Valorization of regional crude glycerol. Culture media optimization for batch docosahexaenoic acid (DHA) production with Aurantiochytrium sp.
Keywords:
crude glycerol, Aurantiochytrium sp., DHA, inoculum, culture mediumAbstract
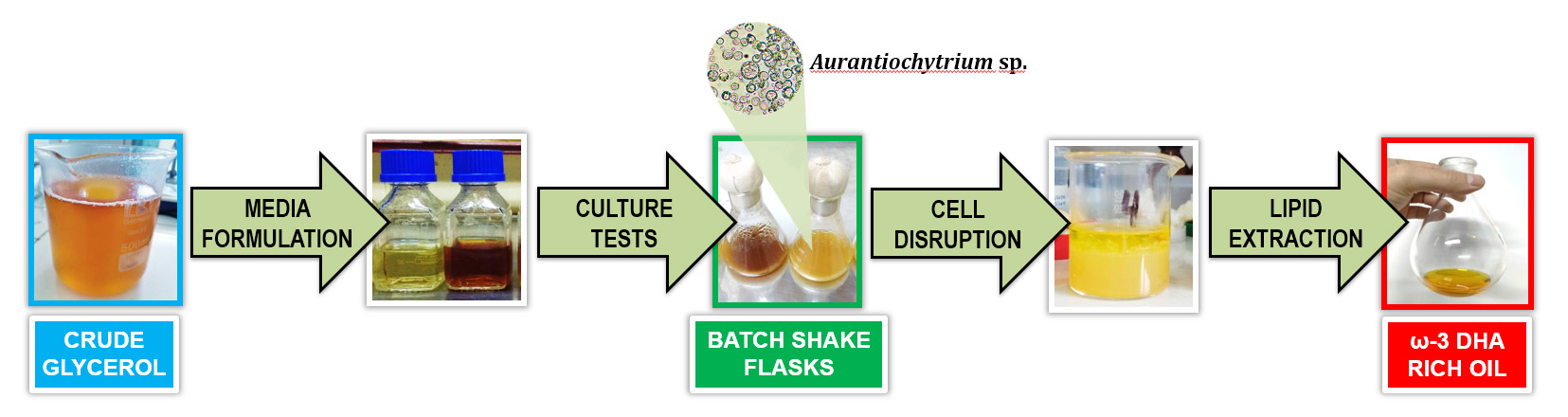
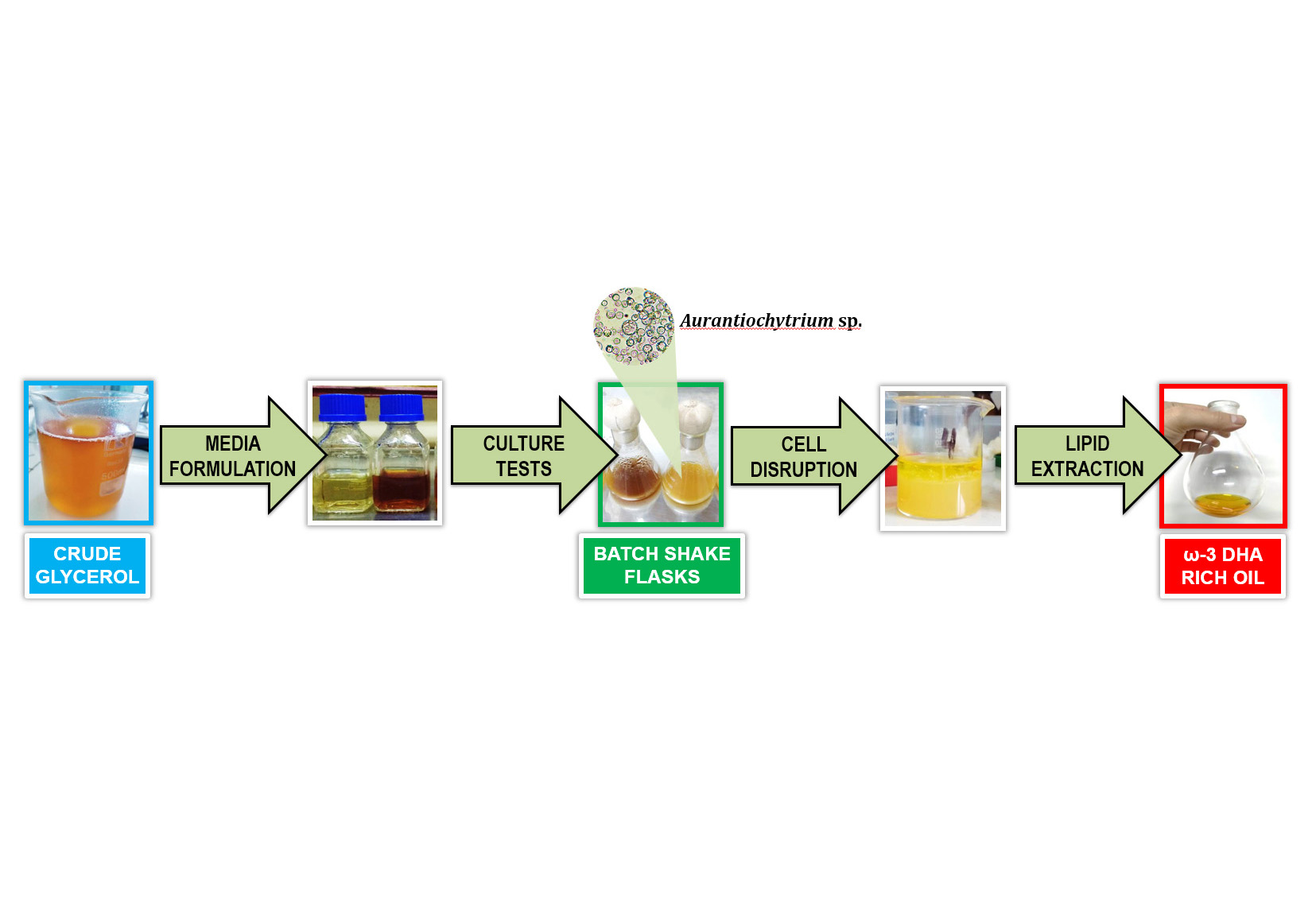
Docosahexaenoic acid (DHA) has many benefits for human health. Commercial DHA sources derive from marine fish but present several production challenges. Aurantiochytrium sp., an abundant marine microalga, becomes an alternative for DHA production. Crude glycerol produced by small-scale biodiesel refineries is a regional, available, and inexpensive waste that can be converted into value-added compounds. This study investigated crude glycerol as a potential carbon source for DHA-rich oil production using an aerobically isolated Aurantiochytrium sp. in batch shake flasks. We also optimized the culture medium formulation by varying carbon and nitrogen sources, thereby reducing medium costs while maximizing DHA production. A larger initial Aurantiochytrium sp. inoculum improved cell concentration and medium carbon depletion, increasing DHA productivity (PDHA). Increasing culture time showed no differences in Aurantiochytrium sp. growth parameters, but reduced DHA production. The absence of yeast extract in the culture media resulted in faster substrate metabolism by Aurantiochytrium sp. and increased DHA production. Crude glycerol yielded the highest PDHA (15.35 mg L-1 h-1) at 120 h. Crude glycerol can be used as a cheaper carbon source in media formulation with Aurantiochytrium sp. cultures for DHA production.
Highlights:
- Crude glycerol is a cheap and highly available carbon source used by Aurantiochytrium for ω-3 DHA-rich oil production.
- Absent yeast extract in culture media enabled faster glucose metabolism, favoring lipid production and improving CTFA and CDHA.
- The largest initial inoculum (10.0% v/v) of Aurantiochytrium improved DCW, substrate depletion and PDHA.
- BCG medium yielded the highest CDHA and PDHA (84 g L-1 and 15.35 mg L-1 h-1, respectively) at 120 h.
- Quality assays of ω-3 DHA-rich oil product showed it is safer for human and animal food formulation.
