Physicochemical Properties, Bioactive Compounds, and Antioxidant Activity of Andean Fruits: Optimization of Extraction by Response Surface Methodology
Palabras clave:
bayas andinas, páramo, polifenoles, vitamina C, actividad antioxidante, RSMResumen
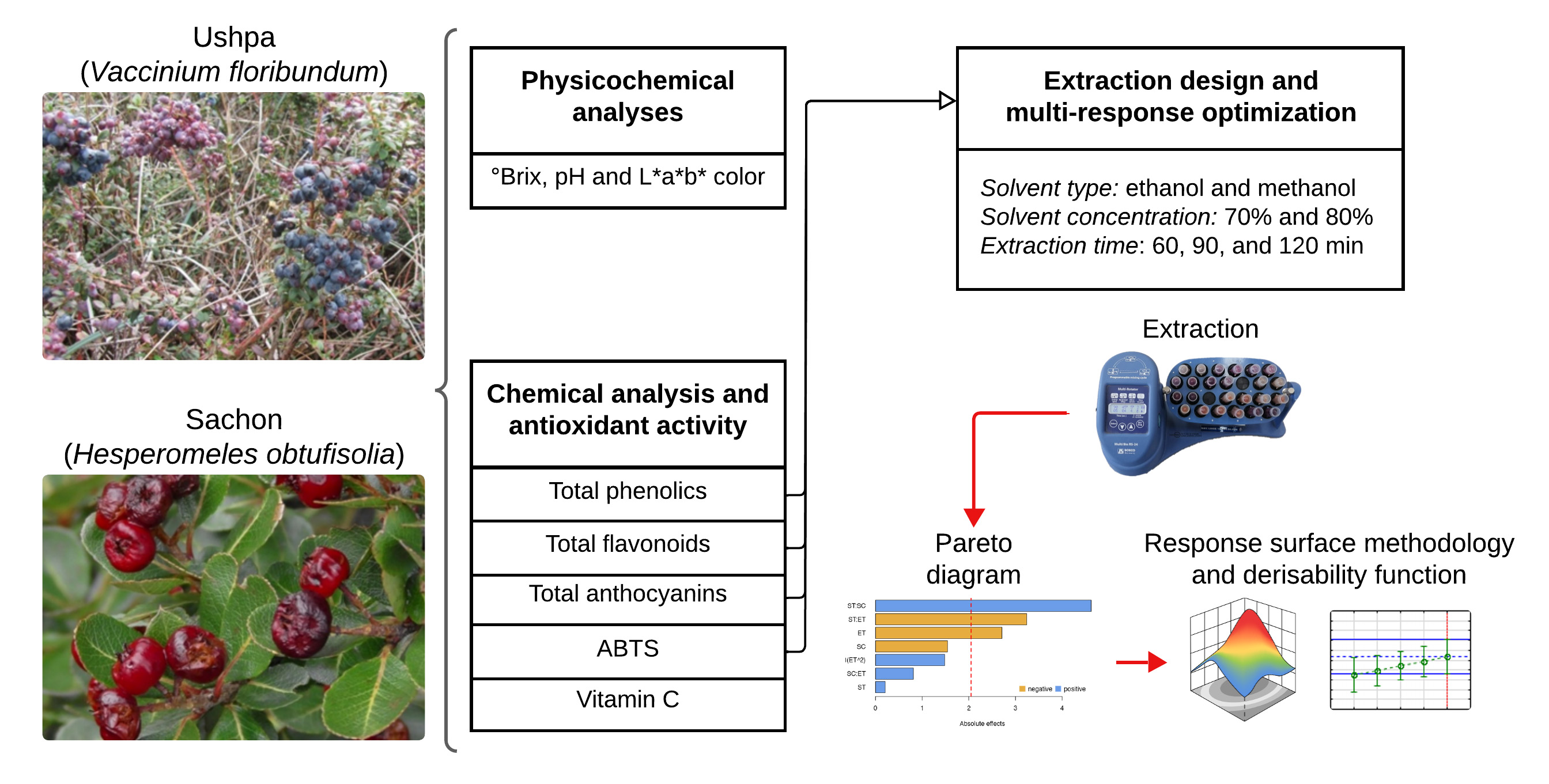
Native fruits from the Peruvian Andes, such as ushpa (Vaccinium floribundum Kunth) and sachon (Hesperomeles obtusifolia (Pers.) Lindl.), grow in high-altitude environments that favor the accumulation of bioactive compounds. However, limited characterization has restricted their sustainable utilization. This study analyzed the physicochemical properties, total phenolic content (TPC), total flavonoid content (TFC), total anthocyanin content (TAC), vitamin C (VC), and antioxidant activity (AA) of both fruits. It also evaluated the effects of solvent type (ethanol and methanol) and concentration (70% and 80%), and extraction time (60, 90, and 120 minutes) on extraction efficiency using response surface methodology. Both fruits exhibited higher levels of bioactive compounds and antioxidant activity compared to other berry species. Optimal conditions for ushpa (64.86% methanol, 139.68 minutes) and for sachon (64.86% ethanol, 90 minutes) yielded TPC = 3,587 and 948 mg GAE/100 g, TFC = 1,821 and 701 mg CE/100 g, TAC = 252 and 8 mg C3G/100 g, and AA = 563 and 501 µmol TE/g, respectively. These findings support further research and potential valorization of these native fruits.
Highlights:
- Ushpa and sachon are two native Peruvian Andean fruits that have not yet been commercialized.
- Ushpa, in particular, exhibited an outstanding chemical composition and antioxidant activity compared to commercial berries.
- The high antioxidant capacity was associated with phenolic richness and adaptation to high-altitude ecosystems.
- Response surface methodology and the desirability function were applied for multi-response optimization.
- The results highlight the potential of ushpa and sachon as functional ingredients.
Descargas
Citas
Abderrahim, F., Huanatico, E., Segura, R., Arribas, S., Gonzalez, M. C., & Condezo-Hoyos, L. (2015). Physical features, phenolic compounds, betalains and total antioxidant capacity of coloured quinoa seeds (Chenopodium quinoa Willd.) from Peruvian Altiplano. Food Chemistry, 183, 83-90. https://doi.org/10.1016/j.foodchem.2015.03.029
Ali, M. N., & Serçe, S. (2022). Vitamin C and fruit quality consensus in breeding elite European strawberry under multiple interactions of environment. Molecular Biology Reports, 49(12), 11573-11586. https://doi.org/10.1007/s11033-022-07849-5
