stage: fruit set; I stage: young fruit; J stage: fruit near final
size; PH: post-harvest, one week after harvest; C: control plants without
gibberellic acid treatment.
Barras
verticales indican error estándar. Los datos corresponden al primer año del
experimento. Estado H: cuajado de frutos; Estado I: frutos jóvenes; Estado J:
frutos próximos al tamaño final; poscosecha: una semana posterior a cosecha;
Control: sin tratamiento.
Figure
2. Evolution of flower/fruit abscission rate
(flower/fruits day-1)
of ‘d’Agen’ plum in response to: (A) 100 ppm gibberellic acid (GA) application
at different phenological stages of the previous growing season, and (B)
different concentrations of GA applied during the pit-hardening stage of the
previous growing season.
Figura 2. Evolución
de la tasa de abscisión de flores/frutos (flores/frutos día-1) del ciruelo ‘d’Agen’ en respuesta a:
(A) aplicación de ácido giberélico (AG) en diferentes estados fenológicos, de
la estación de crecimiento previa; (B) diferentes concentraciones de AG
aplicadas durante el endurecimiento del carozo del fruto, de la estación de
crecimiento previa.
The observations
made in mid-October correspond to the first phase of fruit development, known
as stage I, characterized by cell division (2). Additionally, in
the second year, the occurrence of high temperatures and “Zonda” winds during
the flowering period, followed by a late frost, affected the persistence of
flowers and fruits. Despite these differing environmental conditions, the
period of maximum fruit drop for the ‘d’Agen’ cultivar occurred in mid-October
in both years. Furthermore, the H phase is also characterized by the sprouting
and vegetative growth of the plant. These results are of great agronomic
importance, as they indicate that the H phase is a sensitive period for the
‘d’Agen’ plum plant due to the competition between flower induction and
developing fruits, as well as the increases in vegetative growth (12).
Gibberellins affect
flower differentiation and sexual determination, resulting in abnormalities and
masculinizing effects (16). This could
explain the increase in flower/fruit drop in treatments with higher GA
concentrations (75 and 100 ppm). However, this effect was only observed in
treatments applied at the time of maximum sensitivity to flowering inhibition
(H stage).
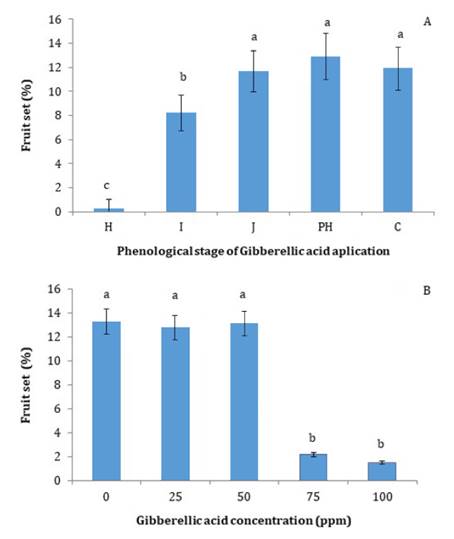
Fruit
Set
Application of GA
at the phenological stage H not only reduced flower density but also led to a
marked decrease in final relative fruit set, which was less than 1% of the
initial number of flowers. GA applications at the later phenological stage
(stage I) also reduced fruit set by about 30% compared to later treatments,
which achieved a fruit set percentage of around 12% (figure 3A).
Means
with different letters within columns indicate significant differences
according to the DGC test (P < 0.05). Vertical bars represent
standard error. H stage: fruit set; I stage: young fruit; J stage: fruit near
final size; PH: post-harvest, one week after harvest; C: control, plants not
treated.
Medias
con diferentes letras en las columnas indican diferencias significativas según
test DGC (P < 0,05). Barras verticales corresponden al error
estándar. Estado H: cuajado de frutos; Estado I: frutos jóvenes; Estado J:
fruto alcanzando el tamaño final; Estado PH: poscosecha, una semana posterior a
la cosecha; C: Control, plantas sin tratamiento con AG.