Downloads
References
Attarbachi, T.; Kingsley, M. D.; Spallina, V. 2023. New trends on crude glycerol purification: A review. Fuel 340: 127485. DOI: 10.1016/j.fuel.2023.127485
Band Schmidt, C. J. 2007. Aislamiento, purificación y mantenimiento de cepas de microalgas. In: Arredondo-Vega, B. O.; Voltolina, D. (Eds.), Métodos y herramientas analíticas en la evaluación de la biomasa microalgal. La Paz (Mexico), Centro de Investigaciones Biológicas del Noroeste. p. 1-11.
Bannon, C. D.; Breen, G. J.; Craske, J. D.; Hai, N. G.; Harper, N. L.; O’Rourke, K. L. 1982. Analysis of fatty acid methyl esters with high accuracy and reliability. III. Literature review of and investigations into the development of rapid procedures for the methoxide-catalysed methanolysis of fats and oils, J. Chromatogr. 247(1): 71-89. DOI: 10.1016/S0021- 9673(00)84857-8
Bautista-Martínez, Y.; Granados-Rivera, L. D.; Jimenez-Ocampo, R.; Maldonado-Jáquez, J. A. 2024. Morphostructural composition and meat quality in local goat kids from the northeastern region of Mexico. Revista de la Facultad de Ciencias Agrarias. Universidad Nacional de Cuyo. Mendoza. Argentina. 56(1): 127-137. DOI: https://doi.org/10.48162/rev.39.129
Campoy, C.; Escolano-Margarit, M.; Anjos, T.; Szajewska, H.; Uauy, R. 2012. Omega-3 fatty acids on child growth, visual acuity neurodevelopment. Br. J. Nutr. 107: S85-106. DOI: 10.1017/ S0007114512001493
Chauton, M. S.; Reitan, K. I.; Norsker, N. H.; Tveteras, R.; Kleivdal, H. T. 2015. A techno-economic analysis of industrial production of marine microalgae as a source of EPA and DHA-rich raw material for aquafeed: research challenges and possibilities. Aquaculture. 436: 95-103. DOI: 10.1016/j.aquaculture.2014.10.038
Chen, X.; Sen, B.; Zhang, S.; Bai, M.; He, Y.; Wang, G. 2021. Chemical and physical culture conditions significantly influence the cell mass and docosahexaenoic acid content of Aurantiochytrium limacinum strain PKU#SW8. Mar. Drugs. 19(12): 671. DOI: 10.3390/md19120671
Du, F.; Wang, Y. Z.; Xu, Y. S.; Shi, T. Q.; Liu, W. Z.; Sun, X. M.; Huang, H. 2021. Biotechnological production of lipid from thraustochytrids. Biotechnol. Adv. 48(8): 107725. DOI: 10.1016/j. biotechadv.2021.107725
EFSA Panel on Nutrition. Novel Foods and Food Allergens. 2020. Safety of Schizochytrium sp. oil as a novel food pursuant to regulation EU 2015/2283. EFSA J 18(10): e06242. DOI: 10.2903/j. efsa.2020.6242
Furlan, V.; Maus, V.; Batista, I.; Bandarra, N. 2017. Production of docosahexaenoic acid by Aurantiochytrium sp. ATCC PRA-276. Braz. J. Microbiol. 48(2): 359-365. DOI: 10.1016/j. bjm.2017.01.001
González-Martinez, A.; Angón, E.; González, M. A.; Rodríguez Tobar, J. M.; Barba Capote, C.; García Martínez, A. R. 2021. Effect of rearing system and sex on the composition and fatty acid profile of Andinoacara rivulatus meat from Ecuador. Revista de la Facultad de Ciencias Agrarias. Universidad Nacional de Cuyo. Mendoza. Argentina. 53(2): 232-242. DOI: https://doi.org/10.48162/rev.39.056
Hermida, L. G.; Gallardo, G. 2015. Food applications of microencapsulated omega-3 oils. In: Sagis L.M.C. (Ed.), Microencapsulation and microspheres for food applications. Academic Press. p. 271-299. DOI: 10.1016/B978-0-12-800350-3.00018-2
Janssen, C. I.; Kiliaan, A. J. 2014. Long-chain polyunsaturated fatty acids (LC-PUFA) from genesis to senescence: the influence of LC-PUFA on neural development, aging, and neurodegeneration, Prog. Lipid Res. 53: 1-17. DOI: 10.1016/j.plipres.2013.10.002
Kamal Eldin, A. 2010. Methods to determine the extent of lipid oxidation in foods. In: Decker, E.; Elias, R.; McClements, D. (Eds.). Oxidation in foods and beverages and antioxidant applications. Understanding mechanisms of oxidation and antioxidant activity. Sawston (UK), Woodhead Publishing. p. 181-195.