Aliman, J., Michalak, I., Bušatlić, E., Aliman, L., Kulina, M., Radović, M., & Hasanbegović, J. (2020). Study of the physicochemical properties of highbush blueberry and wild bilberry fruit in central Bosnia. Turkish Journal of Agriculture and Forestry, 44(2), 156-168. https://doi. org/10.3906/tar-1902-36
Bayang, J. P., Laya, A., Kolla, M. C., & Koubala, B. B. (2021). Variation of physical properties, nutritional value and bioactive nutrients in dry and fresh wild edible fruits of twenty-three species from Far North region of Cameroon. Journal of Agriculture and Food Research, 4, 100146. https://doi.org/10.1016/j.jafr.2021.100146
Bezerra, M., Ribeiro, M., Cosme, F., & Nunes, F. M. (2024). Overview of the distinctive characteristics of strawberry, raspberry, and blueberry in berries, berry wines, and berry spirits. Comprehensive Reviews in Food Science and Food Safety, 23, e13354. https://doi. org/10.1111/1541-4337.13354
Cerino, M. C., Pinela, J., Caleja, C., Saux, C., Pereira, E., & Barros, L. (2023). Dynamic Maceration of Acerola (Malpighia emarginata DC.) Fruit Waste: An Optimization Study to Recover Anthocyanins. Agronomy, 13, 2202. https://doi.org/10.3390/agronomy13092202
Cuesta-Riaño, C. S., Castro-Guascaa, M. P., & Tarazona-Díaz, M. P. (2022). Anthocyanin Extract from Blackberry Used as an Indicator of Hydrogen Potential. International Journal of Fruit Science, 22(1), 224–234. https://doi.org/10.1080/15538362.2022.2037036
Debnath, S. C., & An, D. (2019). Antioxidant properties and structured biodiversity in a diverse set of wild cranberry clones. Heliyon, 5(4), e01493. https://doi.org/10.1016/j.heliyon.2019. e01493
El Mannoubi, I. (2021). Effect of extraction solvent on phenolic composition, antioxidant and antibacterial activities of skin and pulp of Tunisian red and yellow–orange Opuntia Ficus Indica fruits. Journal of Food Measurement and Characterization, 15(1), 643–651. https:// doi.org/10.1007/s11694-020-00673-0
Frías-Moreno, M. N., Parra-Quezada, R. Á., Ruíz-Carrizales, J., González-Aguilar, G. A., Sepulveda, D., Molina-Corral, F. J., Jacobo-Cuellar, J. L., & Olivas, G. I. (2021). Quality, bioactive compounds and antioxidant capacity of raspberries cultivated in northern Mexico. International Journal of Food Properties, 24(1), 603–614. https://doi.org/10.1080/10942912.2021.19 08352
Giusti, M. M., & Wrolstad, R. E. (2001). Characterization and Measurement of Anthocyanins by UV-Visible Spectroscopy. Current Protocols in Food Analytical Chemistry, 0, F1.2.1-F1.2.13. https://doi.org/https://doi.org/10.1002/0471142913.faf0102s00