Figure
3. Fruit set (%) of ‘d’Agen’ plum plants: (A) treated
with 100 ppm gibberellic acid (GA) at different phenological stages during the
previous growing season, and (B) in response to different concentrations of
gibberellic acid (GA) treatments applied at the phenological stage H of the
previous season.
Figura
3. Cuajado de frutos (%) de plantas de
ciruelo ‘d’Agen’: (A) tratadas con 100 ppm de ácido giberélico (AG) en
diferentes estados fenológicos en la estación de crecimiento previa, y (B) en
respuesta a tratamientos con diferentes concentraciones de AG aplicadas en el
estado fenológico H (cuajado de frutos) durante la estación de crecimiento
previa.
In the second year,
at different doses of GA during the H stage, the 75-ppm GA treatment exhibited
the same negative effect on fruit set as the 100-ppm treatment during the
two-year observation period. Conversely, lower concentrations of GA (25 and 50
ppm) did not affect fruit set (figure 3B). This reduction in fruit set can be attributed to the
masculinizing effects of gibberellins, as previously discussed (16). In ’Patterson’
apricot (15), fruit set was not affected by GA
applications; however, in ‘Opal’ plum, fruit set for the following year was
significantly reduced for all GA treatments compared to the control (11).
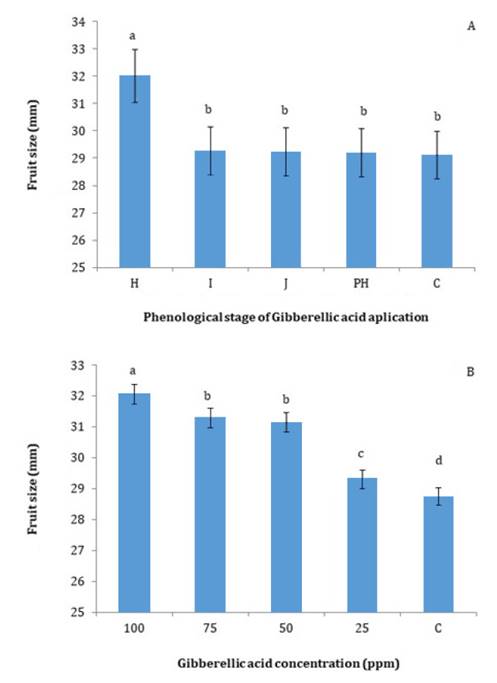
Fruit
Size and Yield
In the first year
of the trial, the application of 100 ppm GA during fruit set (stage H) improved
fruit size at harvest in the following growing season by approximately 3 mm
compared to later applications, which did not differ from each other or from
the control (figure
4A).
Furthermore, according to the regulations of the Plum Exporters Committee of
Mendoza (CECIM), the application of gibberellins at stage H resulted in an
improved fruit size category from ‘small’ (39-62 fruits per kg) to ‘medium’
(35-48 fruits per kg).
Columns
with different letters indicate significant differences according to DGC test (P
< 0.05). Vertical bars represent standard error. H stage: fruit set; I
stage: young fruit; J stage: fruit near final size; Post-harvest: one week
after harvest; Control: untreated plants.
Medias
con diferentes letras en las columnas indican diferencias significativas según
test DGC (P < 0,05). Barras verticales corresponden al error
estándar. Estado H: cuajado de frutos; Estado I: frutos jóvenes; Estado J:
fruto alcanzando el tamaño final; Estado PH: poscosecha, una semana posterior a
la cosecha; C: Control, plantas sin tratamiento con AG.
Figure
4. Fruit size (mm) at harvest of ‘d’Agen’ plums, (A)
treated with 100 ppm gibberellic acid (GA) at different phenological stages
during the previous growing season, and (B) as a function of different GA
concentrations (ppm) applied at the pit-hardening stage of the previous season.