Li, J.; Liu, R.; Chang, G.; Li, X.; Chang, M.; Liu, Y.; Jin, Q.; Wang, X. 2015. A strategy for the highly efficient production of docosahexaenoic acid by Aurantiochytrium limacinum SR21 using glucose and glycerol as the mixed carbon sources. Biores. Technol. 177: 51-57. DOI: 10.1016/j.biortech.2014.11.046
Liu, Y.; Zhong, B.; Lawal, A. 2022. Recovery and utilization of crude glycerol, a biodiesel byproduct. RSC Adv. 12: 27997-28008. DOI: 10.1039/D2RA05090K
Lu, Q.; Li, H.; Xiao, Y.; Liu, H. 2021. A state-of-the-art review on the synthetic mechanisms, production technologies, and practical application of polyunsaturated fatty acids from microalgae. Algal Res. 55: 102281. DOI: 10.1016/j.algal.2021.102281
Mariam, I.; Kareya, M. S.; Nesamma, A. A.; Jutur, P. P. 2021. Delineating metabolomic changes in native isolate Aurantiochytrium for production of docosahexaenoic acid in presence of varying carbon substrates. Algal Res. 55: 102285. DOI: 10.1016/j.algal.2021.102285
Martínez-Reyes, I.; Chandel, N. S. 2020. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 11(1): 102. DOI: 10.1038/s41467-019-13668-3
Martins, D.; Custódio, L.; Barreira, L.; Pereira, H.; Ben-Hamadou, R.; Varela, J.; Abu-Salah, K. 2013. Alternative sources of n-3 long-chain polyunsaturated fatty acids in marine microalgae. Marine Drugs. 11(7): 2259-2281. DOI: 10.3390/md11072259
Nazir, Y.; Halima, H.; Al-Shorgani, N. K. N.; Manikan, V.; Hamid, A. A.; Song, Y. 2020. Efficient conversion of extracts from low-cost, rejected fruits for high-valued docosahexaenoic acid production by Aurantiochytrium sp. SW1. Algal Res. 50(5528): 101977. DOI: 10.1016/j. algal.2020.101977
Negro, E.; González, M. A.; Bernal, C. A.; Williner, M. R. 2016. Saturated and trans fatty acids content in unpackaged traditional bakery products in Santa Fe city, Argentina: nutrition labeling relevance. Int. J. Food Sciences Nutr. 68(5): 546-552. DOI: 10.1080/09637486.2016.1268100
Ochsenreitheri, K.; Gluck, C.; Stressler, T.; Fischer, L.; Syldatk, C. 2016. Production strategies and applications of microbial single cell oils. Front. Microbiol. 7: 1539. DOI: 10.3389/ fmicb.2016.01539
Oliver, L.; Dietrich, T.; Marañón, I.; Villarán, M.; Barrio, R. 2020. Producing Omega-3 polyunsaturated fatty acids: A review of sustainable sources and future trends for the EPA and DHA market. Resources 9(12): 148. DOI: 10.3390/resources9120148
Park, W. K.; Moon, M.; Shin, S. E.; Cho, J. M.; Suh, W. I.; Chang, Y. K.; Lee, B. 2018. Economical DHA (Docosahexaenoic acid) production from Aurantiochytrium sp. KRS101 using orange peel extract and low cost nitrogen sources. Algal Res. 29: 71-79. DOI: 10.1016/j. algal.2017.11.017
Patel, A.; Rova, U.; Christakopoulos, P.; Matsakas, L. 2020. Mining of squalene as a value-added byproduct from DHA producing marine thraustochytrid cultivated on food waste hydrolysate, Sci. Total Environ. 736: 139691. DOI: 10.1016/j.scitotenv.2020.139691
Patterson, H. B. W. 2011. Quality and control. In: List, G.R.; King, J.W. (Eds.). Hydrogenation of fats and oils. Theory and practice. AOCS Press. p 329-350.