Gomes, V., Rendall, R., Reis, M. S., Mendes-Ferreira, A., & Melo-Pinto, P. (2021). Determination of sugar, ph, and anthocyanin contents in port wine grape berries through hyperspectral imaging: An extensive comparison of linear and non-linear predictive methods. Applied Sciences, 11, 10319. https://doi.org/10.3390/app112110319
Guevara-Terán, M., Padilla-Arias, K., Beltrán-Novoa, A., González-Paramás, A. M., Giampieri, F., Battino, M., Vásquez-Castillo, W., Fernandez-Soto, P., Tejera, E., & Alvarez-Suarez, J. M. (2022). Influence of Altitudes and Development Stages on the Chemical Composition, Antioxidant, and Antimicrobial Capacity of the Wild Andean Blueberry (Vaccinium floribundum Kunth). Molecules, 27, 7525. https://doi.org/10.3390/molecules27217525
King, E. S., Cho, J., Li, H., Jiang, X., Madler, A. K., Weishair, M. K., Glenn, S., Brand, M. H., Xu, C., & Bolling, B. W. (2021). Time of harvest affects United States-grown Aronia mitschurinii berry polyphenols, °Brix, and acidity. Journal of Agriculture and Food Research, 6, 100248. https://doi.org/10.1016/j.jafr.2021.100248
Lebedev, V. G., Lebedeva, T. N., Vidyagina, E. O., Sorokopudov, V. N., Popova, A. A., & Shestibratov, K. A. (2022). Relationship between Phenolic Compounds and Antioxidant Activity in Berries and Leaves of Raspberry Genotypes and Their Genotyping by SSR Markers. Antioxidants, 11, 1961. https://doi.org/10.3390/antiox11101961
León-Roque, N., Romero Guzmán, B. M., Oblitas-Cruz, J. F., & Hidalgo-Chávez, D. W. (2023). Optimization of total polyphenol extraction and flavonoid screening by mass spectrometry in mango (Mangifera indica L.) waste from Peru. Food Science and Technology, 43(e105322). https:// doi.org/10.1590/fst.105322
Li, Y., Sun, H., Li, J., Qin, S., Yang, W., Ma, X., Qiao, X., & Yang, B. (2021). Effects of genetic background and altitude on sugars, malic acid and ascorbic acid in fruits of wild and cultivated apples (Malus sp.). Foods, 10, 2950. https://doi.org/10.3390/foods10122950
Liu, Z., Zhang, J., Lu, S., Tang, W., Zhou, Y., & Quek, S. Y. (2022). Effects of different drying methods on phenolic components and in vitro hypoglycemic activities of pulp extracts from two Chinese bayberry (Myrica rubra Sieb. et Zucc.) cultivars. Food Science and Human Wellness, 11(2), 366–373. https://doi.org/10.1016/j.fshw.2021.11.014
Magalhães, L. M., Santos, F., Segundo, M. A., Reis, S., & Lima, J. L. F. C. (2010). Rapid microplate high-throughput methodology for assessment of Folin-Ciocalteu reducing capacity. Talanta, 83(2), 441-447. https://doi.org/10.1016/j.talanta.2010.09.042