Figura
4. Tamaño de frutos (mm) a cosecha del
ciruelo ‘d’Agen’, (A) tratados con 100 ppm de ácido giberélico (AG) en
diferentes estados fenológicos durante la estación de crecimiento previa, y (B)
en función de diferentes concentraciones (ppm) de AG aplicadas en el estado de
endurecimiento de carozo durante la estación de crecimiento previa.
In the second year,
the range of fruit sizes in the treatments was similar to that observed in the
first year (figure
4A and 4B), despite the large difference in crop load between the two
growing seasons. Fruit size differed significantly among GA concentrations,
except between the 50 and 75 ppm treatments (figure 4B). According to the
Plum Exporters Committee of Mendoza (CECIM), GA concentrations of 50, 75, and
100 ppm resulted in ‘medium-sized’ plums, whereas the 0 and 25 ppm treatments
produced ‘small-sized’ fruits.
Fruit size is influenced by multiple factors, but it is well
established that there is an inverse relationship between the number of fruits
per tree and their final size (5).
Fruit thinning reduces carbohydrate competition among the remaining fruits,
promotes cell division and elongation, and thus ensures a commercially
appropriate fruit size (2).
By reducing flower density through gibberellic acid applications, competition
between reproductive structures is decreased from the outset. As a result, this
technique has the potential to produce larger fruit compared to traditional
fruit thinning methods.
The reduction in
flower density and fruit set percentage induced by 100 ppm GA applied at the
phenological stage H in the previous growing season resulted in a decrease in
the number of fruits per tree and total fruit yield, which decreased from over
30 kg per tree in the control to just over 8 kg per tree in the GA-treated
trees (table
1).
In contrast, the reduction in fruit set induced by GA at stage I had a
significant effect on the number of fruits per plant and per unit of TCSA, but
no effect on fruit weight or total fruit yield (table 1).
Table 1.
Yield components of ‘d’Agen’ plums, (A) treated with 100 ppm gibberellic acid
(GA) at different phenological stages during the previous growing season and
(B) treated with different concentrations of GA in the previous growing season
at fruit set (phenological stage H).
Tabla
1. Componentes del rendimiento del ciruelo ‘d’Agen’, (A)
tratamientos con 100 ppm de ácido giberélico (AG) en diferentes estados
fenológicos durante la estación de crecimiento previa, y (B) tratamientos con
diferente concentración de AG en la estación de crecimiento previa en el estado
fenológico de cuajado de frutos (estado H)
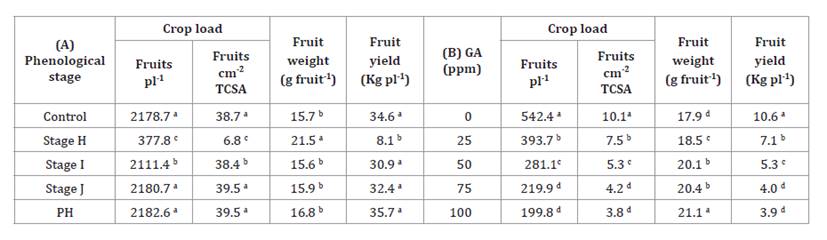
Different
letters in the rows indicate significant differences between treatments
according to DGC test (P<0.05).
References:
H stage: fruit set; I stage: young fruit; J stage: fruit near final size; PH:
post-harvest, one week after harvest; Control: plants not treated; GA:
gibberellic acid.
Letras
distintas en las celdas de cada columna indican diferencias significativas
entre tratamientos según el test DGC (P<0,05).
Referencias:
Estado H: cuajado de frutos; Estado I: frutos jóvenes; Estado J: fruto
alcanzando el tamaño final; PH: poscosecha, una semana posterior a la cosecha;
C: Control, sin tratamiento; GA: ácido giberélico.