Pleissner, D.; Lam, W. C.; Sun, Z.; Lin, C. S. 2018. Food waste as nutrient source in heterotrophic microalgae cultivation. Bioresour. Technol. 137: 139-146. DOI: 10.1016/j.biortech.2013.03.088
Plua Montiel, J.; Neira Mosquera, J. A.; Sanchez Llaguno, S. N.; Aldas Morejon, J. P.; Revilla Escobar, K. Y.; Caicedo-Álvarez, E. (en prensa). omparison of fatty acid profiles of sacha inchi oil (Plukenetia huayllabambana), sesame oil (Sesamum indicum), and peanut oil (Arachis hypogaea) using two extraction methods for food purposes. Revista de la Facultad de Ciencias Agrarias. Universidad Nacional de Cuyo. Mendoza. Argentina.
Rosa, S.; Soria, M.; Vélez, C.; Galvano, M. 2010. Improvement of a two-stage fermentation process for docosahexaenoic acid production by Aurantiochytrium limacinum SR21 apply statistical experimental designs and data analysis, Biores. Technol. 101(7): 2367-2374. DOI: 10.1016/j.biortech.2009.11.056
Santos Jesus, S.; Maciel Filho, R. 2010. Modeling growth of microalgae Dunaliella salina under different nutritional conditions. Am. J. Biochem. Biotechnol. 6(4): 279-283. DOI: 10.3844/ ajbbsp.2010.279.283
Sartori, M.; García, D.; Grossi Vanacore, M. F.; Fessia, A.; Nesci, A. (en prensa). Biofungicide formulation based on Bacillus velezensis EM-A8 for control of maize foliar diseases. Revista de la Facultad de Ciencias Agrarias. Universidad Nacional de Cuyo. Mendoza. Argentina.
Siriwardhana, N.; Kalupahana, N. S.; Moustaid-Moussa, N. 2012. Health benefits of n-3 polyunsaturated fatty acids: eicosapentaenoic acid and docosahexaenoic acid. Adv. Food Nutr. Res. 65: 211-222. DOI: 10.1016/B978-0-12-416003-3.00013-5
Verified Market Research. 2023. Global glycerin market size. Report ID 17400. 202 p. www. verifiedmarketresearch.com/product/glycerin-market. Accessed December 2023.
Vonshak, A. 1986. Laboratory techniques for the cultivation of microalgae. In: Richmond, A. (Ed.), Handbook of microalgal mass culture. CRC Press. p. 117-145.
Wolff, R. L. 1995. Content and distribution of trans-18:1 acids in ruminant milk and meat fats. Their importance in European diets and their effect on human milk. J. Am. Oil Chem. Soc. 72(1): 259-272. DOI: 10.1007/BF02541081
Yu, X. J.; Liu, J. H.; Sun, J.; Zheng, J. Y.; Zhang, Y. J.; Wang, Z. 2016. Docosahexaenoic acid production from the acidic hydrolysate of Jerusalem artichoke by an efficient sugar-utilizing Aurantiochytrium sp. YLH70. Ind. Crops Prod. 83: 372-378. DOI: 10.1016/j. indcrop.2016.01.013
Published
How to Cite
Issue
Section
License
Copyright (c) 2018 Revista de la Facultad de Ciencias Agrarias UNCuyo

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License.
Aquellos autores/as que tengan publicaciones con esta revista, aceptan las Políticas Editoriales.










.jpg)