Mattson, M. G., Sozzi, A., Corfield, R., Gagneten, M., Franceschinis, L., Schebor, C., & Salvatori, D. (2022). Colorant and antioxidant properties of freeze-dried extracts from wild berries: use of ultrasound-assisted extraction method and drivers of liking of colored yogurts. Journal of Food Science and Technology, 59(3), 944–955. https://doi.org/10.1007/s13197-021- 05096-3
MIDAGRI. (2022). Situación actual de las frutas y verduras en el Perú: Producción, exportación e importación. https://repositorio.midagri.gob.pe/handle/20.500.13036/1227
Monge-Sevilla, R. D., Fernández, L., Espinoza-Montero, P. J., Méndez-Durazno, C., Cisneros-Pérez, P. A., Romero-Estévez, D., Bolaños-Méndez, D., Alvarez-Paguay, J., & Jadán, M. (2024). Chemical composition and antioxidant properties of native Ecuadorian fruits: Rubus glabratus Kunth, Vaccinium floribundum Kunth, and Opuntia soederstromiana. Heliyon, 10, e30593. https://doi.org/10.1016/j.heliyon.2024.e30593
More, P. R., & Arya, S. S. (2021). Intensification of bio-actives extraction from pomegranate peel using pulsed ultrasound: Effect of factors, correlation, optimization and antioxidant bioactivities. Ultrasonics Sonochemistry, 72, 105423. https://doi.org/10.1016/j.ultsonch.2020.105423
Orsavová, J., Juríková, T., Bednaříková, R., & Mlček, J. (2023). Total Phenolic and Total Flavonoid Content, Individual Phenolic Compounds and Antioxidant Activity in Sweet Rowanberry Cultivars. Antioxidants, 12, 913. https://doi.org/10.3390/antiox12040913
Oyarzún, P., Cornejo, P., Gómez-Alonso, S., & Ruiz, A. (2020). Influence of Profiles and Concentrations of Phenolic Compounds in the Coloration and Antioxidant Properties of Gaultheria poeppigii Fruits from Southern Chile. Plant Foods for Human Nutrition, 75(4), 532-539. https://doi. org/10.1007/s11130-020-00843-x
Pascariu, O. E., Dias, L. G., & Israel-Roming, F. (2024). Optimization of Extraction Method of Bioactive Compounds from Elderberries (Sambucus nigra L.) and Testing Extract Stability. Horticulturae, 10(7). https://doi.org/10.3390/horticulturae10070743
Pérez, B., Endara, A., Garrido, J., & Ramírez-Cárdenas, L. (2021). Extraction of anthocyanins from mortiño (Vaccinium floribundum) and determination of their antioxidant capacity. Revista Facultad Nacional de Agronomia Medellin, 74(1), 9453–9460. https://doi.org/10.15446/ rfnam.v74n1.89089
Re, R., Nicoletta, P., Anna, P., Ananth, P., Min, Y., & Catherine, R.-E. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology and Medicine, 26(9-10), 1231-1237.