In the second year, significant differences were observed among
GA treatments for all yield components. Treatments with higher GA
concentrations resulted in greater reductions in crop load, both expressed as
fruits per tree and per unit TCSA, as well as in total fruit yield (table
1). The 75 and 100 ppm GA treatments showed no significant
differences in yield components, except for fruit weight. In contrast, the 25
and 50 ppm GA treatments and the control trees differed from each other and
from the higher GA treatments in most of the parameters evaluated.
Notably, crop load in the second year was about a quarter of
that observed in the first year. This reduction was attributed to high
temperatures and “Zonda” winds during full flowering, followed by spring frosts
at the fruit set stage, which is the most sensitive period to frost (4).
These events hindered the establishment of optimal gibberellin concentration
for ‘d’Agen’ plums in our trials. In a year marked by extreme weather
conditions, the reduction in flower density resulting from the application of
GA at flower induction had a detrimental effect on the fruit yield per tree.
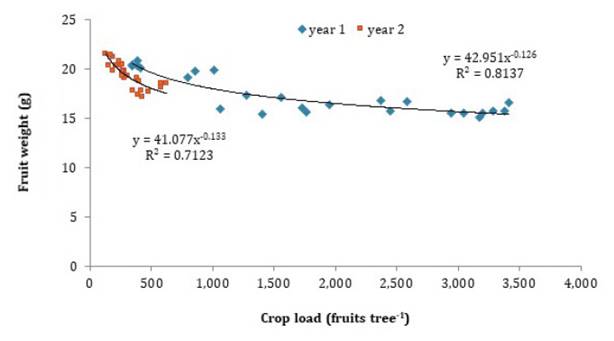
When data on fruit number and size from both years are plotted
on a single graph, it is observed that the initial phase of the experiment was
characterized by a high crop load (2,178 fruits per tree in the control), with
a negative linear correlation between fruit size and crop load (figure
5). This behavior has been observed in plums (5),
and peaches (6, 12).
Data from the second year showed a tendency on the left side of the graph, with
fruit weight values slightly below the trend observed in the first year, but
with increased variation in fruit size relative to crop load (figure
5). This response can be explained by the greater sensitivity of
fruit size to change in the low crop load range of the response curve compared
to the high crop load range, where fruit size tends to stabilize at lower
values (11).
Figure
5. Relationship between crop load (fruits per plant)
and fruit weight (g) over two growing seasons in ‘d’Agen’ plum. Data were
collected during the 2019/2020 and 2020/2021.
Figura 5. Relación
entre carga (frutos por planta) y peso de fruto (g) de dos estaciones de
crecimiento de ciruelo ‘d’Agen’. Datos correspondientes a 2019/2020 y
2020/2021.
Fruit load in the
second year was nearly one-quarter of that measured in the first year, while
fruit size in the control treatment increased by only 2.2 g (14%) compared to
the first year. In general, a reduction in flowering density in stone fruit
trees allows for an increase in fruit size, although this typically results in
a decrease in yield. This phenomenon has been observed in peach (6), nectarine, and
cherry (2), as well as in European and Japanese
plums (11).
Growth of
individual plant organs can be constrained by assimilation capacity (source
limitation) or the ability to utilize assimilates (sink limitation) (2). Fruit capacity to
absorb assimilates is considered the primary factor influencing competition for
these resources. This capacity is initially determined by flower quality, which
is influenced by the nature of the inflorescence and the number of flowers produced
per tree (5). Furthermore, the sink capacity may
be affected by late frosts or other unfavorable environmental conditions that
influence embryo growth. Such damage may result in premature fruit drop or
become apparent during the ripening process. These factors can influence the
shape, appearance, or size of the fruits (4). This may explain
the limited response in fruit size during the second year of the study. Despite
a reduction in crop loads compared to the previous year, no discernible
difference in fruit size was observed between the two years. In situations
where crop loads are low and the growing environment is conducive, source
limitations are considered negligible, and sink strength becomes the main
determinant of growth. This is representative of the second year of the study,
during which each fruit tends to achieve its potential size or weight.
Consequently, it is reasonable to hypothesize that the climatic adversities
experienced during the second year affected sink strength and constrained fruit
size, despite the low competition among developing fruits.
A positive
correlation (r²= 0.84) was observed between floral density and fruit set for
the two growing seasons. However, when the 75 and 100 ppm gibberellin
treatments, which directly affected fruit set, were excluded from the analysis,
fruit set percentage was not influenced by floral density. This pattern was
consistent across a wide range of flowering densities, from 40 to 250 flowers
per square centimeter of branch. This finding is consistent with those reported
for peach (15). Consequently, a
positive linear relationship was evident between floral density and fruit yield
in the ‘d’Agen’ plum trees (y = 0.1496x + 1.65; r2 = 0.86).
To achieve a yield
of between 20,000 and 25,000 kg ha-1 (30–35 kg per tree) for
‘d’Agen’ plums, it is necessary to have a crop load of between 2,000 and 2,500
fruits per plant. This corresponds to an average fruit size of approximately 29
mm or between 16.0 and 16.5 g per fruit, as evidenced by the data obtained in
this experiment. These values align with the typical dimensions of a small
plum, and achieving at least 20 grams per fruit is necessary to reach the
medium size category.
To reach the target
yield components mentioned above, a floral density of over 150 flowers per cm²
of branch is required. The lowest gibberellin dose used in the present study
(25 ppm) resulted in a reduction in floral density of approximately 60%, with
values falling below 100 flowers per cm2.
This is insufficient to achieve the anticipated yield components. Therefore,
reducing floral density to improve fruit size of ‘d’Agen’ plum without a
significant reduction in yield would require the use of a gibberellin
concentration lower than 25 ppm, not evaluated in this study.
On the other hand,
the most consistent response in increased fruit size was observed with a crop
load under 1,000 fruits per tree (figure 5), insufficient for an acceptable fruit yield. Therefore, the
reduction in floral density should be less drastic than that achieved in this
study. Moreover, agronomic management should be complemented with practices
that promote the final fruit size. These included adjustments to pruning
techniques, fertilization, irrigation during critical periods of the crop, and
direct techniques aimed at improving fruit size (12).
Conclusions
The reduction of flowering density with gibberellic acid
application during the previous growing season allowed the determination that
the phenological stage of fruit set (stage H, occurring five weeks after full
bloom) corresponds to the moment of floral induction for ‘d’Agen’ plums.
Gibberellic acid application effectively reduced floral density and modified
fruit size at harvest. However, all tested concentrations excessively reduce
the floral density needed to achieve an acceptable fruit yield. Therefore,
future research should focus on evaluating gibberellic acid concentrations
below 25 ppm, and refining this technique in response to interannual
variability.
Acknowledgements
This study was funded by INTA in collaboration with Universidad
Nacional del Litoral (CAI+D 2024, 85420240100028LI; and PEICID-2023-045).
1. Agustí, M.;
Reig, C.; Martínez-Fuentes, A.; Mesejo, C. 2022. Advances in Citrus flowering:
A review. Frontiers in plant science. 13:868831. DOI: 10.3389/fpls.2022.868831
2. Cerri, M.;
Rosati, A.; Famiani, F.; Reale, L. 2019. Fruit size in different plum species
(genus Prunus L.) is determined by postbloom developmental processes and
not by ovary characteristics at anthesis. Scientia Horticulturae. 255: 1-7.
DOI:10.1016/j.scienta.2019.04.064
3. Di Rienzo, J.
A.; Casanoves, F.; Balzarini, M. G.; González, L.; Tablada, M.; Robledo, C. W.