Rigolon, T. C. B., de Barros, F. A. R., Vieira, É. N. R., & Stringheta, P. C. (2020). Prediction of total phenolics, anthocyanins and antioxidant capacity of blackberry (Rubus sp.), blueberry (Vaccinium sp.) and jaboticaba (Plinia cauliflora (Mart.) Kausel) skin using colorimetric parameters. Food Science and Technology, 40, 620–625. https://doi.org/10.1590/fst.34219
Robles-Apodaca, S. M., González-Vega, R. I., Ruíz-Cruz, S., Estrada-Alvarado, M. I., Cira-Chávez, L. A., Márquez-Ríos, E., Del-Toro-Sánchez, C. L., Ornelas-Paz, J. de J., Suárez-Jiménez, G. M., & Ocaño-Higuera, V. M. (2024). Optimization of Extraction Process for Improving Polyphenols and Antioxidant Activity from Papaya Seeds (Carica papaya L.) Using Response Surface Methodology. Processes, 12(2729). https://doi.org/10.3390/pr12122729
Saad, A. G., Azam, M. M., & Amer, B. M. A. (2022). Quality Analysis Prediction and Discriminating Strawberry Maturity with a Hand-held Vis–NIR Spectrometer. Food Analytical Methods, 15(3), 689–699. https://doi.org/10.1007/s12161-021-02166-2
Sai-Ut, S., Kingwascharapong, P., Mazumder, M. A. R., & Rawdkuen, S. (2023). Optimization of extraction of phenolic compounds and antioxidants from passion fruit and rambutan seeds using response surface methodology. Journal of Agriculture and Food Research, 14(100888). https://doi.org/10.1016/j.jafr.2023.100888
Seki, H., Murakami, H., Ma, T., Tsuchikawa, S., & Inagaki, T. (2024). Evaluating Soluble Solids in White Strawberries: A Comparative Analysis of Vis-NIR and NIR Spectroscopy. Foods, 13(2274). https://doi.org/10.3390/foods13142274
Serea, D., Constantin, O. E., Horincar, G., Stănciuc, N., Aprodu, I., Bahrim, G. E., & Râpeanu, G. (2023). Optimization of Extraction Parameters of Anthocyanin Compounds and Antioxidant Properties from Red Grape (Băbească neagră) Peels. Inventions, 8(59). https://doi. org/10.3390/inventions8020059
Torres-Guevara, F. A., Yupanqui, M. L. G., & Suárez-Rebaza, L. A. (2020). Sustancias bioactivas y actividad antioxidante de frutales nativos de páramos y bosques de neblina del norte peruano. Revista Peruana de Medicina Integrativa, 5(4), 129–134. https://doi.org/10.26722/ rpmi.2020.54.185
Torres-Guevara, F. A., Ganoza-Yupanqui, M. L., Mantilla-Rodriguez, E., Suárez-Rebaza, L. A., & Bussmann, R. W. (2023). Ethnobotany of fruit species native to paramos and cloud forests of Northern Peru. Ethnobotany Research and Applications, 25(10). https://doi.org/10.32859/ era.25.10.1-15
Varo, M. Á., Martín-Gómez, J., Mérida, J., & Serratosa, M. P. (2021). Bioactive compounds and antioxidant activity of highbush blueberry (Vaccinium corymbosum) grown in southern Spain. European Food Research and Technology, 247(5), 1199–1208. https://doi.org/10.1007/ s00217-021-03701-5
Vega, E. N., García-Herrera, P., Ciudad-Mulero, M., Dias, M. I., Matallana-González, M. C., Cámara, M., Tardío, J., Molina, M., Pinela, J., C. S. P. Pires, T., Barros, L., Fernández-Ruiz, V., & Morales, P. (2023). Wild sweet cherry, strawberry and bilberry as underestimated sources of natural colorants and bioactive compounds with functional properties. Food Chemistry, 414(135669). https://doi.org/10.1016/j.foodchem.2023.135669
Descargas
Publicado
Cómo citar
Número
Sección
Licencia
Derechos de autor 2018 Revista de la Facultad de Ciencias Agrarias UNCuyo

Esta obra está bajo una licencia internacional Creative Commons Reconocimiento-NoComercial-CompartirIgual 3.0.
Aquellos autores/as que tengan publicaciones con esta revista, aceptan las Políticas Editoriales.










.jpg)