2020. InfoStat 2020. Centro de Transferencia InfoStat. FCA. Universidad
Nacional de Córdoba. Argentina. http://www.infostat.com.ar
4. Drepper, B.;
Bamps, B.; Gobin, A.; Van Orshoven, J. 2021. Strategies for managing spring
frost risks in orchards: effectiveness and conditionality-A systematic review
protocol. Environmental Evidence. 10: 32. DOI:10.1186/s13750-021-00247-7
5. Erogul, D.; Sen,
F. 2015. Effects of gibberellic acid treatments on fruit thinning and fruit
quality in Japanese plum (Prunus salicina Lindl.). Scientia
Horticulturae. 186: 137-142. DOI:10.1016/j.scienta.2015.02.019
6. Giovanaz, M. A.;
Fachinello, J. C.; Spagnol, D.; Weber, D.; Carra, B. 2016. Gibberellic acid
reduces flowering and time of manual thinning in “Maciel” peach trees. Revista
Brasileira de Fruticultura. 38(2): e-692. DOI:10.1590/0100-29452016692
7. IDR (Instituto
de Desarrollo Rural). 2015. Informe por producto: Panorama del Sector Ciruela
Deshidratada de Mendoza. Argentina http://www.idr.org.ar/wp-content/
uploads/2016/04/Panorama-Ciruela-deshidratada-2015-.pdf/ (Access: 29 April
2025).
8. IDR (Instituto
de Desarrollo Rural). 2020. Informe anual: Fenología de frutales 2020. Mendoza.
Argentina. https://www.idr.org.ar/fenologia-de-frutales/ (Access: 29 April
2025).
9. IDR (Instituto
de Desarrollo Rural). 2021. Censo de ciruela para industria. Producción
primaria. Recolección geoespacial de producción primaria e industrial. Mendoza.
Argentina https://www.idr.org.ar/wp-content/uploads/2022/10/censo_produccion_
primaria.pdf (Access: 29 April 2025).
10. Kaur, A.;
Maness, N.; Ferguson, L.; Deng, W.; Zhang, L. 2021. Role of plant hormones in
flowering and exogenous hormone application in fruit/nut trees: a review of
pecans. Fruit Research. 1: 15. DOI:10.48130/FruRes-2021-0015
11. Lammerich, S.;
Kunz, A.; Damerow, L.; Blanke, M. 2020. Mechanical crop load management (CLM)
improves fruit quality and reduces fruit drop and alternate bearing in European
plum (Prunus domestica L.). Horticulturae. 6: 52. DOI:10.3390/horticulturae6030052
12. Reginato, G.;
Sotomayor, J. P. 2020. Cómo cosechar para obtener fruta de calidad en ciruelo
europeo. Extension UC Davis Chile.
https://www.plataformaextension.cl/ciclos/ciruelo-europeo/ (Access: 29 April
2025).
13. Sidhu, R. S.;
Bound, S. A.; Hunt, I. 2022. Crop load and thinning methods impact yield,
nutrient content, fruit quality, and physiological disorders in ‘Scilate’
apples. Agronomy. 12: 1989. DOI:10.3390/agronomy12091989
14. Wang, S.; Wang,
Q.; Jiang, W.; Wang, Y.; Yan, J.; Li, X.; Wang, J.; Guan, Q.; Ma, F.; Zhang,
J.; Zheng, Q.; Zou, Y.; Xu, J. 2024. Evaluating the sustainable cultivation of
‘Fuji’ apples: suitable crop load and the impact of chemical thinning agents on
fruit quality and transcription. Fruit Research. 4: e009. DOI:
10.48130/frures-0024-0002
15. Yañez Toro, R.
M. 2019. Regulación de la carga frutal en duraznero y nectarino (Prunus
pérsica) cvs. Elegant Lady y Ruby Diamond mediante el uso de giberelinas y
su efecto sobre el retorno floral bajo las condiciones de Chile central.
Master’s thesis, Agronomy and Forest Engineering Course. Pontificia Católica de Chile University.
16. Zhebentyayeva, T.; Shankar, V.; Scorza, R.; Callahan, A.;
Ravelonandro, M.; Castro, S.; DeJong, T.; Saski, C. A.; Dardick, C. 2019.
Genetic characterization of worldwide Prunus domestica (plum) germplasm
using sequence-based genotyping. Horticulture Research. 6: 12. DOI:10.1038/s41438-018-0090-6